Membrane Potential
Inside of cell = high potassium
Outside of cell = high sodium
2 Force Gradients :
electrical
chemical = mass = concentration
Lipid bilayer = insulator , separates 2 conductors. extracellular and intracellular
Asymmetries of ion concentration across lipid bilayer = voltage = membrane potential
Nernst Equation :
calculates membrane potential
The Nernst potential is the voltage that would exist if the membrane were only permeable to one ion
Electrochemical Equation :
accounts for how an ion behaves under the actual membrane potential , which is influenced by all ions and the currents across the membrane.
So how to find the actual membrane potential
its a "weighted average" of all the membrane potentials
It uses the "permeability" of some ion channel , aka its "conductance"
higher permeability = higher rate of ions traveling into or out of cell
So total averaged membrane potential over all the different types of ion channels in the membrane
This gives the true "resting membrane potential"
There is always a balance between the mass ( chemical ) concentration and the ionic concentration
"electrochemical gradient" = final balance of equilibrium between both forces
Start with mass/chemical part first , which way does something move based on mass
Then , assuming it did move to concentration , add up the net of charges you expect on either side now after the movement.
Now we have another movement / force that might pull back the other way now due to the "electro" part of the whole "electrochemical" gradient.
If too many positively charged ions enter the cell, the cell becomes positively charged, and this can push back against the movement of more positive ions into the cell ( since like charges repel ).
Conversely , this charge buildup might attract negatively charged ions to move in the opposite direction ( to balance the charge ).
The Driving Force :
an ion will move down its electrochemical gradient
it wants to move , in or out of the cell , in such a way that the entire membrane potential
remember , sodium , if left to itself , balances out at
potassium , if left to itself ,
ok , so if we take a snapshot of the current membrane potential ,
and lets say the total
for sodium , it would love the cell to be at
so its goin to rush in , to try and bring
but for potassium , it wants the cell to be at
so its going to want to leave the cell , to make the membrane more negative , and closer to
Current :
In reality , or in physics , current is defined as the flow of positive charge
neuro "science" defines current as ion movement
so its the same , just track the flow of positive charge.
however , if you are dealing with an anion , it "flowing" has the opposite physics equivalent
Neuro "science" Interpretations:
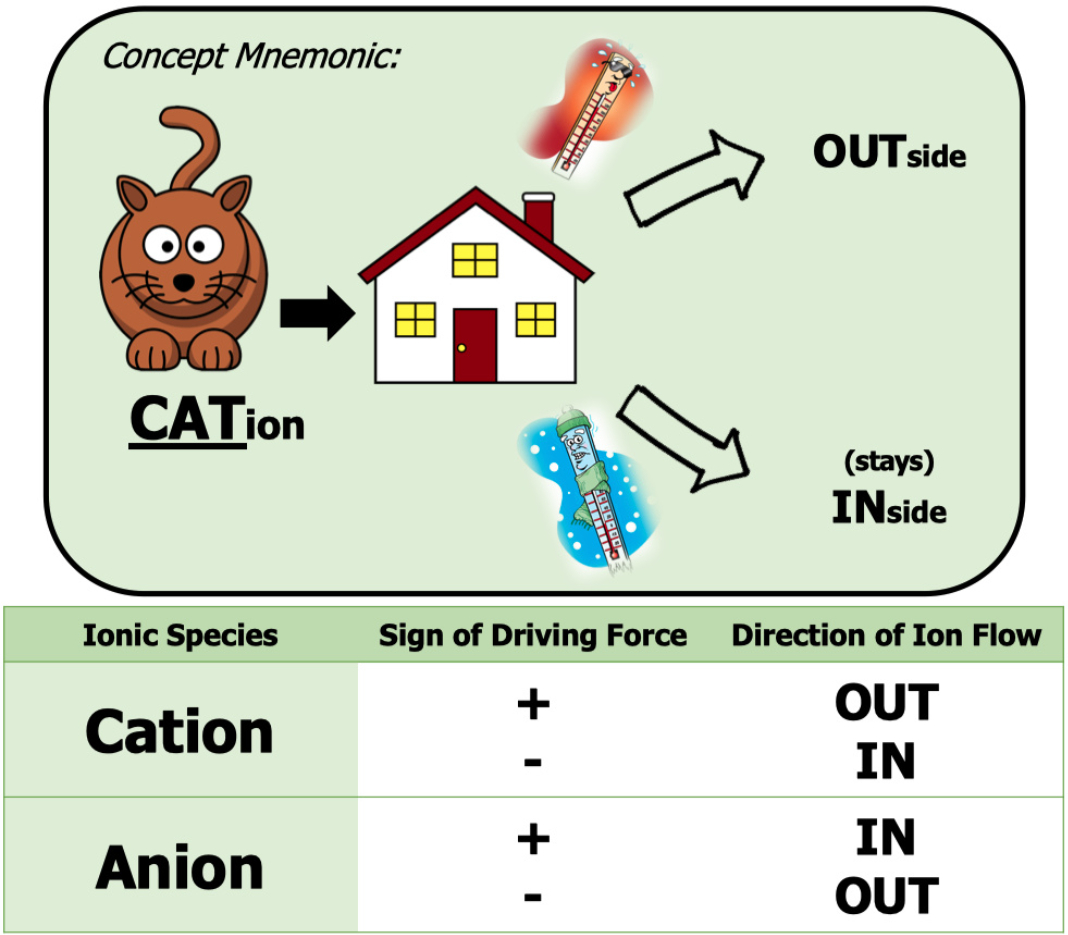
Positive Ion:
leaving the cell = outward current = positive driving force
entering the cell = inward current = negative driving force
Negative Ion:
leaving the cell = inward current = negative driving force
equivalent to positive charge moving in
entering the cell = outward current = positive driving force
has the same effect as positive charge moving out
Liddle's Syndrome :
Symptoms :
tonic and clonic seisures
fever
urine
hypertensive
increased sodium ( hypernatremia )
decreased potassium ( hypokalemia )
because they cells are so hypokalemic , aka reduced potassium inside the cell , aka the cell is hyperpolarized , very negative
now to fire an action potential , a TON more sodium has to enter if its ever going to become positive enough to reach the threshold value for firing.
but sodium just doesn't have enough energy to compensate , so you are left with reduced firing
arrhythmia , seizures , muscle spasms
Transport Mechanisms and Ion Channels
Small and lipophilic molecules
Everything else needs help
transporters and ion channels
Transmembrane protein function and regulation is the essence of cellular communication
changing cellular function by controlling what goes into and out of the cell ,
as well as events that occur inside the cell
Transmembrane Proteins :
Function = cellular communication
control what gets into and out of the cell
receptors have to do this indirectly
3 Major Classes = receptors , ion channels , transport proteins ( ion transporters / carrier mediated transport )
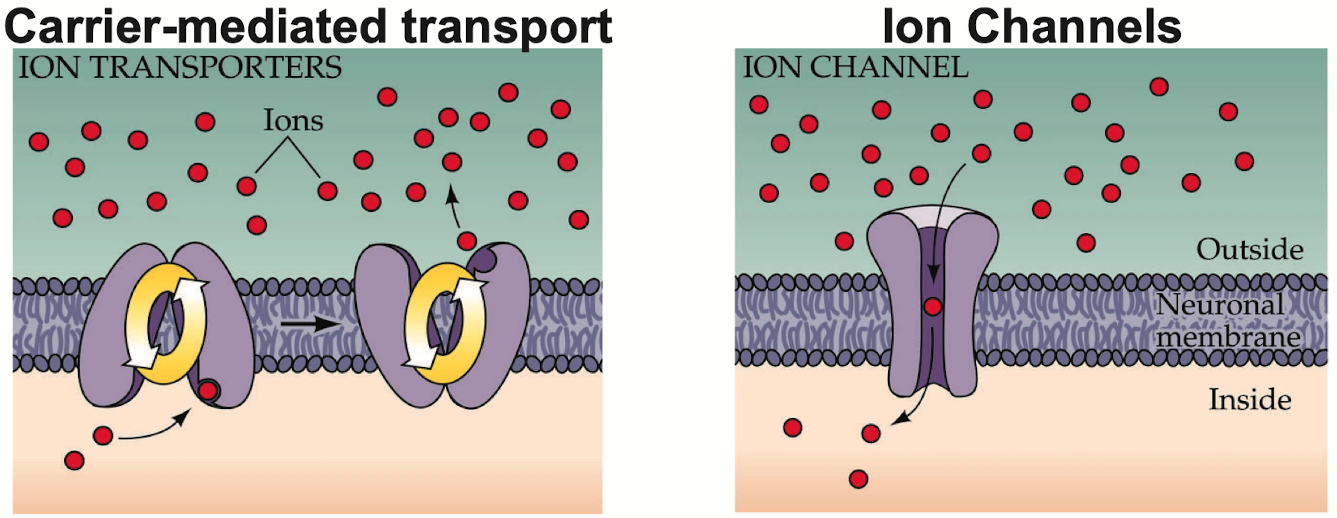
Transport Proteins :
Function = provide a permeation pathway for a specific solute , across the plasma membrane
solute binds to the protein with some affinity ,
transport rate is dependent on solute concentration
Transport Mechanisms :
active transport is much faster than diffusion
rate limited by concentration of solute
following michaelis menten kinetics
facilitated diffusion = passive
most of the time these require a transport protein still , so not 100% passive
example = GLUT
rate limited by number of transport proteins
secondary active transport = coupled transport. 1st entity goes down its gradient. This generates potential energy which then allows for the 2nd entity to go against its gradient
ion exchange ( antiport )
co-transport ( symport )
active transport = requires exogenous energy ( ATP )
example = sodium / potassium ATPase = restores normal equilibrium balance. 3 , 2 , 1 = Nokia
3 = No ( sodium ) = out of the cell
2 = Ki ( potassium ) = into the cell
1 = A ( ATP )
second example = Calcium ATPase
moves calcium against its gradient , back into the sarcoplasmic reticulum of muscle cells
third example = ABC Transporters
Ion Channels :
Active ion channels form aqueous pores through the membrane.
This pore is lined with some type of selectivity filter that allows primarily only specific ions to briefly bind and move through the pore ,
down its electrochemical gradient through the pore
at a rate approaching diffusion through water
Normally , the channel is closed
Some stimulus causes conformational change , which then actually forms the pore
Ion channels are classified based on their activation method :
Leak = always open
often involved in maintaining normal concentrations of ions
responsible for setting the resting membrane potential of the cell
example = ENAC
Stretch Activated
Ligand Gated :
can be activated from inside or outside
Voltage Gated
these can have an inactivation period ( 3rd conformational change )
activation ➡️ de-activation ➡️ in-activation ➡️ activation ➡️ ...
the inactivation is typically caused by some occlusion , blocking of the pore
S4 segment = a conserved transmembrane region :
consists of a series of positively charged amino acid residues that form a spiral of charges traversing the membrane.
It is likely that these S4 segments serve as voltage sensors.
That is, the positive charges “feel” the membrane potential , and move in response to changes in the membrane potential ,
such that channels activate following a depolarization.
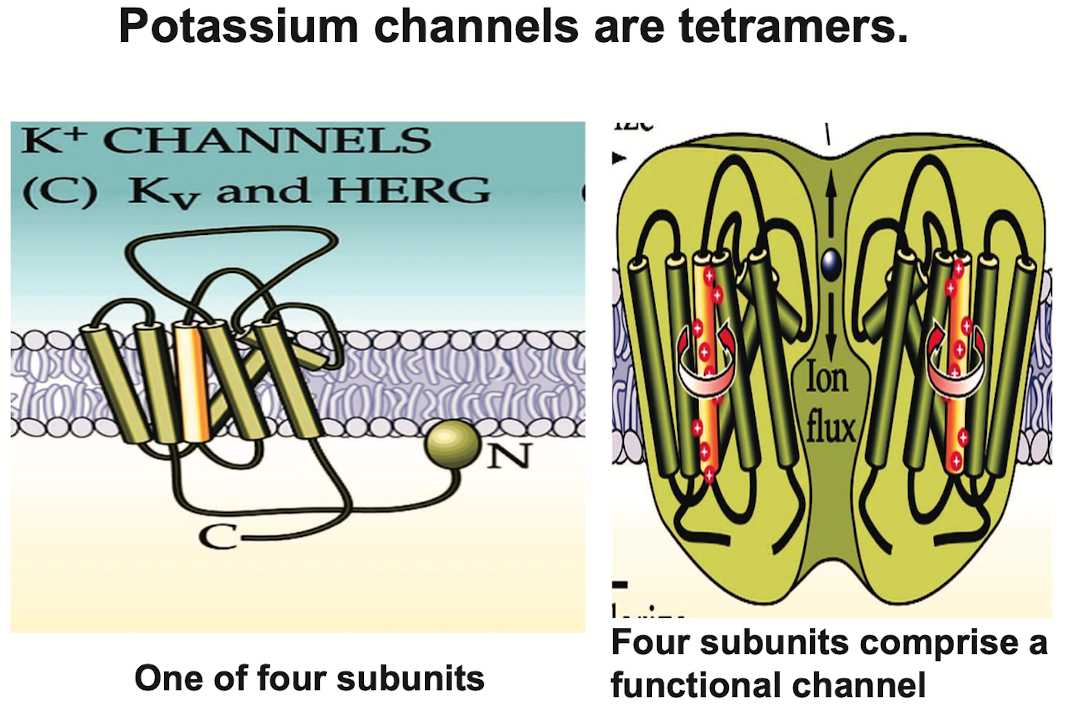
How is
they are tetramers
Ion Channels and Selectivity: Potassium channels are among the most selective ion channels, favoring K⁺ ions over other similarly sized ions like Na⁺ and Ca²⁺. This selectivity is crucial for maintaining cellular membrane potential and facilitating signal transmission.
Ionic Hydration: In solution, ions like potassium are surrounded by a hydration shell of water molecules. This shell helps stabilize the ion as it moves through various biological environments. The challenge for ion channels is to strip away this hydration shell to allow the ion to pass through the narrow pore of the channel.
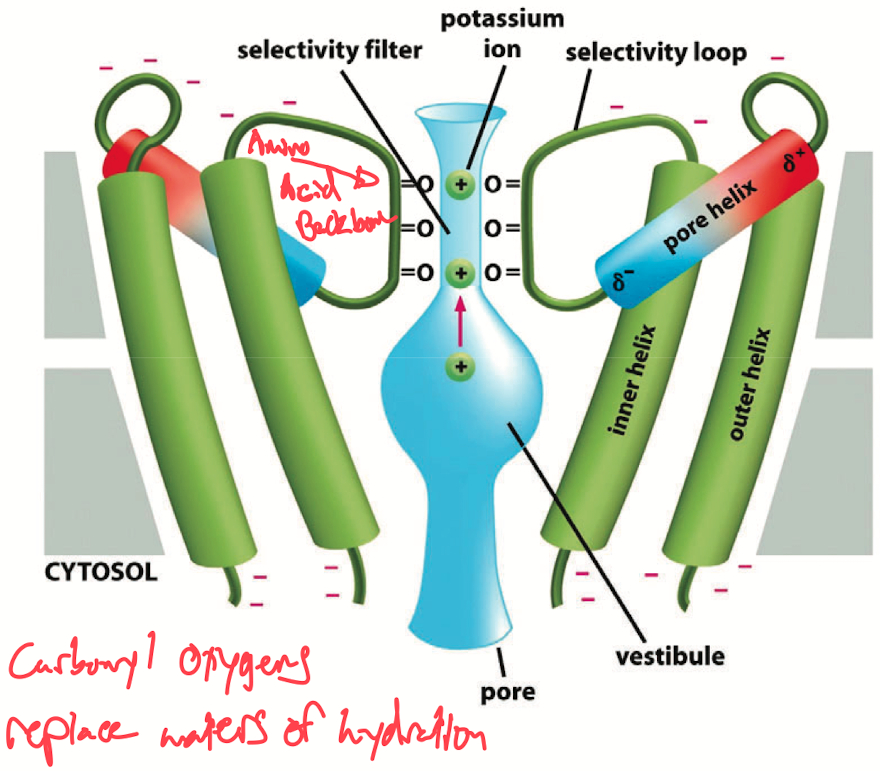
Potassium Channel Structure: The selectivity filter of potassium channels is lined with carbonyl oxygens from the backbone of amino acids. These carbonyl groups form a sort of molecular sieve that is precisely sized to interact with K⁺ ions. The amino acid sequence at the selectivity filter is often GYG (Glycine-Tyrosine-Glycine), which is highly conserved across potassium channels.
Dehydration and Selectivity: The pore of the potassium channel is designed to mimic the hydration environment that normally surrounds a K⁺ ion. When a potassium ion enters the channel, the carbonyl oxygens in the selectivity filter replace the water molecules that normally hydrate the ion. The geometry of the pore is such that it fits a partially dehydrated K⁺ ion exactly, allowing it to pass through with minimal energy expenditure.
Why Sodium (Na⁺) Doesn't Pass: Despite Na⁺ being smaller than K⁺, it cannot pass through the potassium channel. This is because the carbonyl oxygens are spaced perfectly for the larger K⁺ ion. For the smaller Na⁺ ion, the carbonyl groups are too far apart to stabilize it effectively, so the energy cost of dehydrating and passing Na⁺ through the pore is too high, preventing it from permeating the channel.
Ion Permeation and Repulsion: As K⁺ ions move through the channel, they bind at specific sites along the selectivity filter. There are typically four binding sites created by the carbonyl oxygens. When one K⁺ ion enters and binds to a site, it is quickly followed by another K⁺ ion, which binds to the next available site. The electrostatic repulsion between the positively charged K⁺ ions causes them to push each other through the channel. This repulsion mechanism ensures a continuous flow of K⁺ ions through the channel in a coordinated, sequential manner.
Energetics: The process is highly efficient, as the ion’s movement is driven both by the repulsive force between ions and the thermodynamic energy minimized by the selectivity filter. The precise geometry and electrostatic environment of the channel ensure that potassium flows with minimal resistance while excluding other ions.
How is
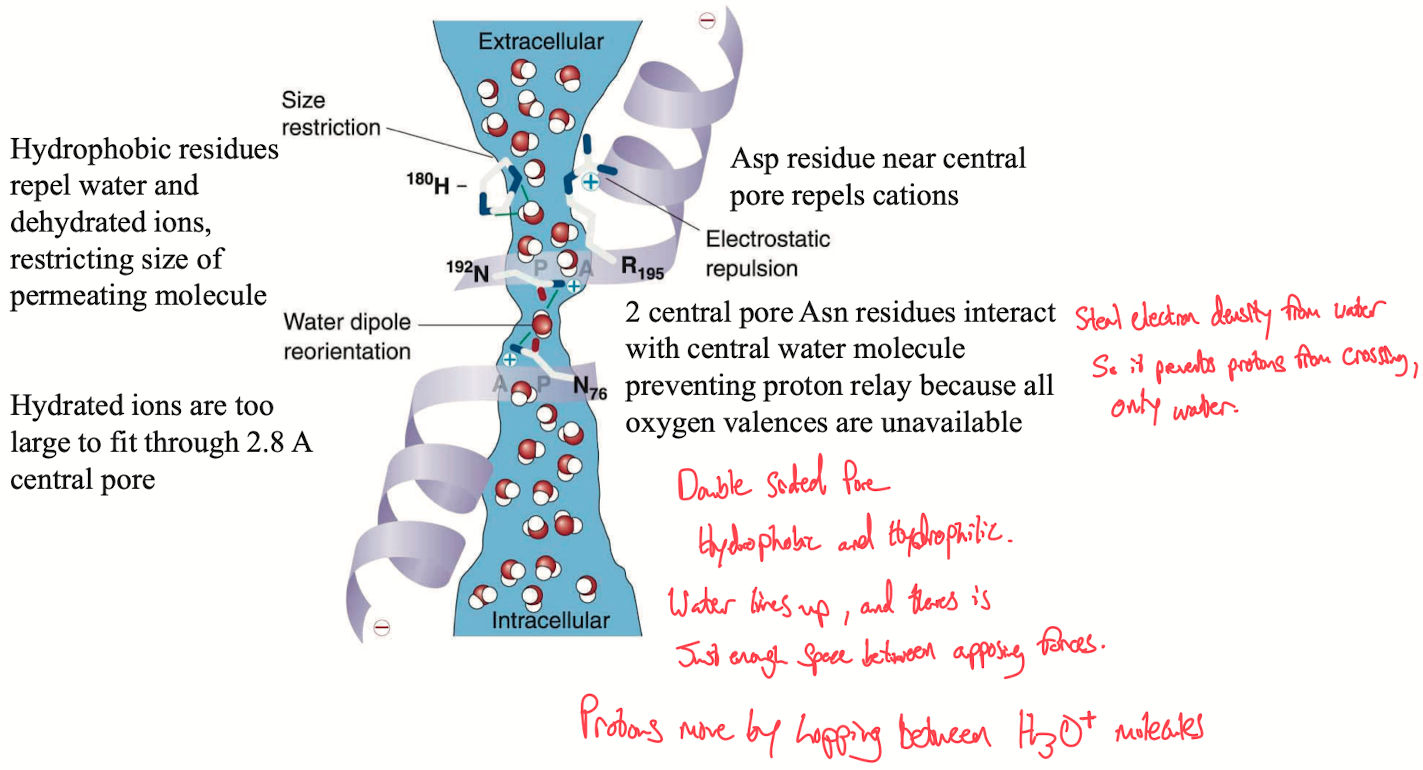
Aquaporins: Specialized water channels embedded in cell membranes, selectively allowing water molecules to pass while blocking ions, especially protons (H⁺). The precision is crucial for maintaining osmotic balance without disrupting cellular pH.
Channel Structure: Aquaporins have both hydrophobic and hydrophilic regions. The hydrophobic sides repel ions like Na⁺, K⁺, and Cl⁻, while the hydrophilic portions facilitate the alignment of water molecules. The water molecules move single-file through the channel, minimizing interactions with other solutes.
Water Passage: Water molecules traverse the pore one at a time, driven by specific interactions with the hydrophilic regions. The pore size and electrostatic environment are finely tuned to only accommodate water, preventing larger or charged molecules from passing through.
Proton Exclusion Mechanism: A critical function of aquaporins is the prevention of proton leakage (H⁺) across the membrane, which would disturb the pH gradient. Protons typically move via the Grotthuss mechanism, where H⁺ transfers rapidly through a chain of hydronium ions (H₃O⁺) and water (H₂O), effectively "hopping" from one molecule to the next.
Disruption of Proton Hopping: Aquaporins prevent this by placing two asparagine residues in the central part of the pore. These asparagine residues interact with the central water molecule, disrupting the hydrogen-bond network necessary for proton hopping (H₃O⁺ ⇌ H₂O). The asparagine residues pull electron density from the water, neutralizing the chain reaction of proton transfer, thus blocking the Grotthuss mechanism within the channel.
Importance: This mechanism ensures that water can be transported efficiently without allowing protons to follow, which would disrupt the cell’s electrochemical gradient and pH homeostasis.
Acetylcholine Receptors ( AChR ) :
hetropentamero
each of the five subunits winds through the plasma membrane 4 times
AChRs are kinked in the middle
when ACTH binds between the residues , the kinks twist in opposite directions around each other
this forms a larger whole , opening the channel
Cholera :
symptoms :
diarrhea
dehydration
physiology :
caused by the bacterium Vibrio cholerae
produces a toxin known as cholera toxin ( CTX )
interferes with normal cellular processes in the small intestine , leading to water and electrolyte loss
How does the bacterial toxin affect normal nutrient absorption ?
The cholera toxin binds to cells lining the small intestine , leading to an overstimulation of adenylate cyclase.
This increases the levels of cyclic AMP ( cAMP ) , which disrupts the normal function of sodium and chloride transporters.
Instead of absorbing these nutrients and water , the intestine secretes large amounts of electrolytes and water into the gut , causing diarrhea.
treatment :
ingest high amount of sodium and glucose
why ?
The sodium-glucose co-transport system in the small intestine allows both sodium and glucose to enter cells together.
Water follows sodium into the cells via osmosis, helping rehydrate the body.