Interpret a Scatchard Plot to Assess the KD of a Compound
the lower the number ( concentration ) , the higher the affinity of a drug for the receptor
Let
Let
Let
Scatchard Plot :
graphical method of analyzing equilibrium ligand-binding data
used for determining :
the number of ligand binding sites on a receptor
whether the sites show cooperative interactions
whether more than one site exists
the respective affinities of each site
Y-Axis =
Y-Intercept =
X-axis =
X-Intercept =
Scatchard Plot Equation for Single Binding System :
density of receptors in the tissue
Also note in this form , its the same as standard form :
"Students will be able to interpret a Scatchard plot to assess the Kd of a compound"
So essentially , you just need to know which term is the slope or y-intercept ( they both have a
term ) , and then solve for or
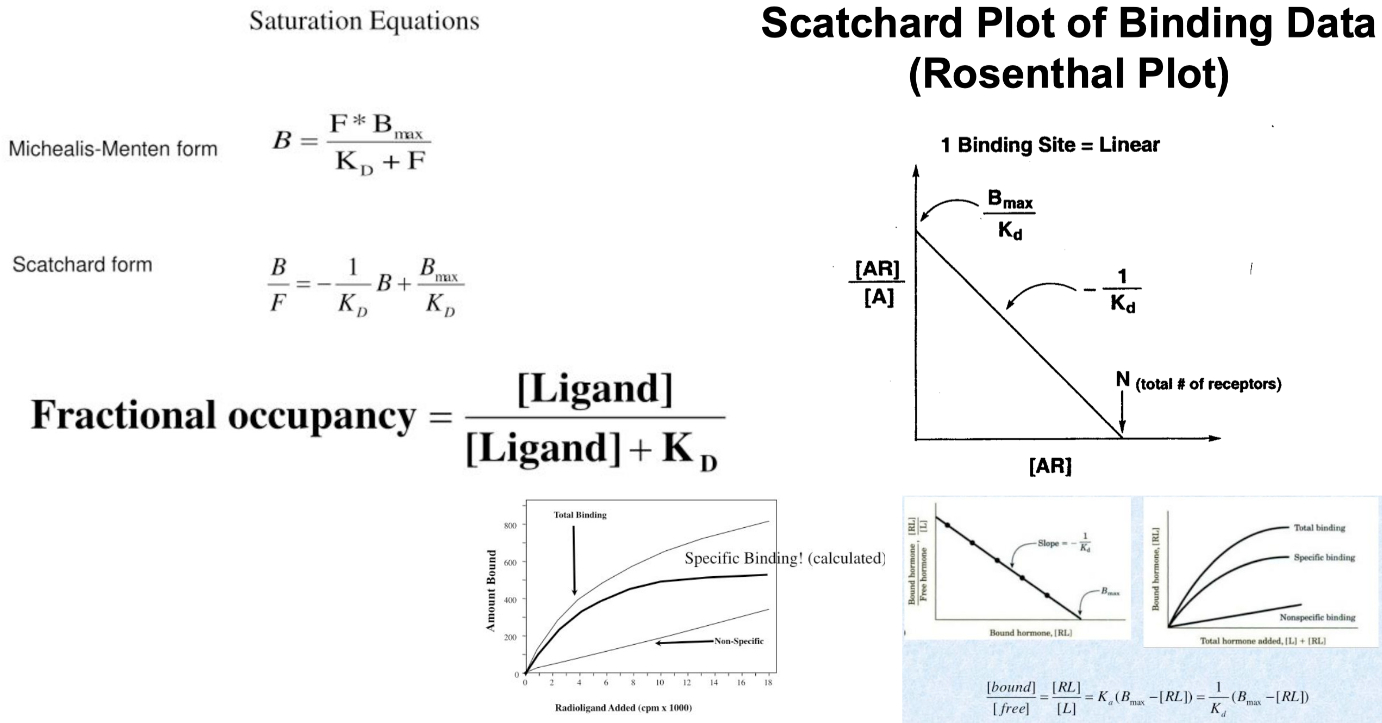
Saturation Equations :
Affinity : How strong is the interaction? (
Capacity : How many sites are there? (
Specificity : Can you distinguish binding of different ligands?
Michaelis-Menten focuses on enzyme kinetics :
It relates substrate concentration to reaction rate and is used to find
Scatchard focuses on binding , not catalysis :
It’s better for studying receptors, antibodies, or protein-ligand interactions where you're interested in how tightly a molecule binds ( affinity ) and how many binding sites are available
Define the Difference Between an Agonist and an Antagonist
Agonist = molecule that binds to a receptor and activates it , producing a biological response
it has both affinity ( binding to the receptor ) and efficacy ( it elicits a biological response )
Antagonist = a molecule that binds to a receptor but does NOT activate it. Instead , it blocks or dampens the effect of an agonist
it has affinity ( it binds ) but no efficacy ( no intrinsic activity )
Define pA2
p = potency
A = antagonist
The pA₂ value tells us how strong the fake ligand ( antagonist ) is at blocking the real ligand ( agonist )
a large pA₂ for the antagonist :
a small amount of antagonist doubles the dose of agonist needed to start the signal
a small pA₂ for the antagonist :
a large amount of the antagonist is needed to block the agonist
Defined as negative logarithm of its molar concentration required to double the agonist dose needed to achieve the same effect.
pharmacological term used to quantify the potency of a competitive antagonist
used as an expression of antagonist potency
where
B = used for antagonist / blocking agents
how tightly the antagonist binds to the receptor
D = used for ligands
Negative Log Approximation :
where
and
Examples :
Thus , a
A
Thus , the larger the
Often calculated from dose-response curves in the presence of various antagonist concentrations
It indicates the concentration at which a competitive antagonist shifts the agonist’s dose-response curve two-fold
i.e., the antagonist concentration that requires doubling of the agonist to achieve the same response
pA2 helps compare relative potencies of different antagonists.
A higher pA2 value indicates a more potent antagonist.
Define the Difference Between Affinity and Efficacy
Affinity = tendency of a drug ( or ligand ) to bind to its receptor
quantified by the
the lower the
Efficacy = the ability of a bound drug to initiate a response
a drug with high efficacy can produce a strong response once bound
Affinity = how well a ligand fits and binds
Efficacy = how effectively that ligand activates the receptor once bound

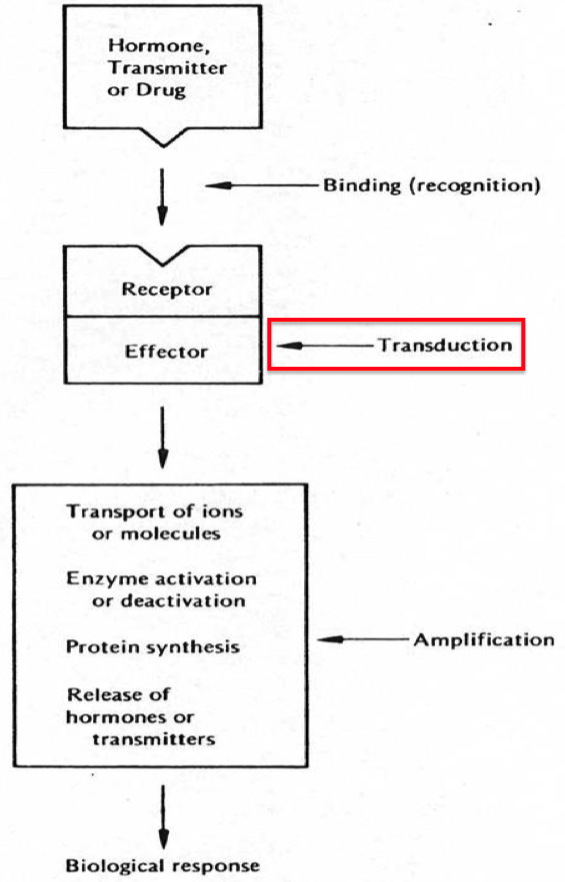
Give Examples of 4 Different Receptor Transduction Mechanisms
What do we mean by "transduction" ?
refers to the process by which the binding of a ligand (e.g., a hormone, neurotransmitter, or drug) to its receptor initiates a series of intracellular events, ultimately leading to a cellular response.
This is a key step in signal transduction , where the extracellular signal (ligand binding) is converted into an intracellular signal that changes the cell's behavior or activity.
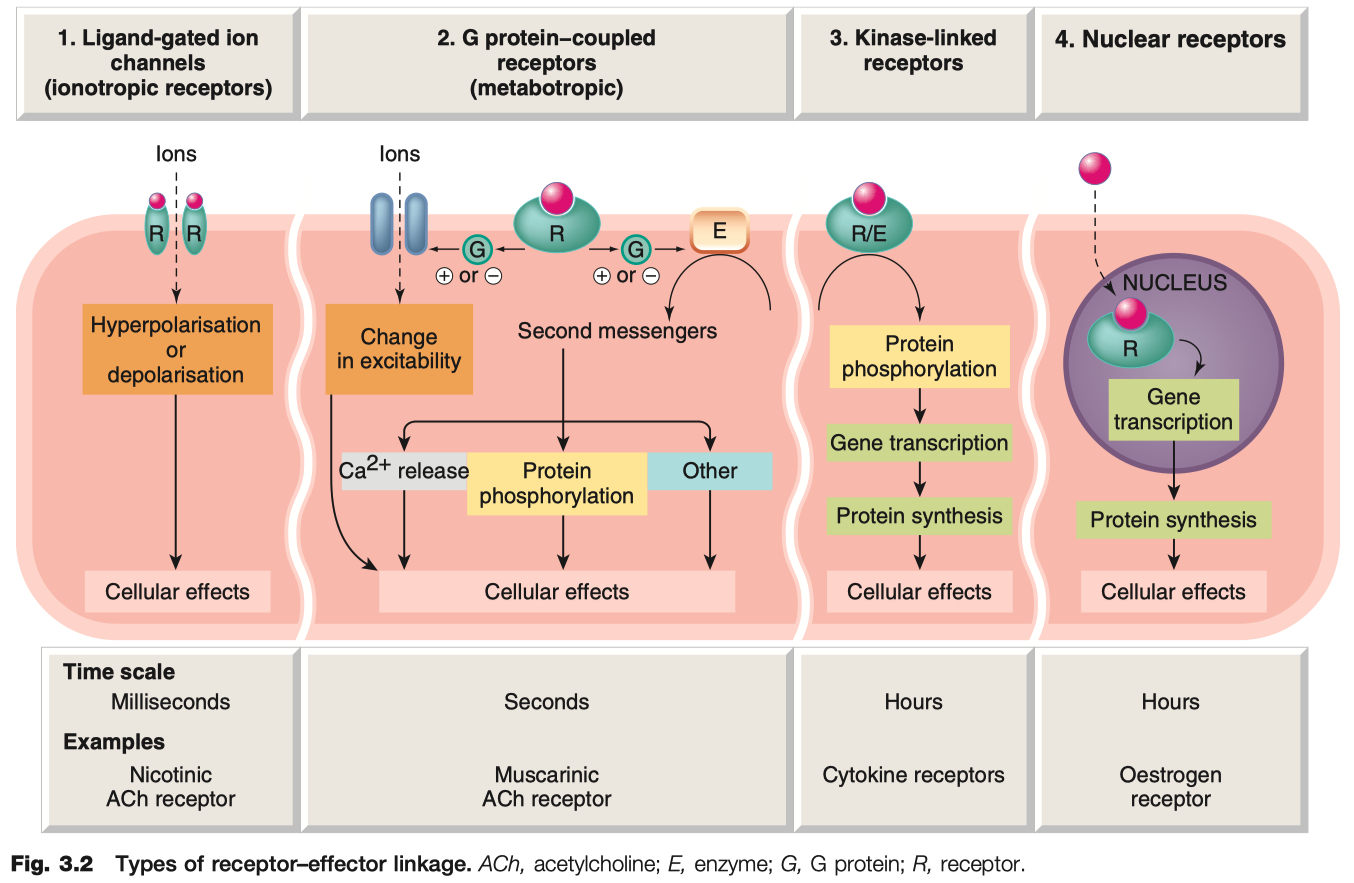
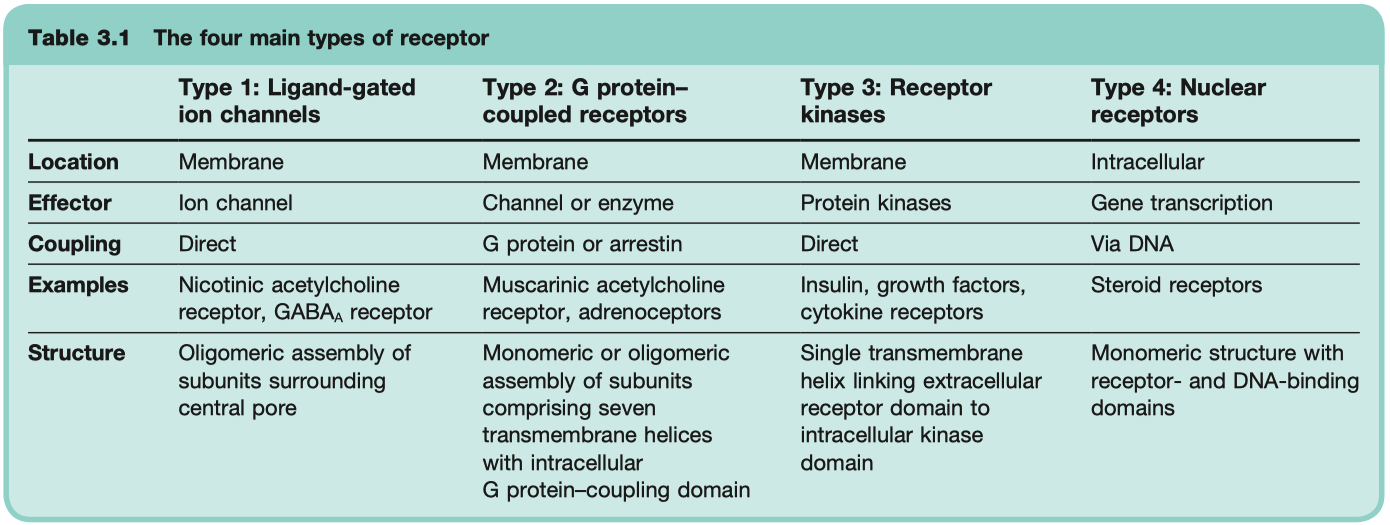
Ligand-Gated Ion Channels
transduce effect in milliseconds
critical for neuronal function
Examples = GABAA Receptor , Nicotinic ACh Receptor , 5HT3 Receptor
Mechanism: Ligand binding → opens ion channel → fast changes in membrane potential or ion flux
G-Protein Coupled Receptors ( GPCR )
critical for neuronal function
Signal Molecule binds and Activates GPCR (
binds and inhibits adenylyl cyclase
reduces PKA activation
reduces cell response ( gene transcription )
Signal Molecule binds and Activates GPCR (
binds and stimulates adenylyl cyclase
increases PKA activation
increases cell response ( gene transcription )
Signal Molecule binds and Activates GPCR (
this depletes levels of PIP2 in the membrane
but voltage gated M-channels ( potassium channels ) need PIP2 in order to open
so M-channels close
this is a SIGNALING event !
the removal of PIP2 closed potassium channels , depolarizes the membrane ,
makes it very easy to fire action potential now
only a little bit of sodium is need to reach the action potential threshold
DAG then activates PKC
IP3 causes calcium to be released from endoplasmic reticulum
the calcium can also reinforce / enhance signaling of PKC
3 Different Ways
Depletion of
Liberation of
Libration of DAG and hence activation of PKC

Describe one alternative pathway that G-protein activation may signal via
Conventional Way = producing signaling molecules
Alternative Way = reduction ! of
Example: β-adrenergic receptors , GABAB , muscarinic ACh , histamine receptors
Mechanism: Ligand binding → G-protein activation ( Gs , Gi , Gq ) → second messenger cascades (e.g., cAMP, IP3/DAG).
Kinase Coupled Receptors
much slower , hours
critical for hormonal function
Example: Insulin receptor ( tyrosine kinase ) , epidermal growth factor receptors , nerve growth factor receptors
Mechanism: Ligand binding → receptor dimerization/activation of intrinsic enzyme activity ( often tyrosine kinase ) → phosphorylation cascades.
Binding of ligand activates intracellular tyrosine kinase domain.
Phosphate from ATP transferred to selected tyrosine residues on side Chains, on receptor and on intracellular signalling proteins.
2 or more receptor oligomers then come together to form dimers or higher multimers.
secondary messenger systems and intermediary products can cross activate with other secondary messenger systems
Nuclear Receptors
very slow , transducer effect in hours
critical for steroid hormone function
Example: Steroid hormone receptors , thyroid hormones , retinoids , and vitamin D
receptors are NOT in the membrane , ONLY in the cytosol
Mechanism: Lipid-soluble ligand enters cell → binds cytosolic or nuclear receptor → alters gene transcription and protein synthesis
receptor generally held in in-active state by inhibitory protein
ligand binds , activating it
receptor moves into nucleus and acts as transcription factor
An important example of hormonal activation of nuclear receptors Is the action of the steroid hormone Cortisol
Misc
Specific signal transduction mechanisms determine the effect that receptor activation has on tissue.
Receptor activation can engage more than one signal transduction mechanism.
Thus the same receptor activated in different tissues may mediate different effects depending on the transduction mechanism it is coupled to.
Thus histamine receptors expressed on a nerve will mediate different effects than the same Histamine receptors on smooth muscle.
The signal transduction pathway may be different (Histamine receptors are terribly promiscuous) and the effectors are almost certainly different.
Which receptors does the excitatory neurotransmitter glutamate act upon?
Ionotorpic Glutamate Receptors = NMDA , AMPA , Kainate
ligand gated
Metabotropic Glutamate Receptors = GPCRs , modulate secondary messanger pathways and synaptic plasticity
Are there selective agonists / antagonists for these receptors? Name some examples
selective agonists = NMDA , Glutamate
selective antagonists = ketamine , memantine
Are any of these drugs used clinically? What for?
Ketamine (NMDA Receptor Antagonist) :
Uses: Dissociative anesthetic, analgesic, and (in lower doses) treatment for treatment-resistant depression.
Mechanism: Non-competitive NMDA receptor blockade, reducing excitatory neurotransmission.
Memantine (NMDA Receptor Antagonist)
Uses: Approved for moderate to severe Alzheimer’s disease.
Mechanism: Uncompetitive (voltage-dependent) NMDA receptor antagonist, helps prevent excessive glutamate-induced excitotoxicity.
Perampanel (AMPA Receptor Antagonist)
Uses: Adjunctive therapy for partial-onset seizures in epilepsy.
Mechanism: Non-competitive AMPA receptor antagonism, reducing excitatory synaptic transmission.
Riluzole (though not a direct, selective receptor antagonist, it modulates glutamate release among other actions)
Uses: Amyotrophic Lateral Sclerosis (ALS) to prolong survival and reduce excitotoxic damage.
Topiramate (broad-spectrum anti-epileptic)
Has multiple mechanisms, including some inhibition of AMPA/kainate receptors.