Electron Transport Chain
What is electron transport and how is it used to make a chemiosmotic gradient of H+ ?
Electron transport moves electrons from high-energy carriers ( NADH and FADH₂ ) through the electron transport chain , releasing free energy as they move to lower energy states towards oxygen.
This free energy is used by protein complexes to actively pump H⁺ ions from the mitochondrial matrix into the intermembrane space, establishing a chemiosmotic gradient.
The proton gradient stores potential energy , which drives protons back into the matrix through ATP synthase , converting the free energy into ATP synthesis.
What are the four members of the electron transport chain and what are their roles? How does ATP synthase make ATP ?
Step 1: NADH Oxidation (Complex I)
NADH: Nicotinamide adenine dinucleotide (reduced form)
H⁺: Proton
CoQ: Coenzyme Q (ubiquinone)
NAD⁺: Nicotinamide adenine dinucleotide (oxidized form)
CoQH₂: Ubiquinol (reduced form of CoQ)
4H⁺ (matrix): 4 protons taken from the mitochondrial matrix
4H⁺ (intermembrane space): 4 protons pumped into the intermembrane space
Step 2: Succinate Oxidation (Complex II, Succinate Dehydrogenase)
2a. Succinate to Fumarate (Citric Acid Cycle Reaction)
Succinate: A substrate in the citric acid cycle
FAD: Flavin adenine dinucleotide (oxidized form)
Fumarate: A product in the citric acid cycle
FADH₂: Flavin adenine dinucleotide (reduced form)
2b. FADH₂ Oxidation and Ubiquinone Reduction
FADH₂: Flavin adenine dinucleotide (reduced form)
CoQ: Coenzyme Q (ubiquinone)
CoQH₂: Ubiquinol (reduced form of CoQ)
Step 3: CoQH₂ Oxidation (Complex III, Q-cycle)
CoQH₂: Ubiquinol
Cyt c(ox): Cytochrome c (oxidized form)
Cyt c(red): Cytochrome c (reduced form)
CoQ: Coenzyme Q (ubiquinone)
4H⁺ (intermembrane space): 4 protons pumped into the intermembrane space
Step 4: Cytochrome c Oxidation (Complex IV, Cytochrome c Oxidase)
Cyt c(red): Cytochrome c (reduced form)
H⁺ (matrix): Protons from the mitochondrial matrix
O₂: Molecular oxygen (final electron acceptor)
Cyt c(ox): Cytochrome c (oxidized form)
H₂O: Water
4H⁺ (intermembrane space): 4 protons pumped into the intermembrane space
Step 5: ATP Synthesis (Complex V, ATP Synthase)
ADP: Adenosine diphosphate
Pi: Inorganic phosphate
ATP: Adenosine triphosphate
H₂O: Water
4H⁺ (intermembrane space): 4 protons moving from the intermembrane space
4H⁺ (matrix): 4 protons moving back to the matrix through ATP synthase
Overall Electron Transport Chain Reaction:
Don't need to know reactions in detail
Know starting and ending molecules though
Based off 1 NADH ,
for how many protons out in Complex 1 from 1 NADH
What are the five electron carriers used during the electron transport chain ?
Flavoproteins (FMN in Complex I, FAD in Complex II):
Accept and donate 2 electrons (FMN/FMNH₂, FAD/FADH₂).
Iron-Sulfur (Fe-S) Proteins (in Complexes I, II, and III):
Transfer one electron at a time using iron atoms in Fe-S clusters.
Cytochromes (in Complexes III, IV, and mobile cytochrome c):
Heme proteins that carry one electron at a time, utilizing iron (Fe²⁺/Fe³⁺) in heme groups.
Ubiquinone (CoQ) (mobile between Complexes I, II, and III):
Transfers both electrons and protons, shuttling electrons between complexes.
Copper Centers (CuA and CuB in Complex IV):
Transfer one electron at a time, with copper ions (Cu⁺/Cu²⁺) facilitating electron transfer and oxygen reduction.
How many H+ does each electron transport chain protein complex pump into the intermembrane space; how does this affect pH; how many H+ are required to make ATP ?
NADH ( Complex I entry ) :
Complex I pumps 4 H⁺
Complex III pumps 4 H⁺
Complex IV pumps 2 H⁺
Total: 10 H⁺ pumped per electron pair from NADH
Total ATP =
FADH₂ ( Complex II entry ) :
Complex II does not pump H⁺ but passes electrons to CoQ
Complex III pumps 4 H⁺
Complex IV pumps 2 H⁺
Total: 6 H⁺ pumped per electron pair from FADH₂
Total ATP =
Effect on pH :
The proton pumping into the intermembrane space causes :
a decrease in pH ( more acidic ) in the intermembrane space
and a relative increase in pH ( more basic ) in the mitochondrial matrix
This pH difference , along with the charge difference ( more positive in the intermembrane space ) , creates the proton motive force
H⁺ Required to Make ATP :
It is estimated that about 4 H⁺ ions are required to synthesize 1 ATP molecule. This includes:
3 H⁺ ions for ATP synthesis through ATP synthase.
1 H⁺ ion to transport ADP and phosphate ( Pi ) into the mitochondrial matrix for ATP synthesis.
Each full cycle of the ETC allows the production of roughly 2.5 ATP per NADH and 1.5 ATP per FADH₂ due to the difference in proton pumping.
What are the steps of cytochrome C reduction by Cytochrome C Reductase ( complex III )
involves a unique mechanism known as the Q cycle
the Q cycle moves 4 H⁺ ions into the intermembrane space and transfers 2 electrons to reduce 2 cytochrome c molecules , while regenerating ubiquinol.
Ubiquinol (QH₂) Oxidation: One QH₂ molecule donates two electrons. One electron reduces cytochrome c, while the other is transferred to cytochrome b, where it reduces ubiquinone (Q) to semiquinone (Q•⁻).
Proton Release: During QH₂ oxidation, 2 H⁺ ions are released into the intermembrane space.
Second Ubiquinol Reaction: A second QH₂ molecule repeats the process, with one electron reducing another cytochrome c and the other fully reducing semiquinone to regenerate QH₂ at the Qi site.
Proton Release Again: Another 2 H⁺ ions are released, contributing to the proton gradient.
A fully reduced coenzyme Q (QH₂) binds to the upper "Q" site near the cytosolic side of the membrane.
A fully oxidized coenzyme Q (Q) binds to the lower "Q₀" site near the matrix side of the membrane.
Cytochrome c binds to Complex III.
QH₂ transfers two protons into the intermembrane space.
QH₂ donates its first electron to cytochrome c via the Rieske iron-sulfur protein and cytochrome c₁.
QH₂ donates its second electron to the fully oxidized Q in the lower Q₀ site, forming a semiquinone (Q•−).
The oxidized coenzyme Q from the upper site is released back into the coenzyme Q pool in the inner membrane.
The reduced cytochrome c, loaded with one electron, leaves Complex III and travels to Complex IV.
The semiquinone (Q•−) remains bound to Complex III.
A second fully reduced QH₂ molecule binds to the upper "Q" site.
Another oxidized cytochrome c binds to Complex III.
QH₂ transfers two protons into the intermembrane space.
QH₂ donates its first electron to cytochrome c via the Rieske iron-sulfur protein and cytochrome c₁.
QH₂ donates its second electron to the semiquinone in the lower Q₀ site, fully reducing it to QH₂.
The oxidized Q from the upper site is released into the coenzyme Q pool.
The reduced cytochrome c leaves for Complex IV.
The fully reduced QH₂ in the lower Q₀ site attracts two protons from the mitochondrial matrix.
The fully reduced QH₂ in the lower site is released back into the coenzyme Q pool, ready to restart the cycle.
Don't really need to know ?
What are the steps of O2 reduction to H2O by cytochrome C oxidase ( complex IV ) ?
Electron Transfer from Cytochrome c:
Cytochrome c (in the intermembrane space) donates an electron, one at a time, to Complex IV.
These electrons are transferred through several cofactors within Complex IV:
First to CuA, a binuclear copper center.
Then to heme a, a heme group within the complex.
Finally to heme a₃ and CuB, which form a catalytic site where O₂ binds.
O₂ Binding:
Molecular oxygen ( O₂ ) binds to the heme a₃-CuB center in Complex IV, forming a peroxo ( O₂²⁻ ) intermediate.
Electron and Proton Transfers:
Four electrons from cytochrome c and four protons ( H⁺ ) from the mitochondrial matrix are needed to fully reduce one O₂ molecule.
Two electrons reduce the O₂ molecule to form the peroxo intermediate.
Two more electrons, along with protons, convert the peroxo intermediate to hydroxyl groups ( OH⁻ ).
Water Formation:
The addition of four protons from the mitochondrial matrix completes the reduction, turning the two hydroxyl groups into two water molecules ( H₂O ), which are then released into the mitochondrial matrix.
Proton Pumping:
While this reduction of O₂ to H₂O occurs, Complex IV also pumps 4 additional protons from the matrix into the intermembrane space, contributing to the proton gradient used for ATP synthesis.
Summary:
4 electrons from cytochrome c and 4 protons from the matrix reduce 1 O₂ molecule into 2 H₂O molecules.
Additionally, 4 protons are pumped into the intermembrane space, further increasing the proton gradient for ATP synthesis.
When glycolysis and the electron transport chain are coupled , how many NADH and FADH2 are created ?
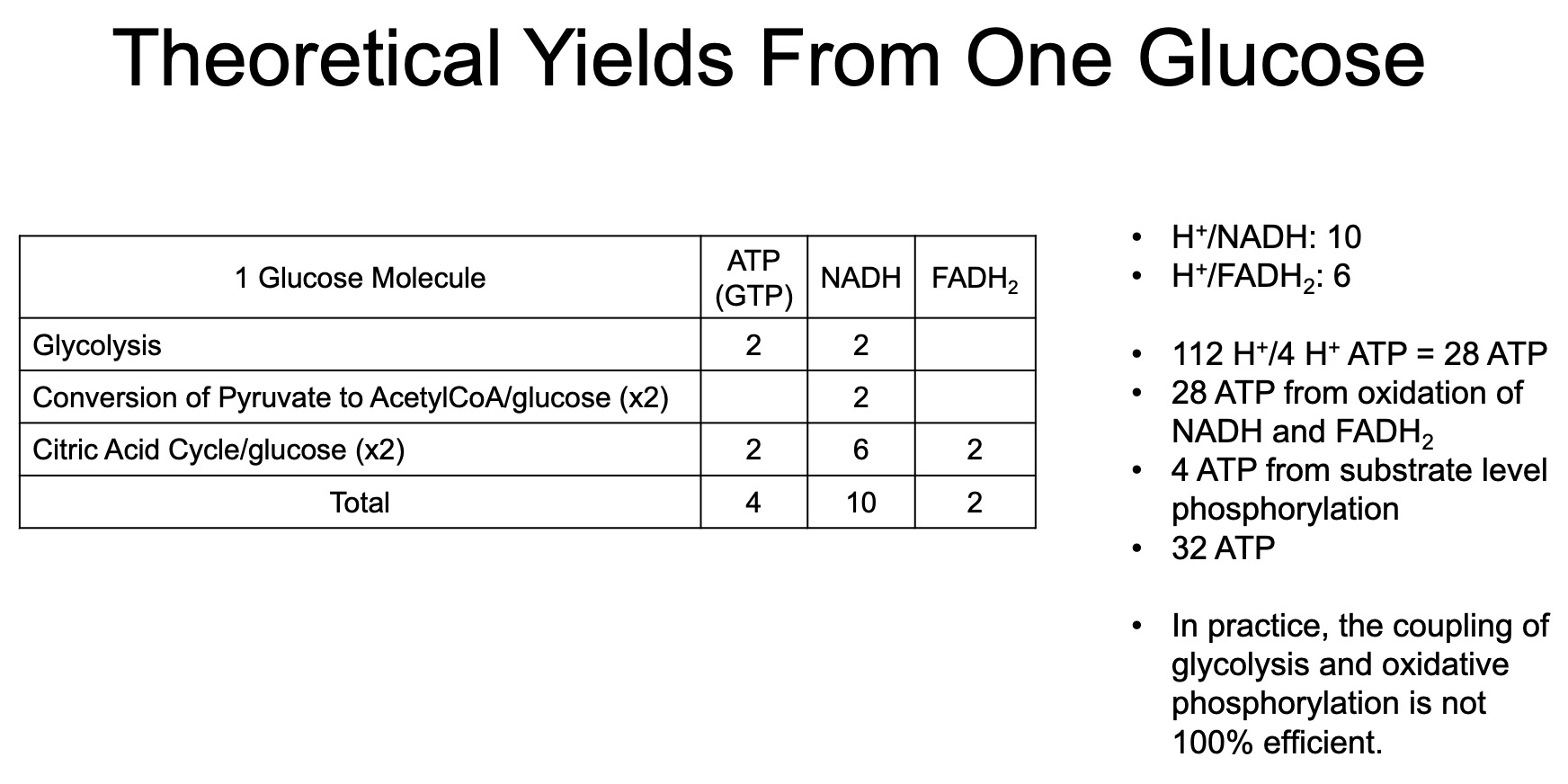
Total ATP from "substrate level phosphorylation" in glycolysis :
Total ATP from "substrate level phosphorylation" in TCA Cycle :
Total NADH from 1 Glucose :
2 from Glycolysis directly
2 from converting the 2 Pyruvate into Acetyl-CoA
6 from running the 2 Acetyl-CoA through TCA
Total = 10
Total FADH2 from 1 Glucose :
2 from running the 2 Acetyl-CoA through TCA
Total = 2
Total ATP from NADH and FADH2 generated from NADH and FADH2 produced by previous glycolysis and TCA :
Combined Total ATP from 1 Glucose :
Coupling :
TCA and ETC are all synchronized by the demand for ATP
If ATP demand is high , glycolysis produces more NADH , which feeds electrons into the ETC to maintain the proton gradient and ATP production.
Conversely , if ATP demand is low , the ETC slows down , which reduces the oxidation of NADH , and in turn , slows glycolysis and other metabolic pathways.
Glycolysis produces NADH by oxidizing glucose to pyruvate.
this NADH must be reoxidized back to NAD⁺ for glycolysis to continue producing ATP.
The ETC is responsible for reoxidizing NADH ( and FADH₂ )
this reoxidation of NADH to NAD⁺ allows glycolysis to continue operating.
ATP Synthase Dimerization :
**ATP synthase can dimerize on the inner mitochondrial membrane
These dimers help to stabilize the curvature of the cristae ( the folds of the inner membrane ) , enhancing the efficiency of proton flow through the enzyme.
This structural organization can lead to the formation of proton "funnels" that allow more effective use of the proton motive force for ATP production.
Dimerized ATP synthases can also increase the capacity for ATP production under conditions of high demand, such as in highly active cells.
By organizing into dimers , ATP synthases enhance the overall efficiency of chemiosmotic coupling, allowing for more ATP to be generated from the proton gradient created by the electron transport chain.
How do folds in the inner mitochondria membrane ( cristae ) improve the efficiency of ATP production ( 3 ) ?
Increased Surface Area:
The cristae provide a larger surface area for the electron transport chain (ETC) proteins, ATP synthase, and other essential components. This allows more electron transport chains to be embedded in the membrane, which can pump more protons (H⁺), thereby supporting a greater production of ATP.
Concentration of Proton Gradient:
Cristae help localize the protons (H⁺ ions) in a smaller volume within the intermembrane space. This enhances the proton gradient, creating a stronger proton motive force that drives more efficient ATP synthesis as protons flow back through ATP synthase.
Efficient Spatial Arrangement of Enzymes:
The folded structure allows for a tight clustering of ATP synthase dimers and other proteins involved in oxidative phosphorylation. This proximity ensures faster and more efficient transfer of protons and electrons, minimizing energy loss and maximizing ATP production.
How are mitochondrial reactive oxygen species created? Know the three discussed metabolic inhibitors.
Mode 1:
In high NADH/NAD+ conditions with low ATP generation, superoxide (O₂⁻•) is produced due to oxygen reacting with fully reduced FMN in Complex I. This can occur when the respiratory chain is inhibited or NADH builds up due to low ATP demand.
Mode 2:
When there is a low ATP production and high proton gradient, and a reduced CoQ pool (CoQH₂), reverse electron transport (RET) can occur, leading to large amounts of superoxide generation. This is commonly associated with ischemia/reperfusion injury.
Both modes describe how different conditions in mitochondrial respiration can lead to varying levels of ROS production, particularly superoxide, which is then detoxified by enzymes like superoxide dismutase (SOD)
ROS are naturally produced by electron leakage in the ETC, particularly at Complex I and Complex III.
SOD plays a vital role in neutralizing superoxide, protecting mitochondria and cells from oxidative stress while ensuring that ROS can fulfill their signaling and immune defense functions.
How ROS Are Produced in Mitochondria (Electron Transport Chain - ETC):
Electron Leakage:
During normal electron transport, a small percentage (1-2%) of electrons "leak" from the chain, particularly at Complex I and Complex III.
These leaked electrons prematurely reduce oxygen (O₂), forming superoxide (O₂⁻•), a reactive oxygen species (ROS).
Partial Reduction of Oxygen:
Instead of being fully reduced to water (H₂O) in Complex IV, oxygen can be partially reduced due to leaked electrons, forming superoxide.
Formation of Other ROS:
Superoxide (O₂⁻•) is further converted to hydrogen peroxide (H₂O₂) by superoxide dismutase (SOD).
Hydrogen peroxide can further react (Fenton reaction) to form highly reactive hydroxyl radicals (•OH), which can cause significant cellular damage.
Ubisemiquinone (CoQ•⁻) and ROS Generation:
During the Q-cycle in Complex III, ubiquinone (CoQ) is reduced to ubiquinol (CoQH₂), and during this process, an unstable intermediate called ubisemiquinone (CoQ•⁻) is formed.
Ubisemiquinone is a radical species, meaning it has an unpaired electron, which makes it more reactive and capable of transferring electrons in unwanted side reactions.
Under normal conditions, ubisemiquinone passes its electron smoothly through the Q-cycle to cytochrome c or back to CoQ at the Qi site, allowing the process to complete without issues.
However, if ubisemiquinone interacts with molecular oxygen (O₂) instead of completing the Q-cycle, it can donate its extra electron to oxygen, partially reducing it to form superoxide (O₂⁻•), a reactive oxygen species (ROS).
3. Temporary Nature of Ubisemiquinone:
Ubisemiquinone (CoQ•⁻) normally exists only temporarily in the Q-cycle as part of the electron transfer mechanism. Its existence is typically short-lived because it is quickly converted either back to ubiquinone (CoQ) or fully reduced to ubiquinol (CoQH₂).
In a well-functioning system, this prevents significant ROS formation because the ubisemiquinone does not linger long enough to react with oxygen.
4. When ROS Production Occurs:
ROS, specifically superoxide (O₂⁻•), is produced only when the ubisemiquinone persists or is present in higher concentrations than usual, giving it time to interact with oxygen.
This can happen under certain conditions, such as:
High mitochondrial membrane potential: When the proton gradient is too large, it can slow down electron flow, leading to electron buildup and increased risk of ubisemiquinone reacting with oxygen.
Low ATP demand: If ATP is not being consumed, electron transport slows down, which increases the likelihood of ROS production.
Damage to the ETC or ischemia: When the respiratory chain is disrupted, electrons may leak more frequently from complexes, producing more ROS.
5. Resolution of ROS Production:
Under normal physiological conditions, the superoxide (O₂⁻•) produced by ubisemiquinone is rapidly converted by superoxide dismutase (SOD) into hydrogen peroxide (H₂O₂), which can then be detoxified by catalase or glutathione peroxidase to form water (H₂O).
Ubiquinol (CoQH₂) itself also has antioxidant properties and can help neutralize ROS directly.
In Summary:
Ubisemiquinone (CoQ•⁻) is a normal intermediate in the ETC, particularly in Complex III during the Q-cycle.
It exists temporarily during electron transfer but can form ROS (superoxide) if it interacts with oxygen before being fully reduced or oxidized.
The ROS production from ubisemiquinone is usually minimal under normal conditions, as it quickly completes the Q-cycle.
Excess ROS generation can occur when the Q-cycle is disrupted (e.g., high membrane potential, low ATP demand), leading to an increase in electron leakage.
ROS, while a byproduct of normal metabolism, can be both good (for signaling and defense) and bad (when excessive, leading to oxidative stress). Maintaining a balance in ROS production is crucial for cellular health.
Rotenone (Complex I Inhibitor):
Action: Rotenone blocks the transfer of electrons from NADH to ubiquinone (CoQ) at Complex I.
Effect: This inhibition stops the electron flow through Complex I, which can lead to an increase in electron leakage and ROS production, particularly because the electrons build up and react with oxygen.
Result: NADH oxidation halts, and ATP production is severely reduced.
Cyanide (CN⁻) and Carbon Monoxide (CO) (Complex IV Inhibitors):
Action: Both cyanide and carbon monoxide inhibit Complex IV (cytochrome c oxidase) by binding to the iron in the heme groups, preventing the transfer of electrons to oxygen.
Effect: This blocks the final step of the ETC, where oxygen is reduced to water. Without this step, electron flow stops, and no proton gradient is generated for ATP synthesis.
Result: Cyanide and CO poisoning leads to a complete shutdown of oxidative phosphorylation, preventing ATP production, and causing cell death if untreated.
Oligomycin (ATP Synthase Inhibitor):
Action: Oligomycin binds to ATP synthase, specifically inhibiting the flow of protons (H⁺) through the enzyme.
Effect: By blocking proton flow, it prevents the synthesis of ATP despite the presence of a proton gradient. The buildup of the proton gradient can also slow down the ETC, leading to increased ROS production.
Result: Without ATP production, cellular energy levels drop, leading to cell dysfunction and death.
Summary of Metabolic Inhibitors:
Rotenone: Inhibits electron flow at Complex I, increasing ROS production and halting ATP synthesis.
Cyanide and Carbon Monoxide: Inhibit Complex IV, preventing oxygen reduction and halting ATP production.
Oligomycin: Inhibits ATP synthase, blocking ATP production by preventing proton flow through the enzyme.
Lipid Metabolism
Understand the different types of lipids and what their structures are. What does it mean to be saturated and unsaturated?
Fatty Acid Lipids :
TAG
Phospholipids
Sphingolipids
Misc Lipids :
Sterols :
Cholesterol
Steroids
Saturated = no double bonds
Un-Saturated = has double bonds
Understand the role of lipases and how lipids are digested and transported, what are the three possible fates of fatty acids in enterocytes, and understand lipid transfer in muscle cells
Lipases : hydrolase = adds water to break ester bond into alcohol + carboxylic acid
Lingual Lipase
Gastric Lipase
Pancreatic Lipsae
Bile-Sensitive Lipase
Hormone-Sensitive Lipase
Monoacylglycerol Lipase
3 Fates of Fatty Acids in Enterocytes :
Reformed into TAGS , packaged into chylomicrons , shipped out
Act as transcription factors
Beta Oxidation
Lipid Transfer in Muscle Cells :
Absorption Option 1 :
Chylomicrons in the bloodstream are broken down by lipoprotein lipase ( LPL ) into FFAs
FFAs enter muscle cells directly via CD36 receptor
Absorption Option 2 :
VLDL in the bloodstream are broken down by LPL into FFAs
FFAs enter muscle cells directly via CD36 receptor
Once Inside :
FFAs enter nucleus and bind to PPARδ
1.1 PPARδ dimerizes with RXR
1.2 PPARδ genes are transcribed ( example = PDK4 )
Pyruvate dehydrogenase kinase 4 ( PDK4 ) phosphorylates pyruvate dehydrogenase
decreases glycolysis
increases fatty acid oxidation
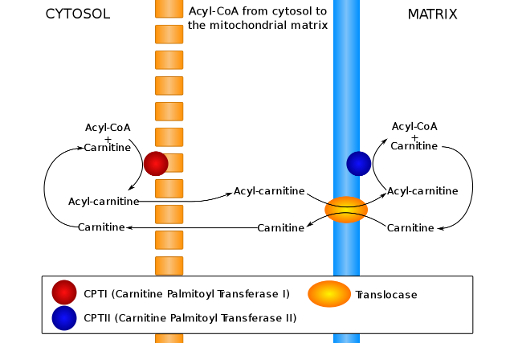
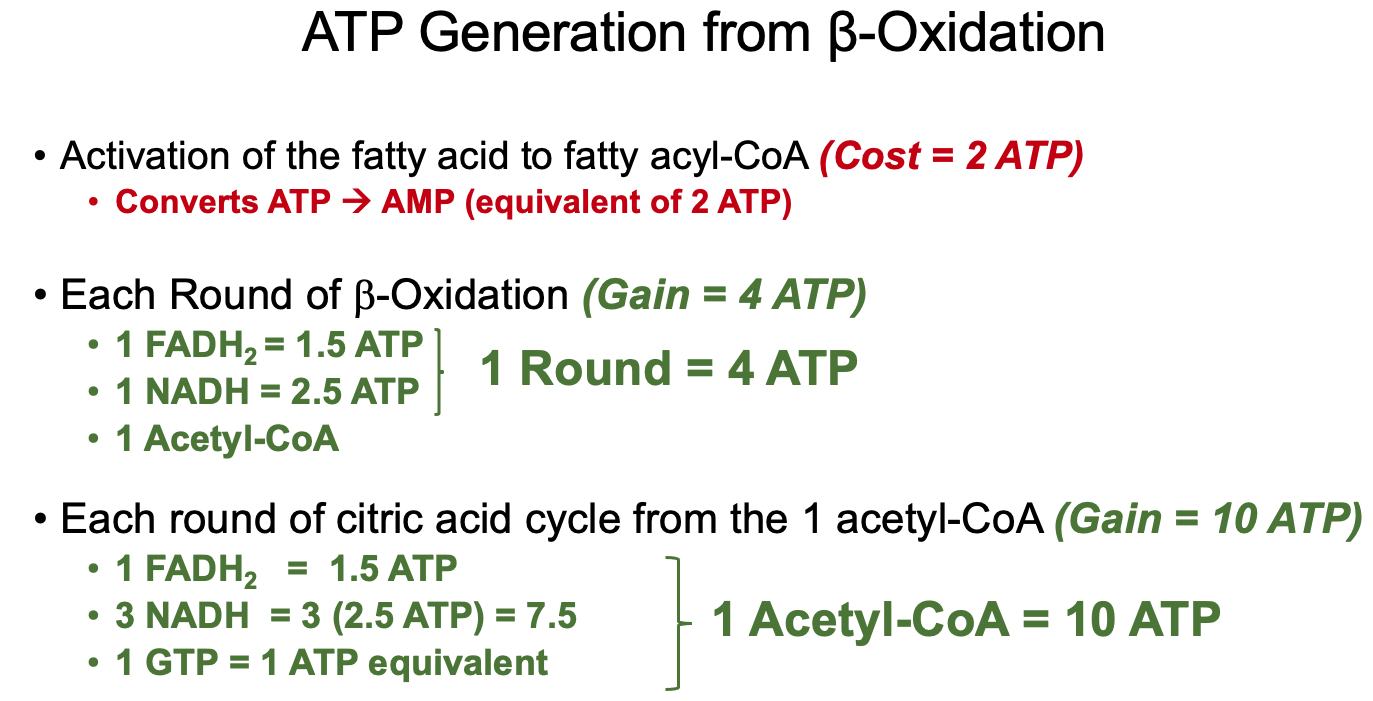
Understand the activation steps necessary for β-oxidation to occur ( fatty acyl-CoA synthesis and carnitine-mediated transport into the mitochondria )
Fatty Acyl-CoA Synthesis :
investment / activation cost = 2 ATP equivalents
Carnitine - Transport into Mitochondria :
Conjugate with Carnitine
CPT1 is an integral membrane protein found in the outer membrane of mitochondria
CPT1 is the rate-limiting enzyme of β-oxidation and is inhibited by malonyl-CoA
Acyl-Carnitine enters mitochondrial matrix using carnitine-acylcarnitine translocase ( CACT )
Acyl-Carnitine is converted back into Fatty Acyl-CoA
Carnitine recycles back to cytosol
Know the steps in β-oxidation – where NADH and FADH2 are produced? What is the net reaction of one round of β-oxidation?
Oxidation – Formation of a double bond in acyl-CoA dehydrogenase step.
Hydration – Addition of water across the double bond in enoyl-CoA hydratase step.
Oxidation – Conversion of hydroxyl to a keto group in L-3-hydroxyacyl-CoA dehydrogenase step.
Thiolysis – Cleavage of the carbon-carbon bond by β-ketothiolase, producing acetyl-CoA and a shortened acyl-CoA.
What does one round of β-oxidation produce, how can you count the numbers of acetyl-CoAs derived from the oxidation of a fatty acid and the number of rounds of β-oxidation possible from a fatty acid of a given length?
One Round of β-Oxidation :
Total Acytl-CoA for Even Numbered Fatty Acid :
Total Rounds of β-Oxidation for Even Numbered Fatty Acid :
How does ATP generation from the oxidation of a fatty acid compare to the ATP generated through the oxidation of glucose?
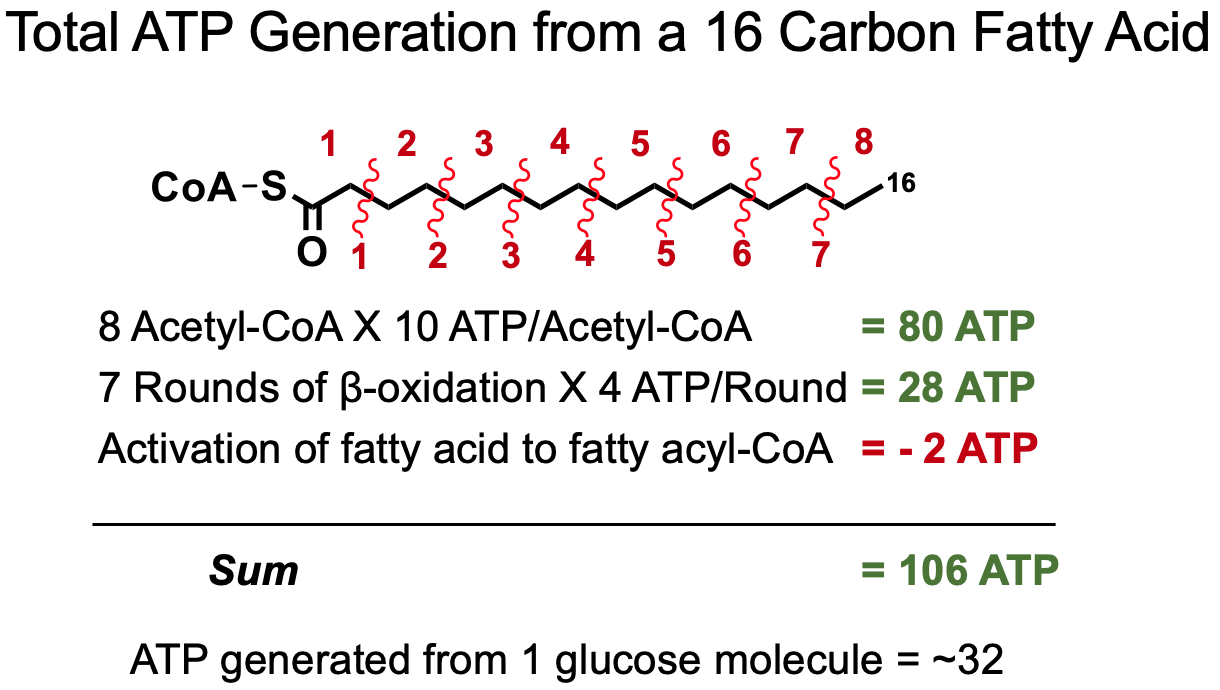
For Unsaturated Fatty Acids :
once it reaches the double bond , it has to be isomerized
this costs
therefore , unsaturated fatty acids yield less ATP than saturated
How is a triglyceride synthesized, what is required, how many steps, what is the rate-limiting enzyme, and how can intermediates impact other cell functions?
Prep :
Glucose enters adipocytes via GLUT-4 ( insulin-dependent )
Dihydroxyacetone phosphate is converted into glycerol-3-phosphate via glycerol-3-phosphate dehydrogenase
TAG Generation :
Rate Limiting Enzyme = step 1 = conversion of glycerol-3-phosphate to 1-acylglycerol-3-phosphate
How can intermediates impact other cell functions ?
1,2-Diacylglycerol ( DAG ) is an important second messenger ( like cAMP ) that is involved in protein kinase C ( PKC ) signaling
Have a basic understanding of how insulin resistance can develop and how increases in plasma free fatty acid and glucose (hyperglycemia) can impact cardiomyocytes
Insulin Resistance :
Type 1 = genetic predisposition , autoimmune , body doesn't produce insulin at all
Type 2 = poor diet , de-sensitization to insulin
pancreatic beta cells also become d-sensitized to glucose
thus leading to a decrease in insulin production
Increases in Plasma Free Fatty Acids and Glucose in Cardiomyocytes :
due to a decrease in insulin , adipocytes interpret this as a lack of energy , so they start breaking apart Chylomicrons and VLDL's into FFAs
high amounts of FFAs impact insulin signaling in other peripheral cells like muscle cells
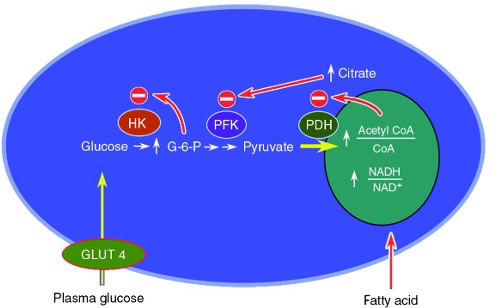
Mechanism 1 :
increased plasma FFA levels
increased beta oxidation , generating increased amounts of NADH , FADH2 , and acetyl-CoA
high NADH inactivates pyruvate dehydrogenase
high acetyl-CoA causes high citrate
high citrate inactivates phosphofructokinase
creates high amount of Glucose-6-Phosphate
results in high amounts of glucose , and decreased glucose uptake
uncouples glucose uptake from insulin signaling
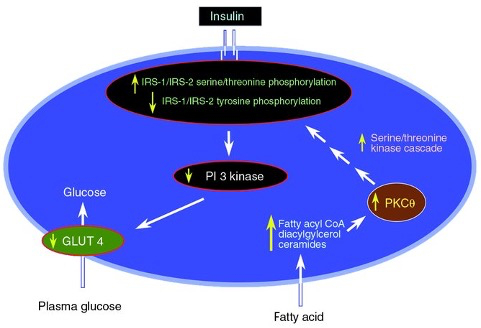
Mechanism 2 :
increased plasma FFA levels
increased intracellular fatty acid metabolites
fatty acyl CoA and 1,2-diacylglycerol ( DAG; an activator of PKC )
these metabolites activate a serine/threonine kinase cascade initiated by protein kinase C
causes serine/threonine phosphorylation of insulin receptor substrates ( IRS-1 and IRS-2 )
reduces the ability of the insulin receptor substrates to activate PI 3-kinase
Tyrosine phosphorylation activates IRS-1 and IRS-2
Serine/threonine phosphorylation inhibit IRS-1 and IRS-2
glucose transport and metabolism downstream of insulin receptor signaling are diminished
Diabetic Cardiomyopathy ( DCM ) :
defined as the presence of abnormal cardiac structure and performance in the absence of other cardiac risk factors
Hyperglycemia, hyperinsulinemia, and insulin resistance mediate the pathological remodeling of the heart
Mechanisms implicated in the pathogenesis of DCM in cardiomyocytes:
Metabolic derangements
Impaired calcium homeostasis
Increased inflammation
Oxidative stress ( increases in ROS production )
Advanced glycation endproducts ( one diagnostic test for diabetes is hemoglobin A1C or HbA1c test )
Diabetes-related increased FFAs impact cardiomyocytes in DCM primarily by altering glucose metabolism and uncoupling glycolysis and oxidative phosphorylation
Diabetes-related hyperglycemia acts on cardiomyocytes in DCM primarily through increased reactive oxygen species ( ROS ) that damages DNA and alters gene expression