HLO
Nuclear Localization Sequences ( NLS ) :
Imports = basic : lysine ( K ) , arginine ( R ) , histidine ( H )
Exports = aliphatic : isoleucine ( I ) , leucine ( L )
LxxxLxxLxL
Nuclear Import Process :
cargo-NLS is complexed with an importin
the complex travels through the Nuclear Pore Complex ( NPC ) from the cytosol , into the nucleus
Ran-GTP binds to the complex , allowing the cargo to be released from the importin
Ran-GTP bound to the important exits the nucleus back into the cytosol
Ran-GTP is hydrolyzed into Ran-GDP and the importin is released
Nuclear Localization Signals use the adapters like importins to facilitate movement into the nucleus
Mitochondria also use signal localization sequences
instead of importins , they use TOM / TIM pore complexes
the localization sequence gets cleaved once its inside
depends on heat shock proteins as chaperones
Peroxisomes also use short signal sequences to get proteins from ER ➡️ peroxisome
Signal sequences are also used to direct partially started ribosomal complexes to the ER wall itself
here it can stabilize , and continue translation , dumping the contents into the ER lumen
N-terminal domain enters into the ER lumen first
Glycosylation is very common for rough ER synthesized proteins
the tags mark the state of protein folding
Improperly folded proteins get ubiquitinated , and then exported into the cytosol , to the be broken down in peroxisomes
Accumulation of Improperly folded proteins ,
triggers gene transcription of chaperones to assist in folding
Vesicle Coatings :
Clathrin = normal endocytosis
COPI = golgi ➡️ ER
retrograde , backward direction
COPII = ER ➡️ golgi
anterograde , forward direction , for export
Dynamin = GTPase that pinches off vesicles
uncoats clathrin
Rab = used to guide endosomes cargo to its target location in the cell
SNARES mediate membrane fusion
they need to be separated after
used in exocytosis
Endocytosis :
clathrin = normal
Process : Cargo binds to receptors, adaptors recruit clathrin, and vesicle formation begins. Dynamin constricts the vesicle neck, enabling release.
Uncoating : ATP-driven disassembly of the clathrin coat for vesicle integration with endosomes.
pinocytosis = water
LDL fats = also use clathrin
Exocytosis :
Continuous : Supplies membrane and secretory proteins without regulation
Stimulus-Dependent : Triggered by external signals, often involving calcium influx binding to synaptotagmin, facilitating vesicle fusion with the plasma membrane.
SNARE Complex :
Components: Synaptobrevin ( V-SNARE ) , Syntaxin , and SNAP-25 ( T-SNAREs )
Parkinson's Disease :
can't release or reuptake dopamine
Parkin 1 = accumulates and prevents synaptic vesicle release
𝛼-synuclein = blocks SNARE complexes , aka vesicle fusion
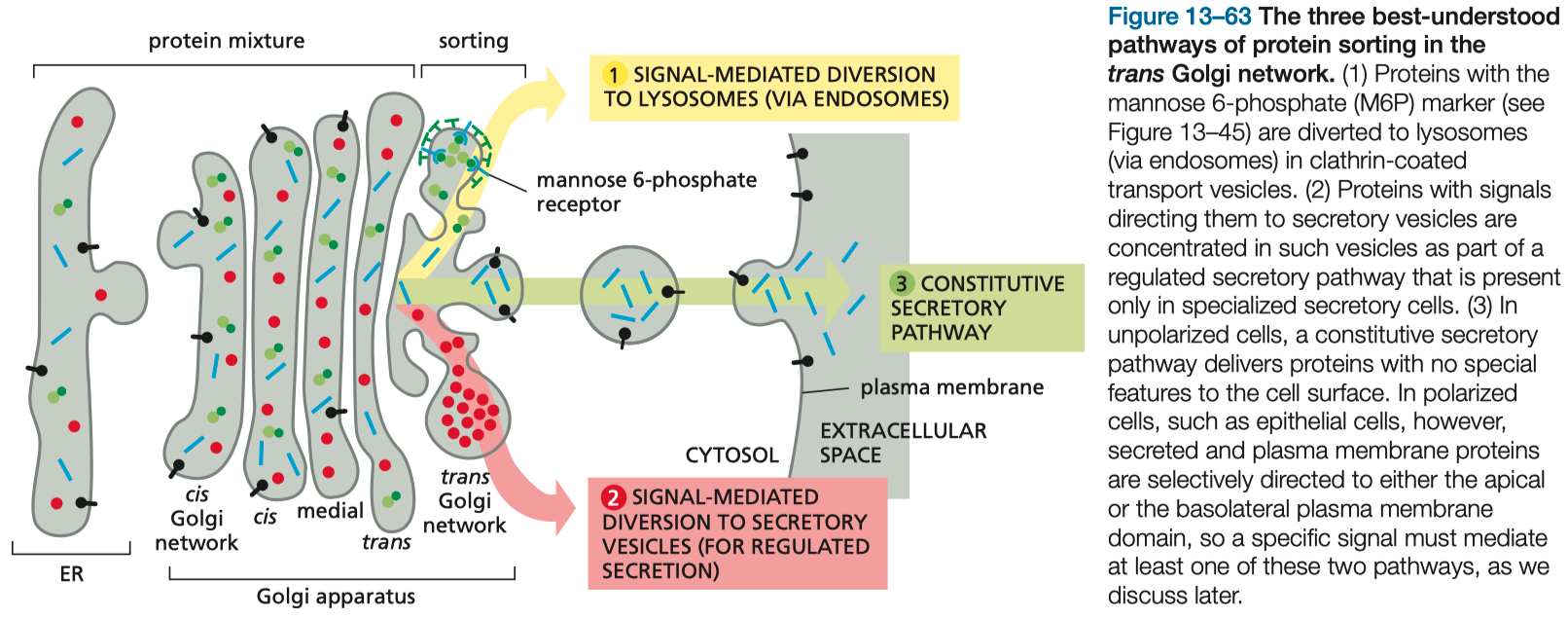
Golgi = sorting device :
lysosomes
secretory vesicles ( signal mediated )
secretory vesicles ( constitutive secretion )
Pathways to a Lysosome :
enocytose , phagocytose , micropinocytose , or autophagy of mitochondrion
Mannose-6-Phosphate Receptors = on golgi , used in early endosome formation
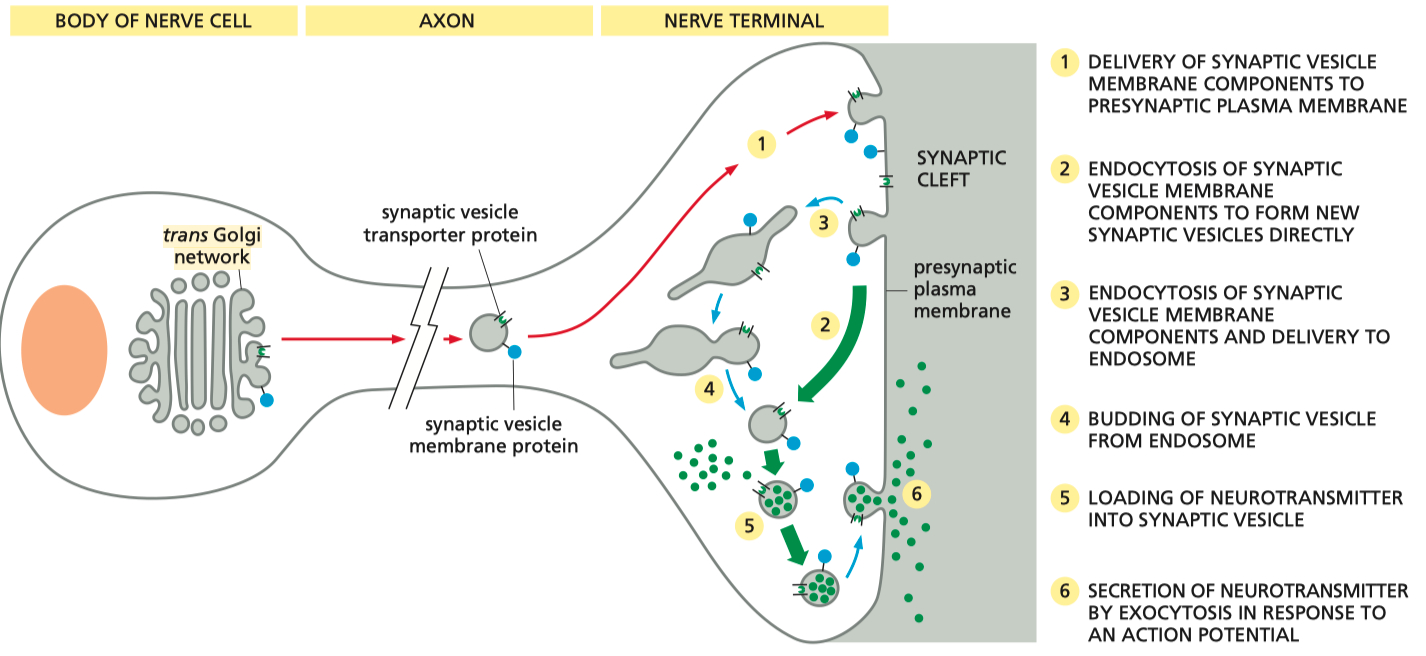
Neurons :
golgi ➡️ synaptic vesicle ( endosome ) ➡️ fuses , offloads contents into synaptic cleft ➡️ contents "re-uptook" / endocytosed back into pre-synaptic terminal
Protein Transport and Sorting Mechanisms
Recognizing Signaling Sequences for Protein Sorting
Function : Signal sequences are short amino acid sequences that function as 'zip codes' to direct proteins to their proper cellular locations.
Examples :
Nuclear Localization Signals ( NLS ) : Directs proteins to the nucleus.
Mitochondrial Targeting Sequences ( MTS ) : Guides precursor proteins to the mitochondria.
Endoplasmic Reticulum ( ER ) Signal Peptides : Target nascent polypeptides to the ER membrane.
Imports = typically , an NLS consists of one or more short stretches of positively charged amino acids, such as lysines ( K ) and arginines ( R )
It can appear as a continuous sequence or in bipartite form ( two basic clusters separated by a few amino acids )
Example = PKKKRKV
Exports = NES sequences are often characterized by a pattern of hydrophobic amino acids, commonly leucine-rich stretches
A canonical NES might look like LxxxLxxLxL , where "L" represents leucines and "x" represents any amino acid
Nuclear Pore Complex ( NPC )
they perforate the nuclear envelope
large , complex structure embedded in the nuclear envelope
act as gatekeepers of the nuclear envelope.
selective gateway for macromolecular traffic
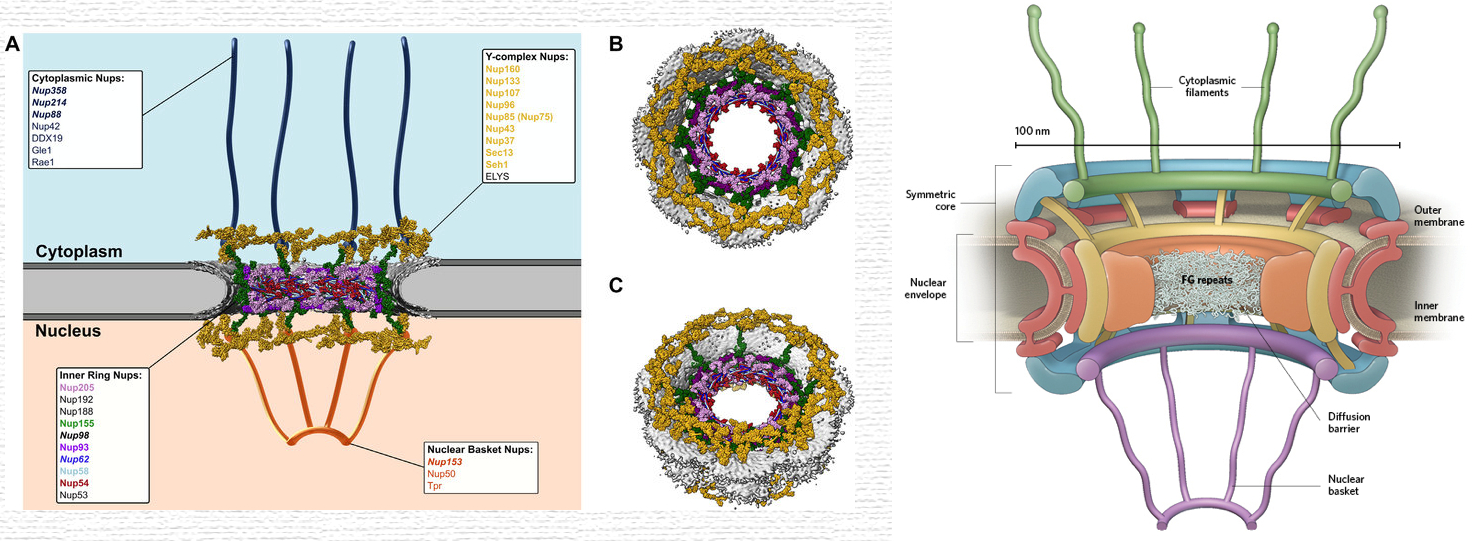
Movement of Proteins Across the Nuclear Pore Complex
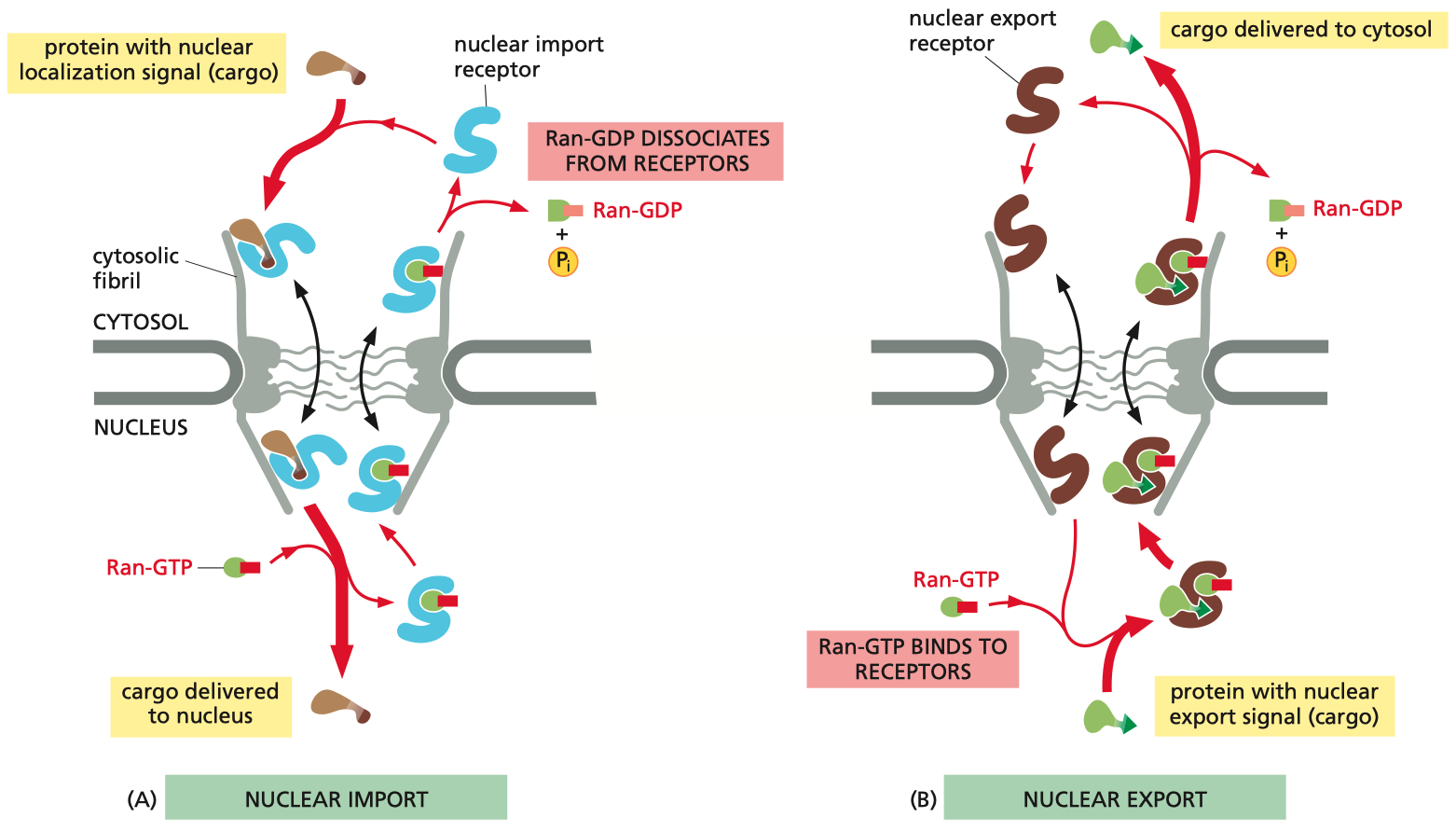
Import Mechanism ( NLS Cargo Proteins ) :
Binding: Cargo proteins with an NLS bind to importins in the cytoplasm.
Transport: The importin-cargo complex translocates through the nuclear pore complex ( NPC ).
Release: In the nucleus, Ran-GTP binds to importins, causing a conformational change that releases the cargo protein.
Recycling: The importin bound to Ran-GTP is transported back to the cytoplasm, where Ran-GAP triggers the hydrolysis of Ran-GTP to Ran-GDP, allowing the importin to be reused.
Export Mechanism ( NES Cargo Proteins ) :
Binding: Cargo proteins with an NES bind to exportins in the nucleus, forming a complex with Ran-GTP.
Transport: The exportin-cargo-Ran-GTP complex translocates through the NPC into the cytoplasm.
Release: In the cytoplasm, Ran-GAP facilitates the hydrolysis of Ran-GTP to Ran-GDP, leading to the release of the cargo protein and exportin.
Recycling: The exportin and Ran-GDP return to the nucleus for reuse, with Ran-GDP being converted back to Ran-GTP by Ran-GEF.
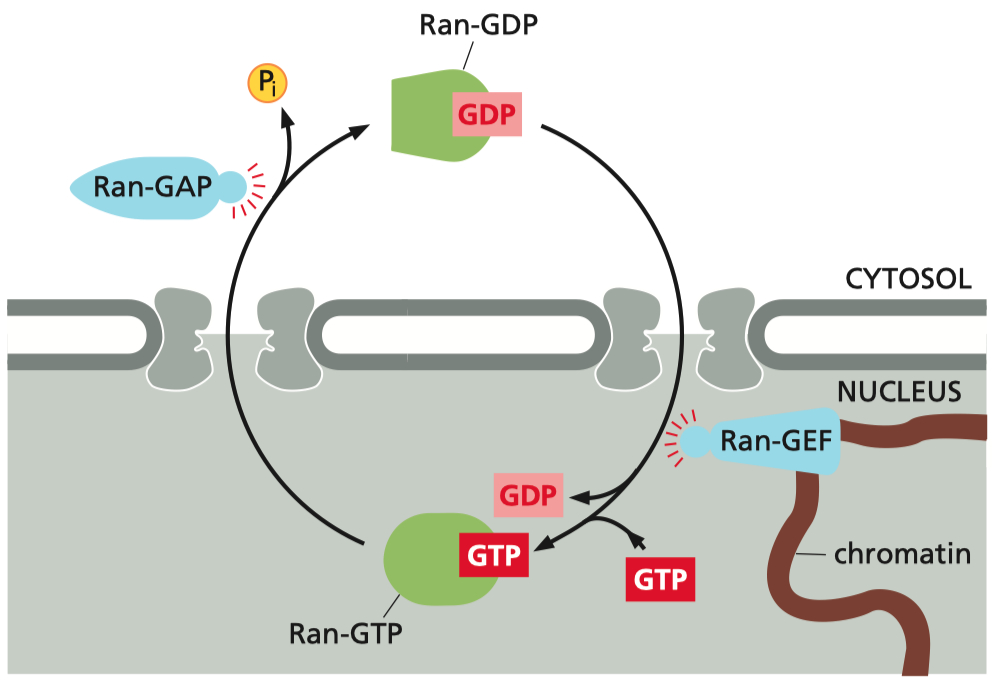
The Role of Ran in Directionality :
The NLS is crucial for recognizing proteins that need to be imported into the nucleus
The NES tags proteins that need to be exported.
The Ran gradient ensures that the binding and release of cargo by importins and exportins occur in the correct compartments ,
maintaining the directionality and efficiency of transport
Ran-GTP binding within the nucleus induces cargo release
Ran-GTP hydrolysis in the cytoplasm resets the receptor
directionality is imposed by the gradient of Ran-GTP and Ran-GDP
Ran-GDP ( inactive form ) : Predominantly located in the cytosol
Ran-GTP ( active form ) : Concentrated in the nucleus
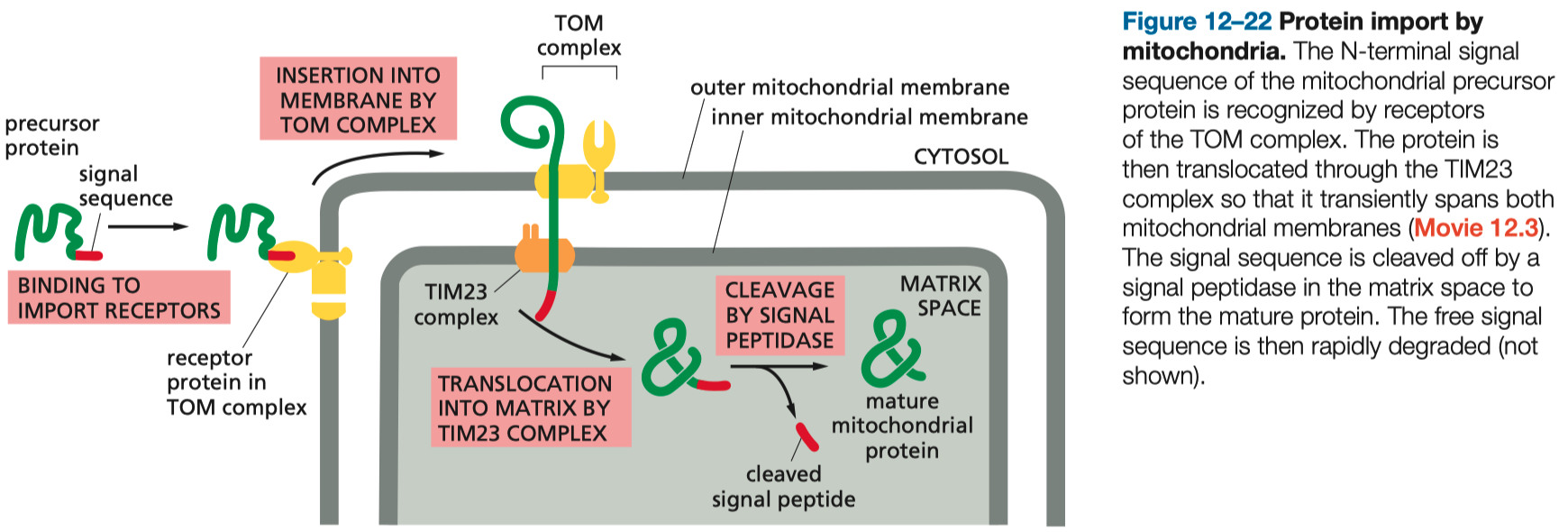
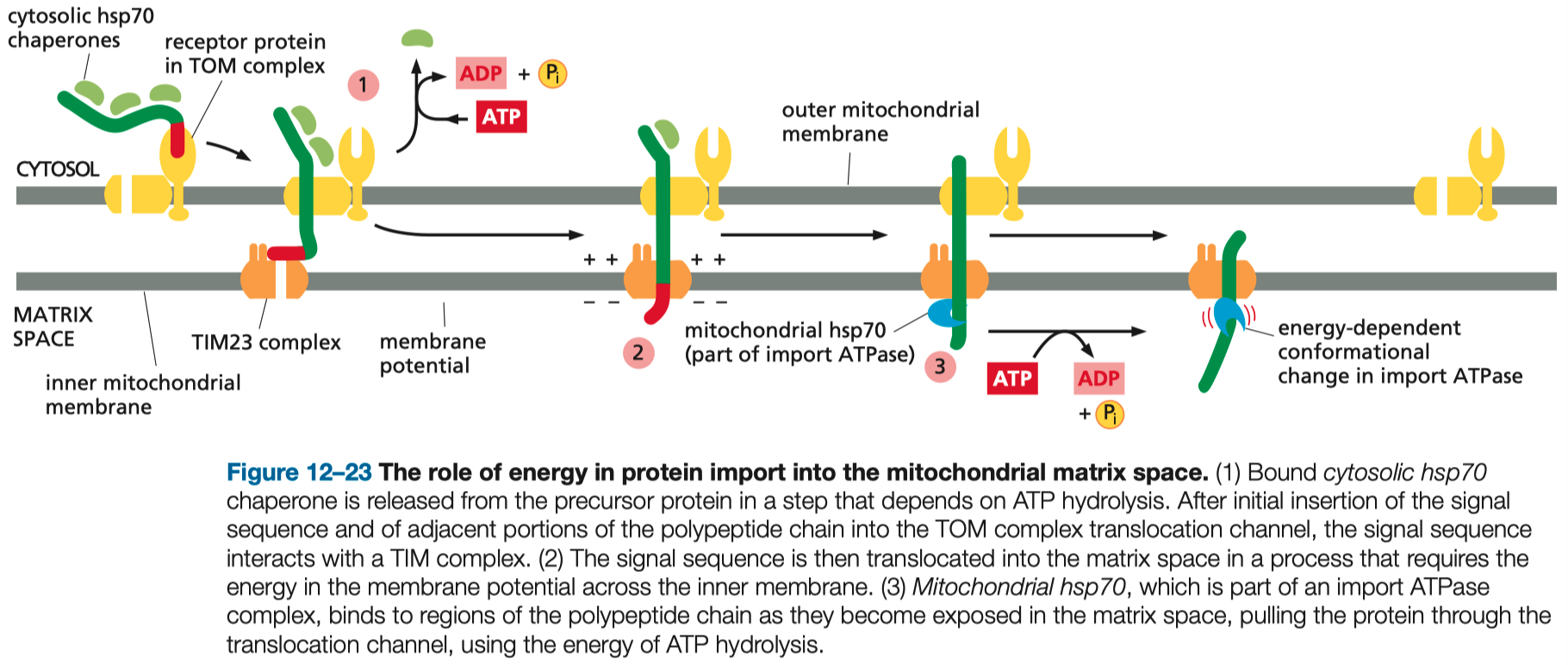
Mitochondrial Protein Import
Signal Sequences: Mitochondrial proteins are tagged with N-terminal presequences that are recognized by mitochondrial translocases.
Key Translocases:
TOM Complex ( Translocase of the Outer Membrane ) : Initial entry site for precursor proteins.
TIM Complexes ( Translocase of the Inner Membrane ) : Facilitate transport across the inner membrane or into the mitochondrial matrix.
Energy Requirements:
ATP Hydrolysis: Required for chaperones assisting protein translocation.
Membrane Potential: Drives precursor proteins through the inner membrane.
Folding Mechanisms:
Proteins must remain unfolded during translocation, mediated by cytosolic and mitochondrial chaperones.
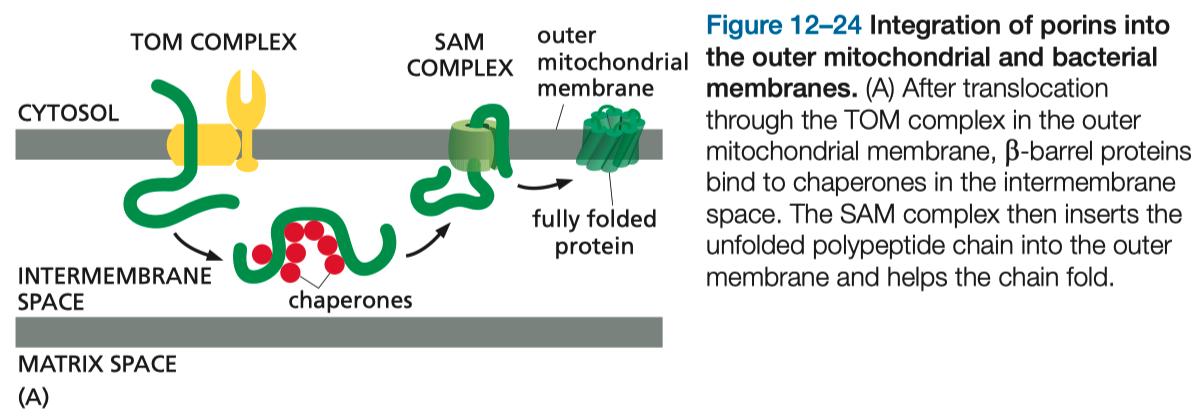
Protein Import into Peroxisomes
A short Signal Sequence Directs the Import of Proteins into Peroxisomes
ER ➡️ Peroxisome Precursors ( empty )
signal recognition sequence on unfolded protein allows entry , forming mature peroxisome
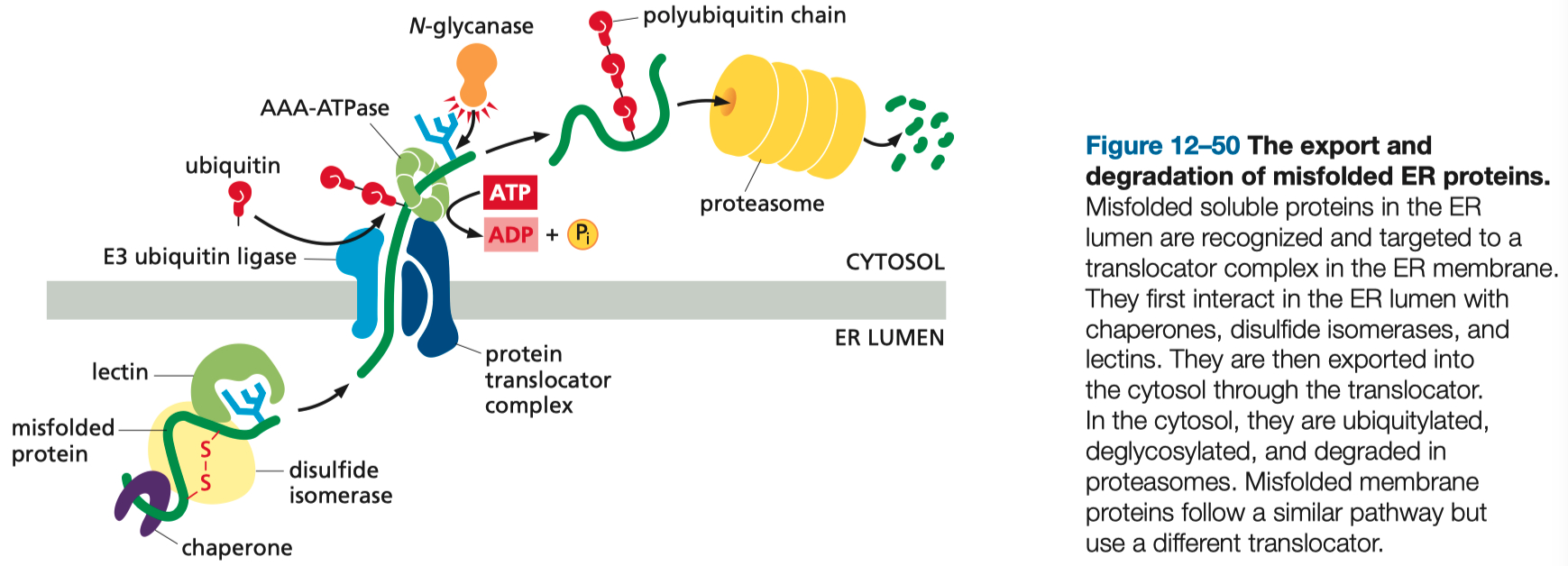
Unfolded Protein Response ( UPR ) in the ER
UPR = includes an increased transcription of genes encoding proteins involved in retrotranslocation and protein degradation in the cytosol, ER chaperones, and many other proteins that help to increase the protein-folding capacity of the ER.
Trigger: Accumulation of misfolded proteins in the ER lumen.
Outcome: Enhanced transcription of chaperones and folding enzymes to manage ER stress. Failure to restore homeostasis can trigger apoptosis.
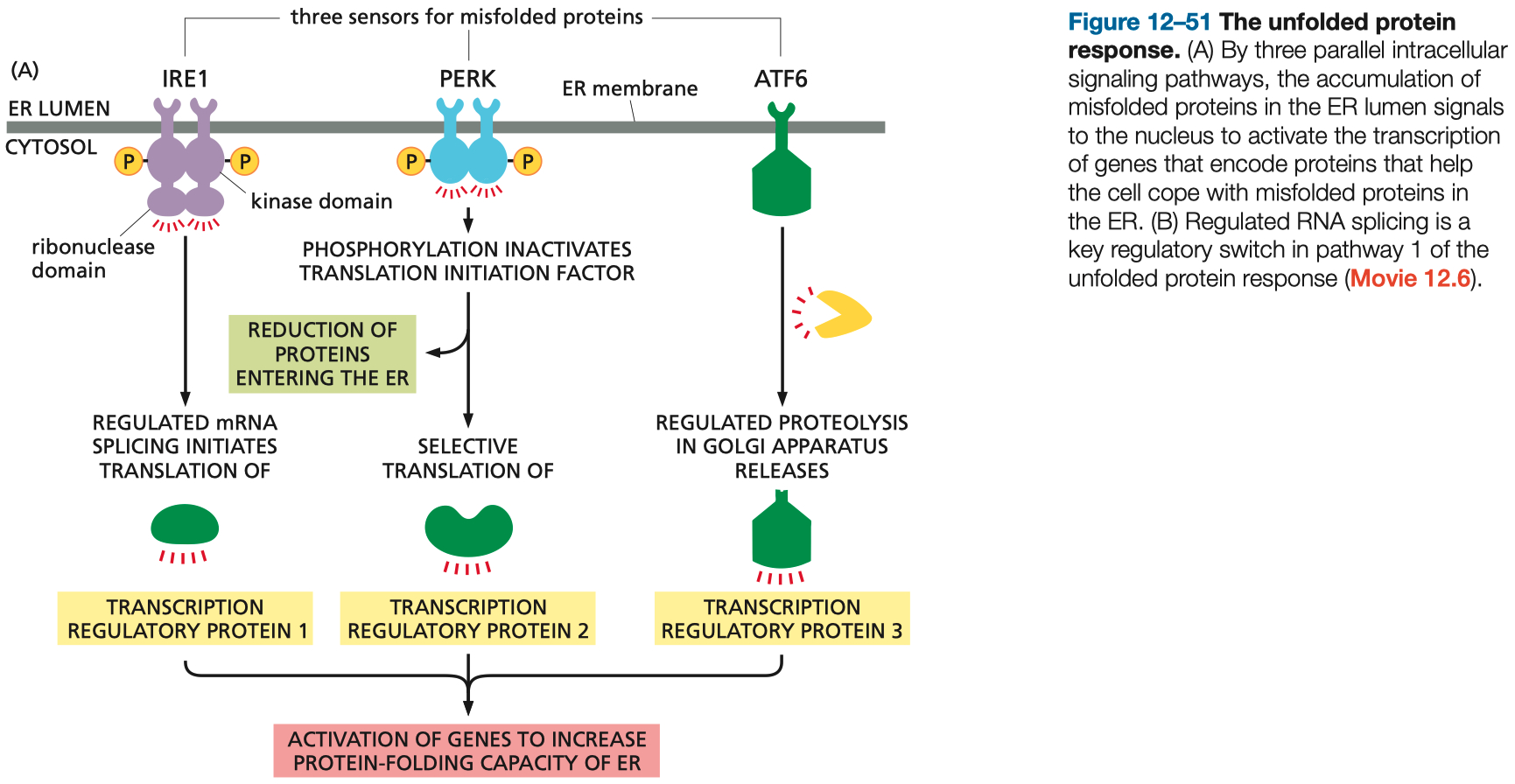
Mechanism :
Misfolded Proteins Sensors ( IRE1 , PERK , ATF6 ) detect high amount of Misfolded Proteins in the ER
Create Transcription Factors
Activation of Genes that aid in Protein Folding ( chaperones )
Signal Sequences were First Discovered in Proteins Imported into the Rough ER
A Signal Recognition Particle Directs the ER Signal Sequence to a Specific Receptor
Most Proteins Synthesized in the Rough ER are Glycosylated by the addition of a common N-linked Oligosacchararide
Oligosaccharides are used as Tags to Mark the State of Protein Folding
Improperly Folded Proteins are Exported from the ER and Degraded in the Cytosol
Translocation across ER membrane does not always require ongoing polypeptide chain elongation
In Single-Pass Transmembrane Proteins, a Single Internal ER Signal Sequence Remains in the Lipid Bilayer as a Membrane spanning alpha Helix
The UPR is a cellular stress response related to the endoplasmic reticulum (ER).
When misfolded proteins accumulate, signaling pathways are activated to enhance protein-folding capacity, degrade misfolded proteins, and reduce general protein synthesis to restore homeostasis.
If unsuccessful, apoptosis may be triggered.
Vesicle Sorting and Transport Pathways
Types of Coated Vesicles :
Clathrin-Coated Vesicles:
Function: Mediate endocytosis from the plasma membrane to endosomes and transport between the Golgi and endosomes.
Adaptor Proteins: Link cargo receptors to clathrin, facilitating vesicle formation.
COPI-Coated Vesicles:
Function: Retrograde transport from the Golgi apparatus back to the ER.
COPII-Coated Vesicles:
Function: Anterograde transport from the ER to the Golgi.
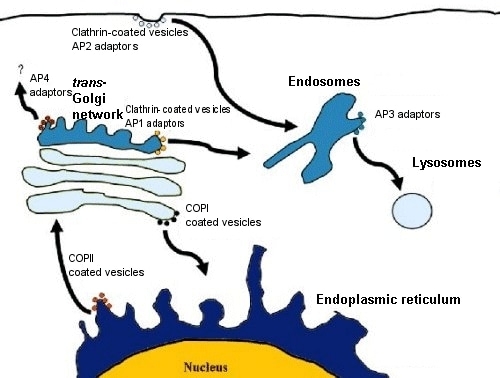
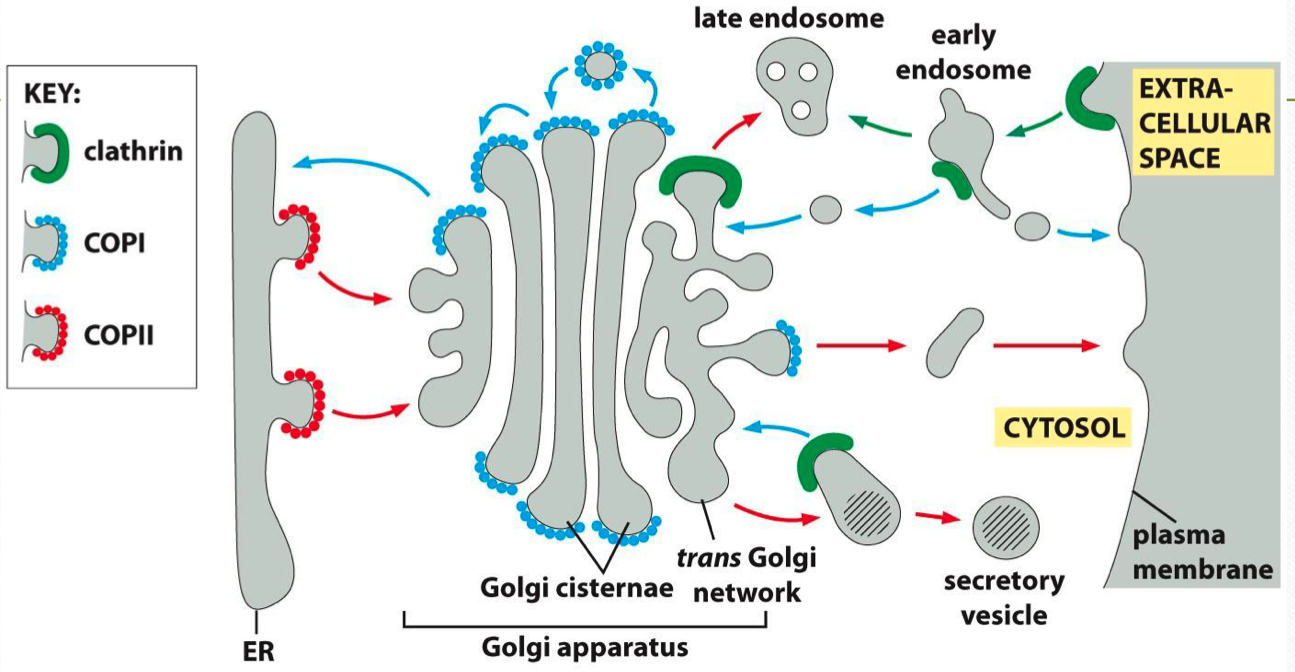
Vesicle Formation and Uncoating :
Adaptor Proteins: Recognize and bind cargo, linking them to coat proteins like clathrin.
Dynamin: A GTPase that pinches off vesicles from donor membranes through GTP hydrolysis.
Rab GTPases: Guide vesicles to their target membranes by tethering vesicles to effector proteins.
SNARE Proteins: Mediate fusion by forming a complex between v-SNAREs on the vesicle and t-SNAREs on the target membrane, facilitating membrane merging.
Endocytosis:
Clathrin-Mediated :
Process : Cargo binds to receptors, adaptors recruit clathrin, and vesicle formation begins. Dynamin constricts the vesicle neck, enabling release.
Uncoating : ATP-driven disassembly of the clathrin coat for vesicle integration with endosomes.
Pinocytosis : Non-clathrin-mediated uptake, common for fluid-phase endocytosis.
LDL Receptor-Mediated : Utilizes clathrin-coated pits for cholesterol transport.
Exocytosis :
Constitutive Pathway :
Continuous: Supplies membrane and secretory proteins without regulation.
Regulated Pathway :
Stimulus-Dependent : Triggered by external signals, often involving calcium influx binding to synaptotagmin, facilitating vesicle fusion with the plasma membrane.
SNARE Complex :
Components: Synaptobrevin ( V-SNARE ) , Syntaxin , and SNAP-25 ( T-SNAREs )
Regulation: Complexin stabilizes the partially assembled SNARE complex, while calcium binding to synaptotagmin catalyzes membrane fusion.
Golgi Apparatus Function and Protein Sorting
Compartmentalization: The Golgi is composed of cis , medial , and trans compartments
Processing:
N-linked Glycosylation: Modified further in the Golgi.
Post-translational Modifications: Includes phosphorylation, sulfation, and addition of sugar moieties.
Sorting Pathways:
Signal-Mediated Diversion to Lysosomes: Involves mannose-6-phosphate tagging and receptor recognition.
Regulated Secretory Pathway: Stores proteins for release upon stimulation.
Constitutive Pathway: Continuous secretion of cellular proteins.
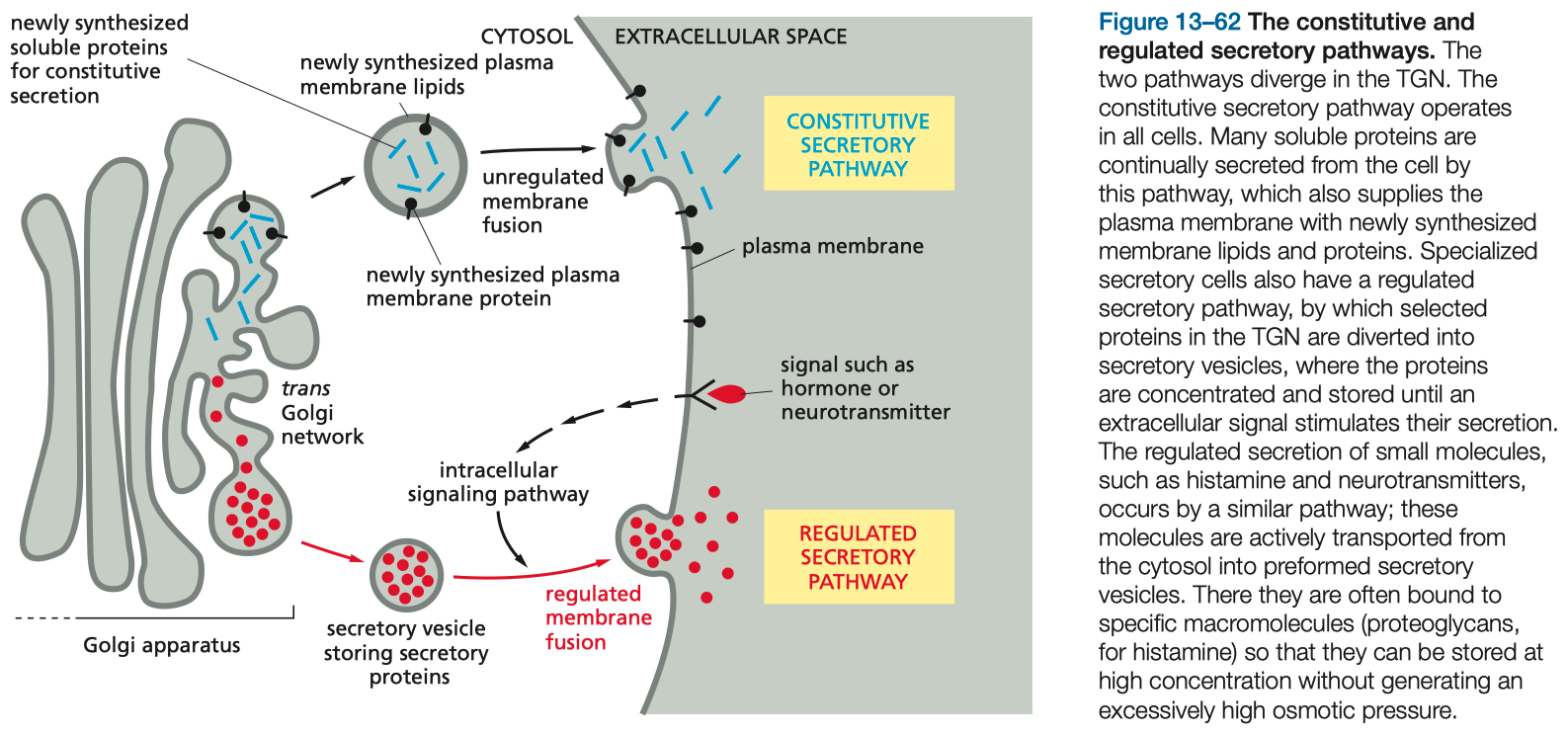
Transport from the ER to the Golgi and Retrieval Pathways
COPII-Coated Vesicles: Facilitate transport from the ER to the Golgi.
Vesicular Tubular Clusters: Form intermediate structures transported along microtubules.
Retrieval Pathway:
COPI-Coated Vesicles: Return ER-resident proteins marked with KDEL sequences to the ER, maintaining cellular protein homeostasis.
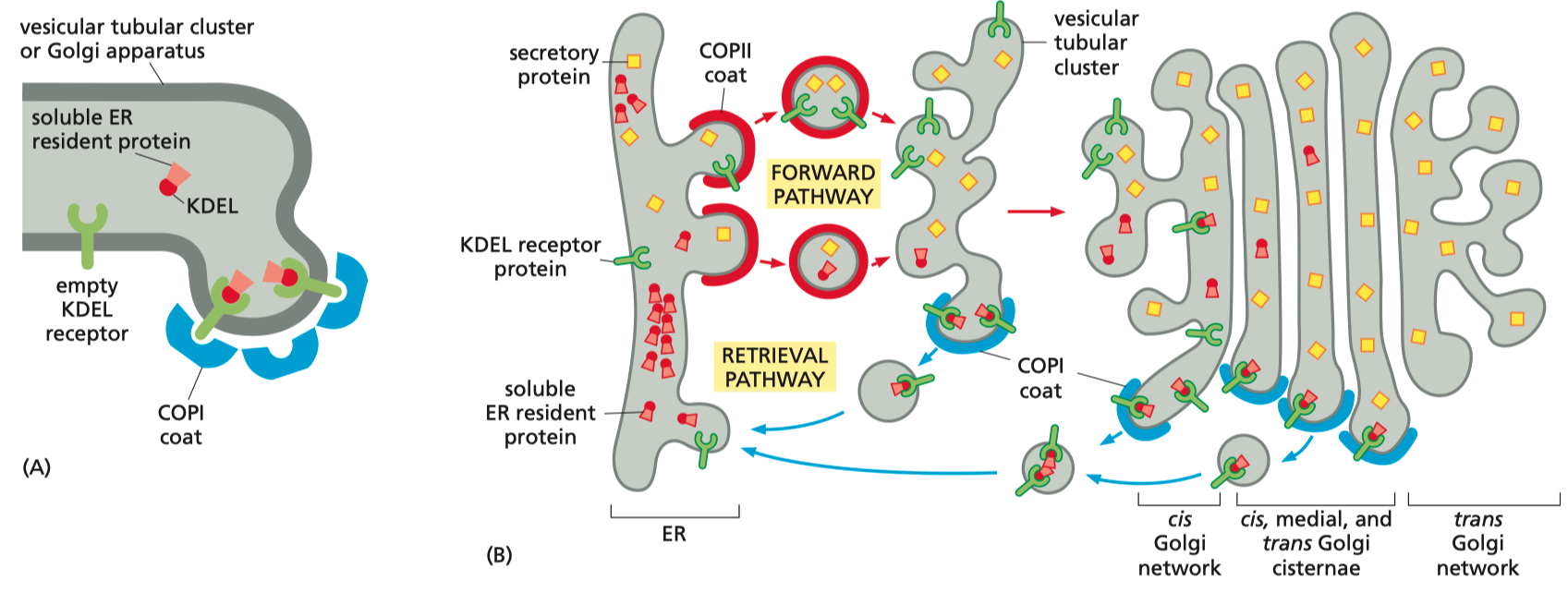
Golgi
The Golgi Sorts Between ( Trans Golgi Network ) :
signal-mediated diversion to lysosomes ( via endosomes )
signal-mediated diversion to secretory vesicles ( for regulated secretion )
constitutive secretory pathway
Parkinsons
Parkin 1 = prevents synaptic vesicle release
ubiquinates Synaptotagmin
Pink 1 = associated with mitochondrial quality control, impacts vesicle recycling.
𝛼-synuclein = blocks vesicle fusion by blocking SNARE complex
Outcome: Dysregulated dopamine release and reuptake, leading to potential dopaminergic neuron toxicity.
Misc
There are Various Types of Coated Vesicles
Adaptor Proteins Select Cargo into Clathrin Coated Vesicles
Dynamin Regulates the Pinching-off and Uncoating of Clathrin Coated Vesicles
Rab Proteins Guide Transport Vesicles to their Target Membrane
Rab protein Guidance to Target Membrane
SNAREs Mediate Membrane Fusion
V-SNARE and T-SNARE
Calcium entry into cell binds to synaptotagmin, resulting in fusion of membranes
SNAREs need to be Separated after Fusion
Golgi Apparatus Consists of an Ordered Series of Compartments
Pathways to lysosomes
endocytose something ➡️ lysosome
phagocytose something ➡️ lysosome
macropinocytosis ➡️ lysosome
autophagy of mitochondria ➡️ lysosome
Manose 6-phosphate Receptor
on golgi
on early endosomes
Pinocytosis : no clathrin coat
LDL uses clathrin mediated endocytosis