Medical Cell Biology - Quiz 1 - Study Guide
Glycolysis
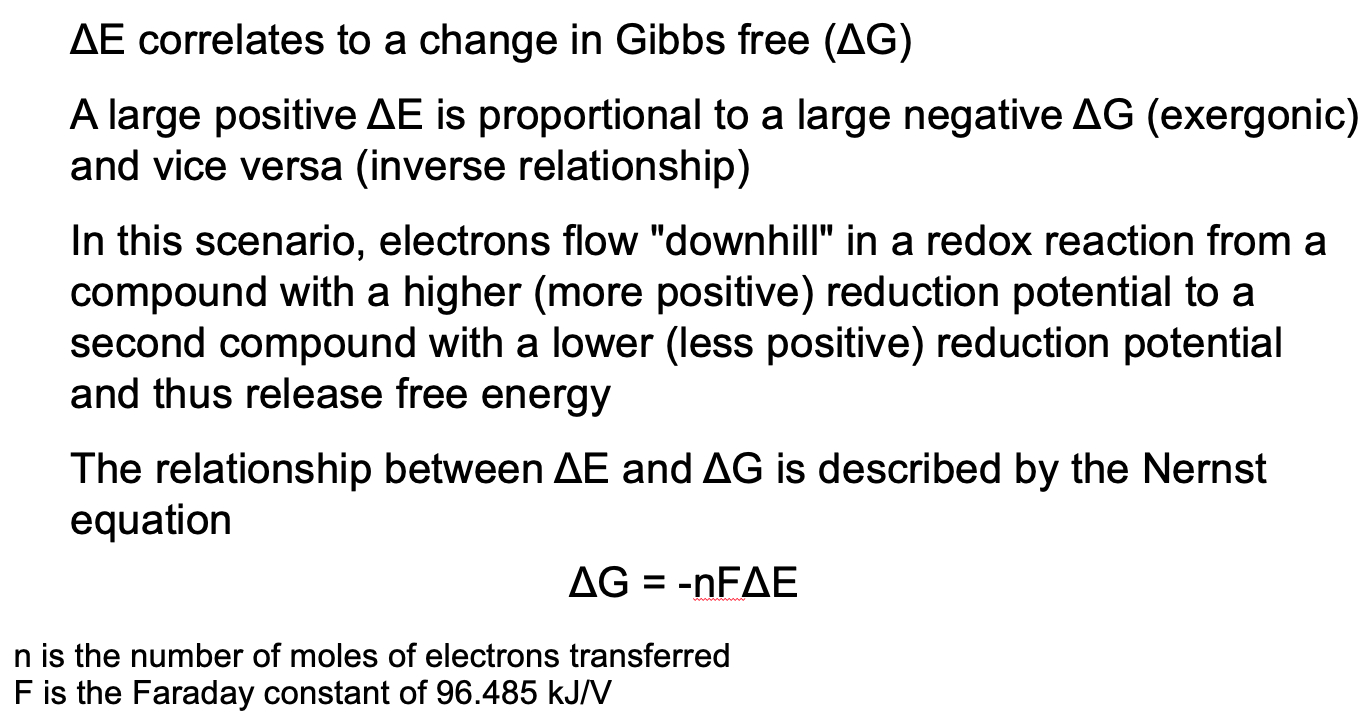
Understand electronegativity and redox reactions and their relationship to changes in free energy

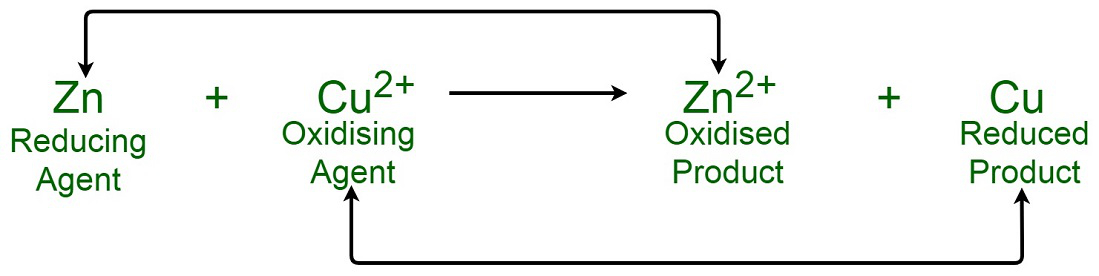
The reduction potentials of the two half reactions allow one to quantify the “ability” of the oxidizing agent to gain electrons
The difference of the two redox potentials (sum when using oxidation potential) can be calculated to determine whether a reaction is favorable (exergonic) or unfavorable (endergonic)
What are the two types of cellular metabolism and what are the different stages of cellular metabolism?
Anabolic vs Catabolic
Stage 1 = large macromolecules are hydrolyzed into their building blocks.
Stage 2 = building blocks are further degraded into a few common metabolites.
Stage 3 = small molecular weight metabolites like acetyl-CoA are degraded yielding ATP.
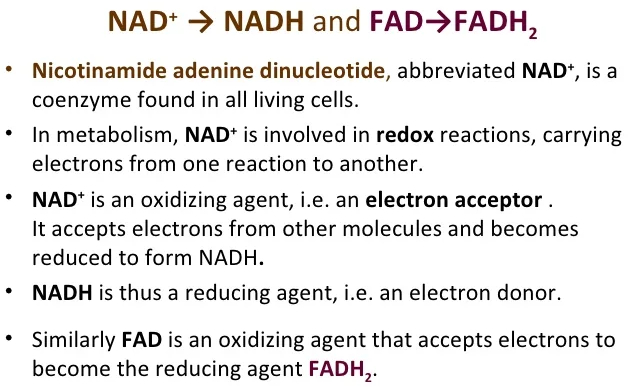
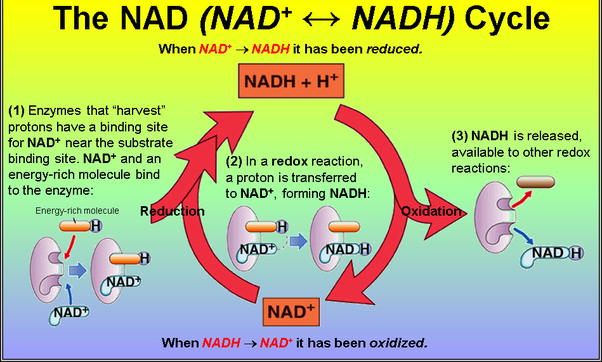
What is the role of ATP in a metabolic reaction? What is the role of electron carriers such as NADH in metabolic reactions?
ATP = High energy carrier
Most Widely Used Activated Carrier Molecule
NADH = electron / proton carrier
NADH and NADPH act as electron carriers because they carry the electrons of a hydride from one region of a cell to another
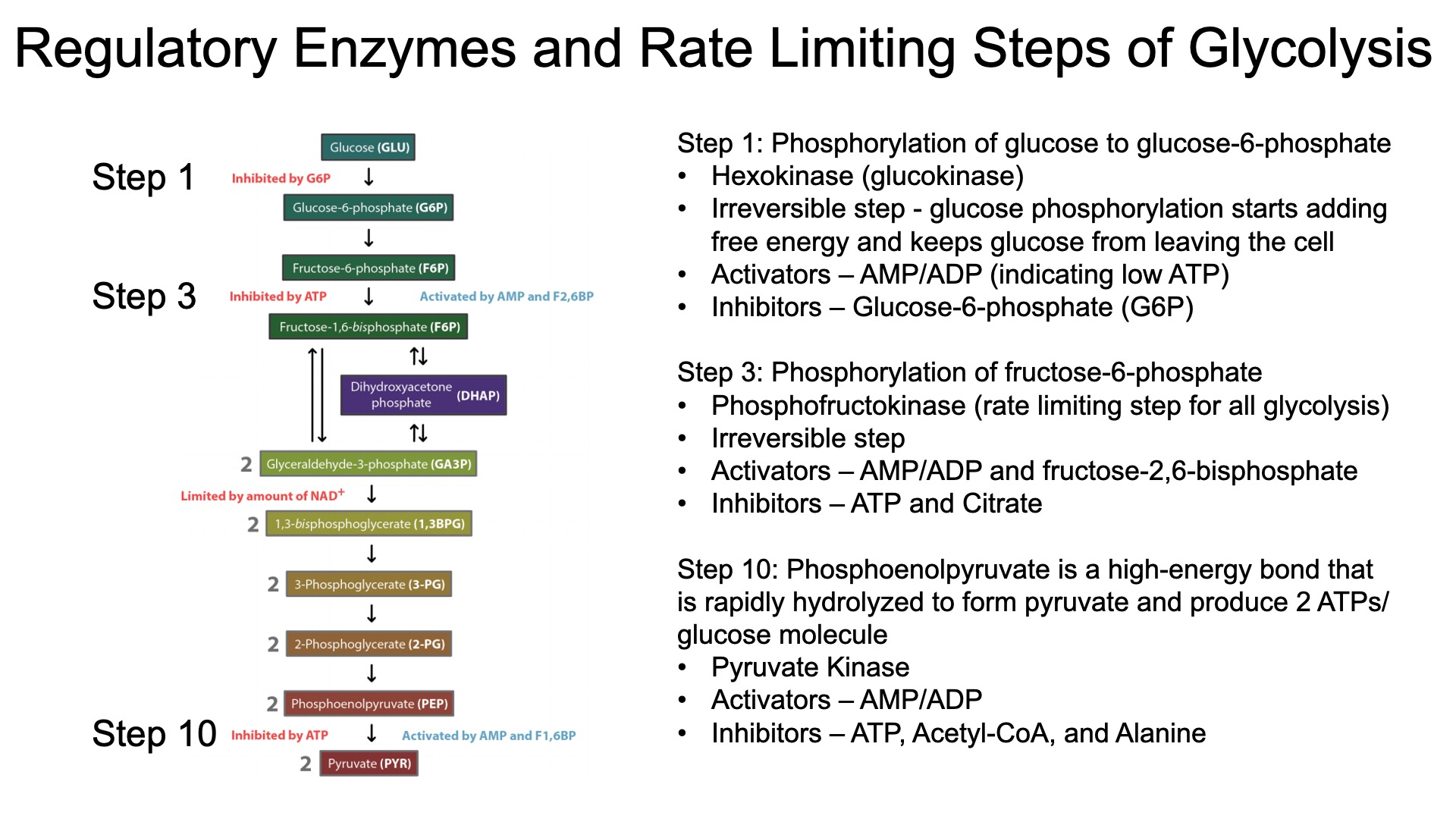
What are the steps in glycolysis and how is glycolysis regulated?
Step Pneumonic Substrate Pneumonic Enzyme 1 Gross Glucose Helen Hexokinase 2 Guys Glucose 6-Phosphate Paints Phosphoglucose Isomerase 3 Favor Fructose 6-Phosphate Pictures Phosphofructokinase 4 Big Butts Fructose 1,6-Bisphosphate Along the Aldolase 5 Dihydroxyacetone Phosphate Training Triose Phosphate Isomerase 6 Good Glyceraldehyde 3-Phosphate Grounds Glyceraldehyde 3-Phosphate Dehydrogenase 7 Boys 1,3-Bisphosphoglycerate Praying Phosphoglycerate Kinase 8 Prefer 3-Phosphoglycerate People Phosphoglycerate Mutase 9 Pretty Girls In 2-Phosphoglycerate Enjoy Enolase 10 Pink Phosphoenolpyruvate Paintings Pyruvate Kinase Pajamas Pyruvate 
In an anaerobic environment, how is ATP creation maintained and how can this impact health?
Fermentation indirectly produces ATP in the absence of oxygen (anaerobic process)
ATP is produced by regenerating NAD+ for glycolysis
This occurs in simple organisms and in the skeletal muscle of complex organisms when oxygen is absent
This also occurs in conditions of ischemia (inadequate blood supply to an organ or part of the body)
Regenerate NAD via lactate dehydrogenase
Anaerobic Exercise: During periods of high-intensity exercise in which oxygen demand exceeds oxygen supply, muscles rely on anaerobic glycolysis for ATP production. Although oxidative phosphorylation produces approximately 15 times more ATP than glycolysis, glycolysis occurs at a rate approximately 100 times faster.
Serum Lactic Acid: Lactic acid levels increase when oxygen demand exceeds oxygen supply/delivery, such as in anemia, heart failure, severe infection (sepsis), and shock. Lactic acid measurements are useful for diagnosing and directing the management of such conditions.
Fibromyalgia: Fibromyalgia is a chronic pain condition characterized by diffuse tender points on the body in the absence of abnormal diagnostic testing. Some studies have revealed an increase in pyruvate and lactate production in individuals with fibromyalgia compared to healthy controls, as well as a decrease in ATP production. Subjects with fibromyalgia also expressed lactate dehydrogenase in lower concentrations.
The Warburg Effect: One hallmark of cancer is the shift from aerobic to anaerobic metabolism seen within tumor cells, referred to as the Warburg Effect. As tumors grow, they expand beyond the capabilities of local blood supply. To combat the inadequate tissue perfusion and oxygenation, cancerous cells shift away from oxidative metabolism and instead rely heavily on anaerobic glycolysis.
How does glucose enter a cell and what is an example of a regulated form of glucose entry?
GLUT and SGLT Family of Transporters
An example of regulated glucose entry is the GLUT4 transporter in muscle and adipose tissues, which is regulated by insulin.
When insulin binds to its receptor, it triggers the translocation of GLUT4 to the cell membrane, increasing glucose uptake.
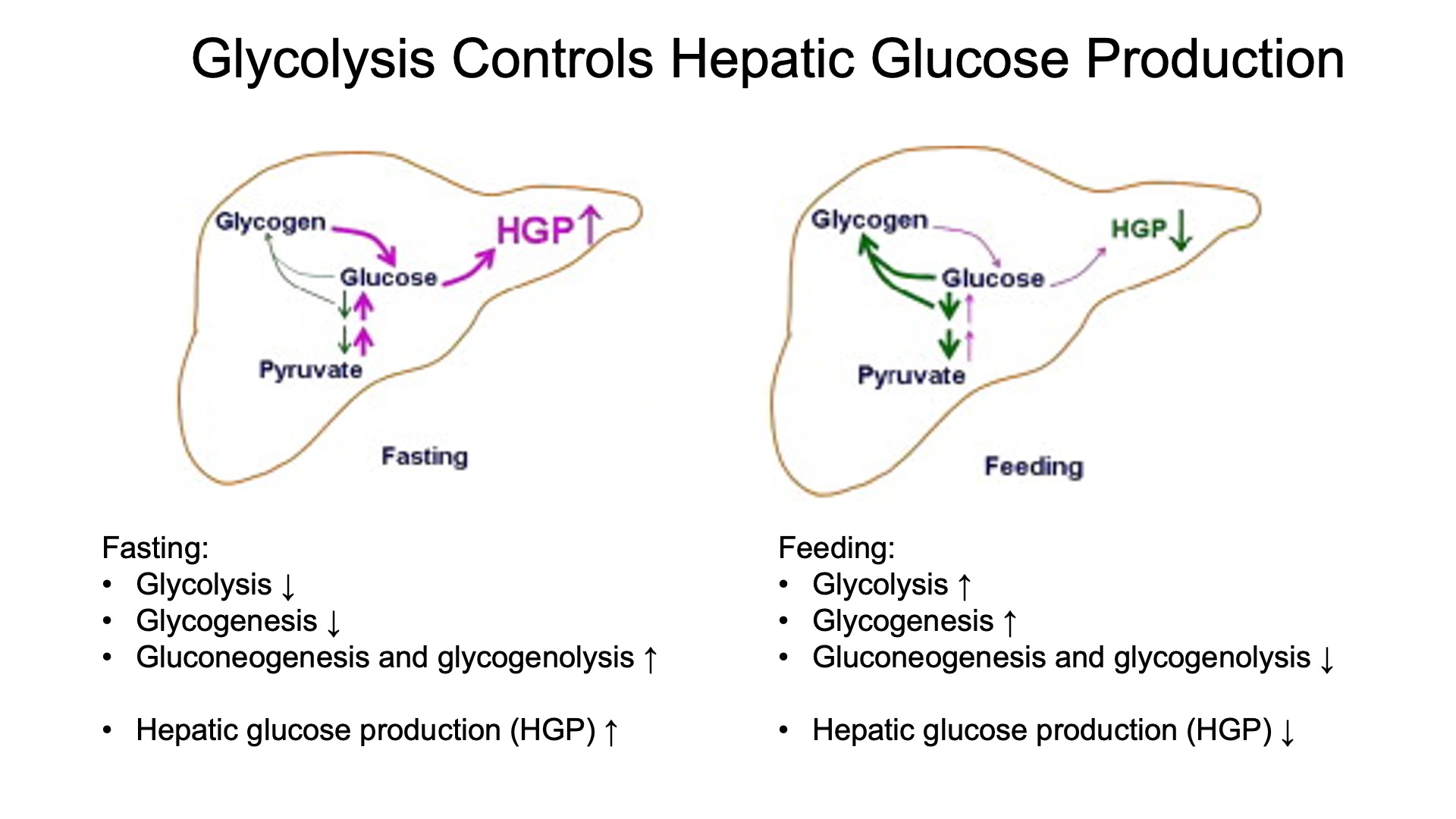
Describe liver glucose metabolism in fasting and fed states.
TCA
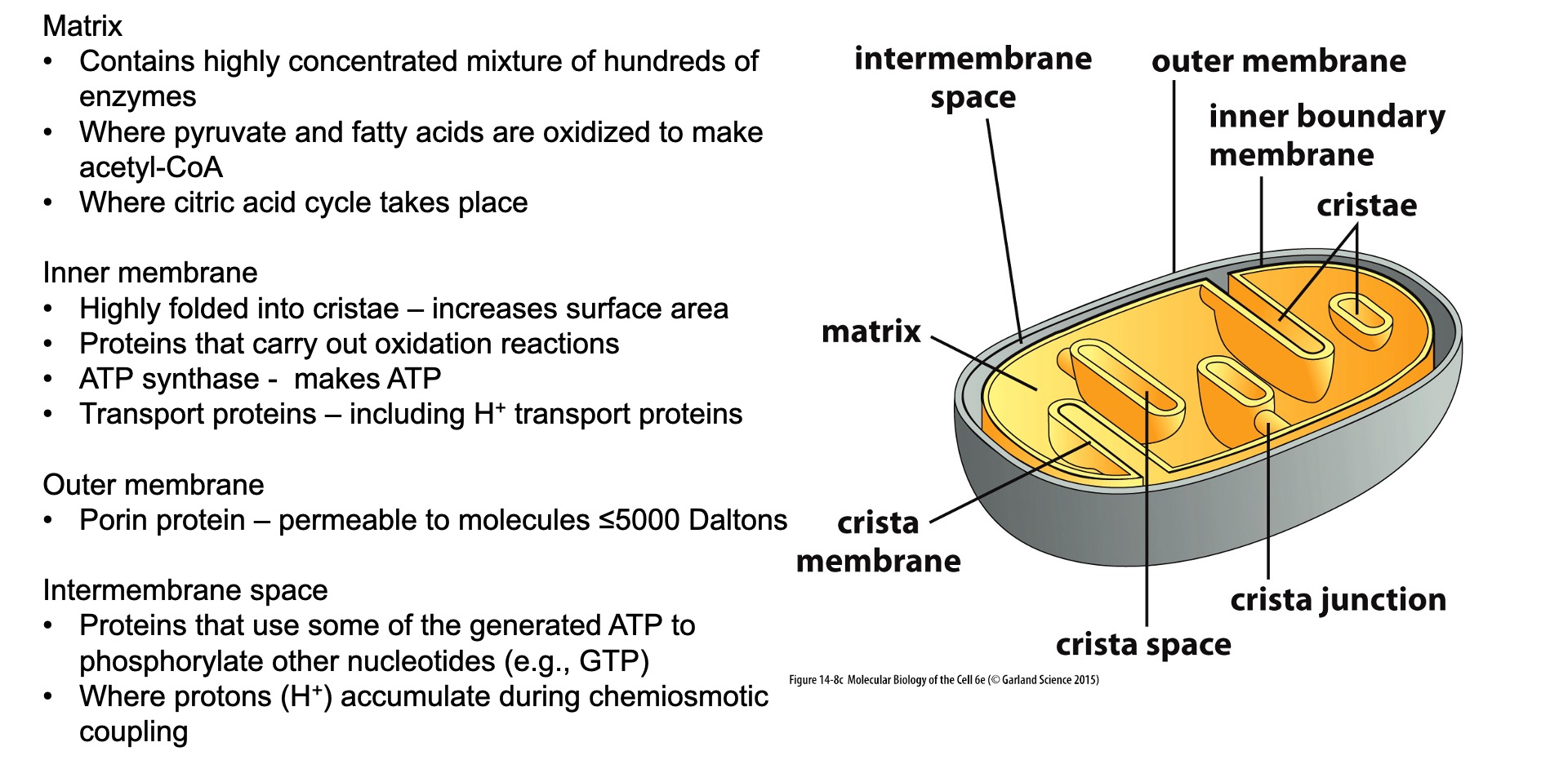
What are the four components of mitochondria and what are their roles?
Outer Membrane:
Structure: A smooth phospholipid bilayer that contains proteins and is permeable to ions and small molecules.
Role: Acts as a barrier between the cytosol and the inner mitochondrial environment. It allows the passage of metabolites and ions, which are essential for mitochondrial function.
Intermembrane Space:
Structure: The space between the outer and inner membranes.
Role: Involved in the storage and release of protons (H+) during electron transport, which contributes to the generation of the proton gradient necessary for ATP synthesis.
Inner Membrane:
Structure: A highly folded membrane that forms cristae, increasing the surface area.
Role: Contains the proteins of the electron transport chain and ATP synthase. It's involved in oxidative phosphorylation and is impermeable to most ions, helping maintain the electrochemical gradient used to produce ATP.
Matrix:
Structure: The innermost part of the mitochondria, enclosed by the inner membrane.
Role: Contains enzymes for the citric acid cycle, fatty acid oxidation, and the mitochondrial DNA and ribosomes. It is the site of key metabolic processes, including the citric acid cycle and parts of amino acid and lipid metabolism.
Where does the citric acid cycle take place, what is first required for its initiation, and what is produced?
Location: The citric acid cycle takes place in the mitochondrial matrix.
Initiation: The cycle is initiated by the condensation of acetyl-CoA (a 2-carbon molecule derived from pyruvate) with oxaloacetate (a 4-carbon molecule) to form citrate (a 6-carbon molecule).
Products: The cycle produces:
2 molecules of CO2
3 molecules of NADH
1 molecule of FADH2
1 molecule of GTP (which can be converted to ATP)
Oxaloacetate, which is regenerated to continue the cycle
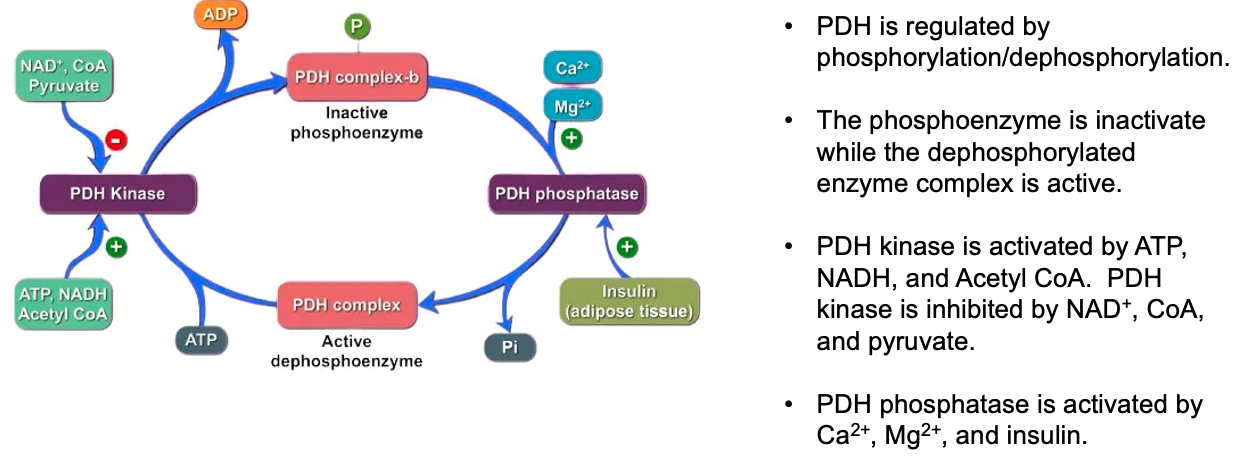
Know the general structure, function, and regulation of pyruvate dehydrogenase
Structure: Pyruvate dehydrogenase (PDH) is a large multi-enzyme complex made up of three enzymes: E1 (pyruvate dehydrogenase), E2 (dihydrolipoamide transacetylase), and E3 (dihydrolipoamide dehydrogenase).
Function: PDH catalyzes the conversion of pyruvate into acetyl-CoA, linking glycolysis to the citric acid cycle. This reaction also produces NADH and releases CO2
Regulation: PDH is regulated by phosphorylation and dephosphorylation.
The PDH complex is inactivated by phosphorylation (by PDH kinase) and activated by dephosphorylation (by PDH phosphatase).
High levels of NADH, acetyl-CoA, and ATP inhibit PDH,
while high levels of ADP and pyruvate activate it.
Know the citric acid cycle, including how the number of carbon molecules change during the eight steps of the citric acid cycle?
Citrate (6C): Formed by the condensation of acetyl-CoA (2C) with oxaloacetate (4C).
Isocitrate (6C): Isomerization of citrate.
α-Ketoglutarate (5C): Decarboxylation of isocitrate, releasing one CO2
Succinyl-CoA (4C): Decarboxylation of α-ketoglutarate, releasing another CO2
Succinate (4C): Conversion of succinyl-CoA.
Fumarate (4C): Oxidation of succinate.
Malate (4C): Hydration of fumarate.
Oxaloacetate (4C): Oxidation of malate, regenerating oxaloacetate.
Step Pneumonic Substrate Pneumonic Enzyme 1 Ahh ! Acetyl-CoA So Citrate Synthase 2 Can Citrate At Aconitase 3 I Isocitrate Disco Isocitrate Dehydrogenase 4 Keep a-Ketoglutarate Devil a-Ketoglutarate Dehydrogenase 5 Selling Succinyl-CoA Slipped Succinyl-CoA Synthase 6 Substances Succinate Down Succinate Dehydrogenase 7 For Fumarate Five Fumarase 8 Money Malate Drinks Malate Dehydrogenase 9 Officer Oxaloacetate 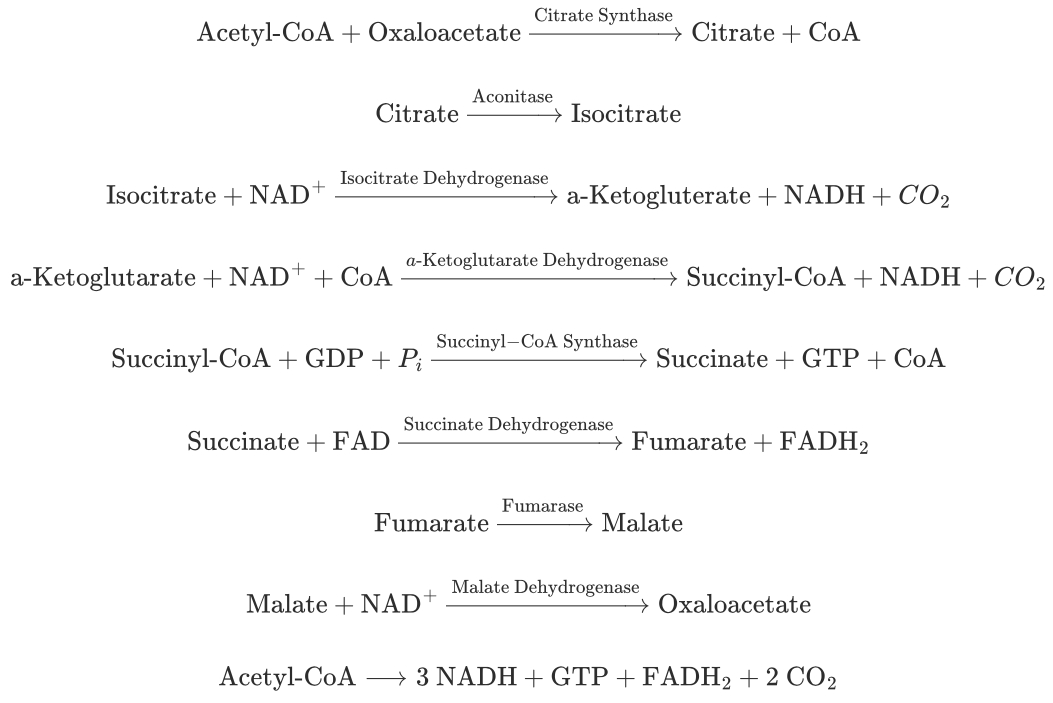
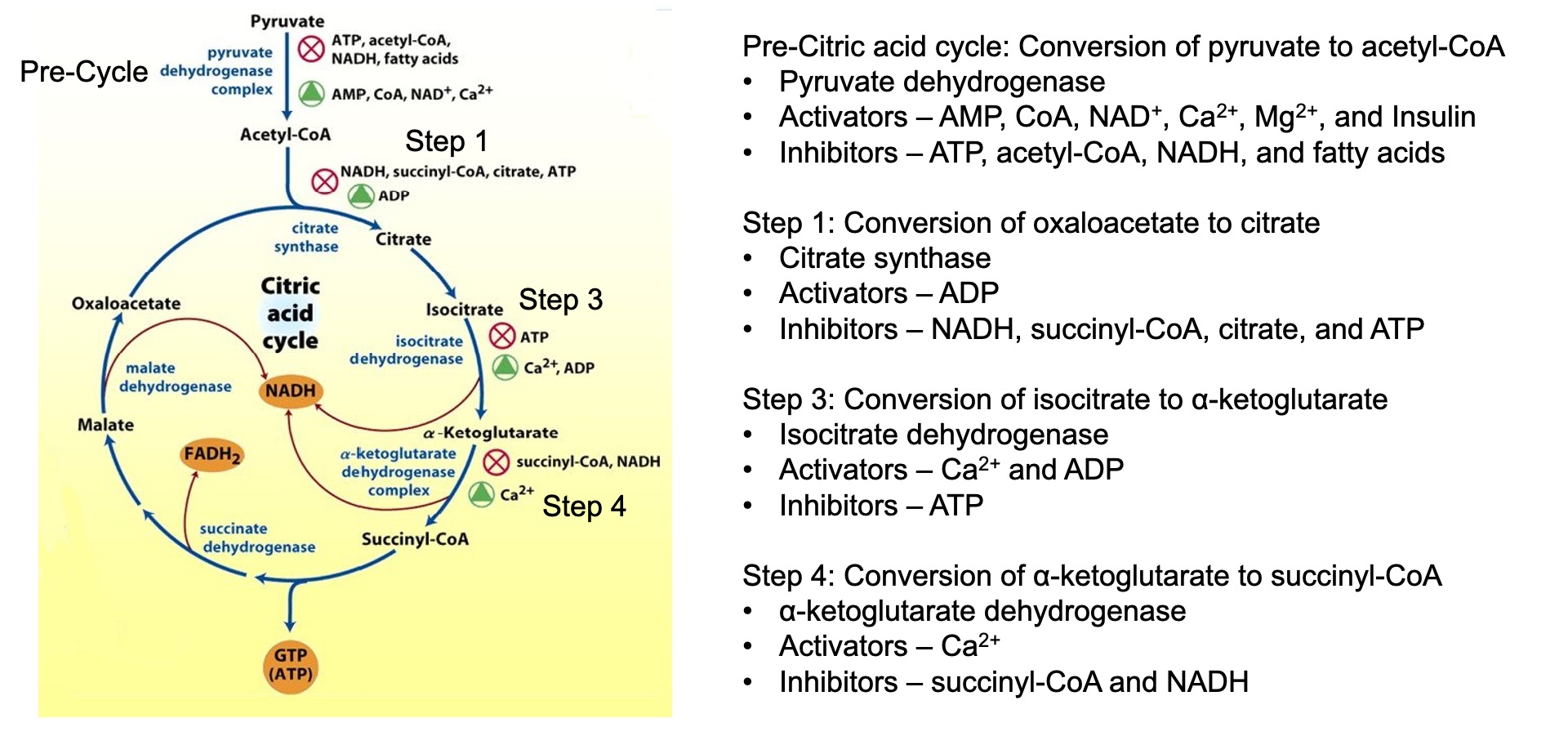
What are the regulatory steps in the citric acid cycle and how does the citric acid cycle regulate glycolysis?
Regulatory Steps:
Citrate Synthase: Inhibited by ATP, NADH, succinyl-CoA, and citrate.
Isocitrate Dehydrogenase: Activated by ADP and
α-Ketoglutarate Dehydrogenase: Activated by
Regulation of Glycolysis: The citric acid cycle indirectly regulates glycolysis through the levels of ATP, NADH, and citrate. High levels of these molecules signal that the cell has enough energy, leading to the inhibition of phosphofructokinase-1 (PFK-1), a key regulatory enzyme in glycolysis.
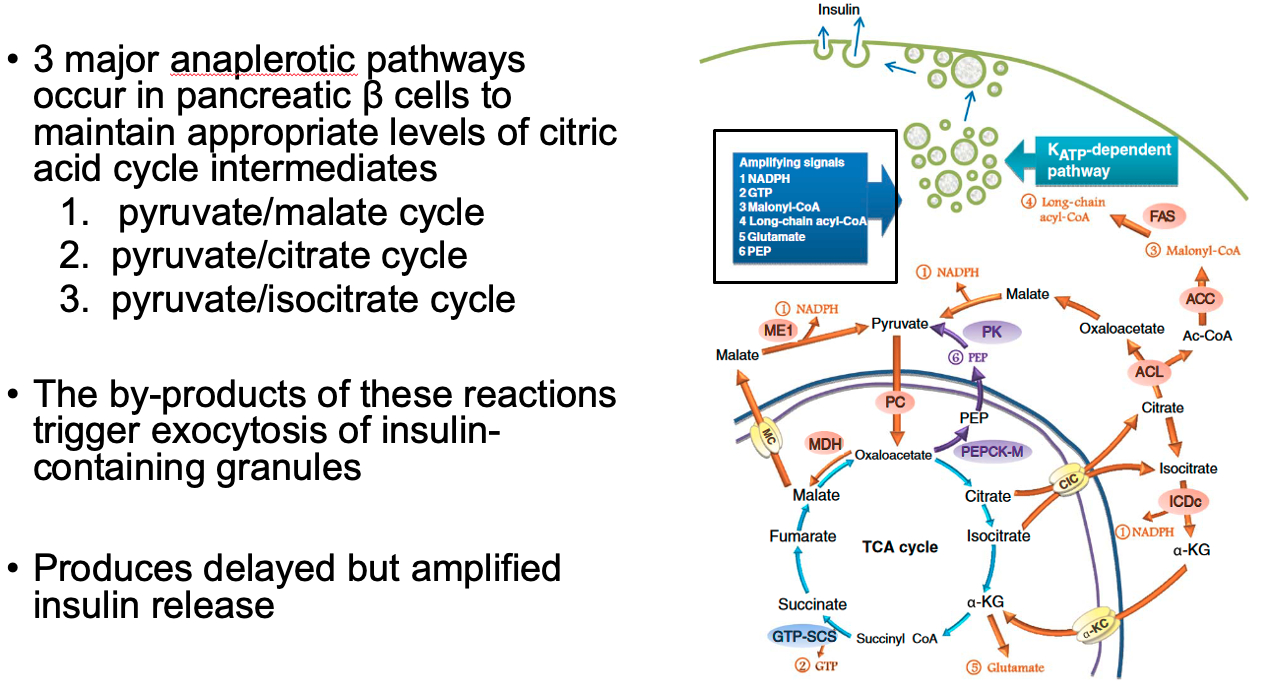
What are the roles of cataplerotic and anaplerotic reactions in the citric acid cycle
Theoretically, reactions of the citric acid cycle provide 100% recovery of the catalytic intermediates
However, citric acid cycle intermediates are used in the formation of other molecules such as amino acids
From most organs, 1-2% of citric acid cycle intermediates “leak” from mitochondria per minute
Cataplerotic Reactions: reactions that remove intermediates from the citric acid cycle
These reactions deplete the cycle's intermediates and can affect its efficiency.
aspartate aminotransferase – oxaloacetate to aspartate
glutamate dehydrogenase – α-ketoglutarate to glutamate
Phosphoenolpyruvate carboxykinase (PEPCK) - oxaloacetate into phosphoenolpyruvate
Anaplerotic Reactions: These reactions replenish citric acid cycle intermediates.
These reactions ensure that the cycle can continue even when intermediates are withdrawn for other processes.
Pyruvate carboxylase - synthesizes oxaloacetate from pyruvate in the mitochondrial matrix
aspartate aminotransferase - oxaloacetate from aspartate
Glutamate dehydrogenase - α-ketoglutarate from glutamate
Describe glucose-stimulated-insulin-release (two phases)
First Phase: Acute Response
Rapid release of pre-stored insulin granules in response to increased blood glucose levels.
This phase lasts for a few minutes and quickly lowers blood glucose.
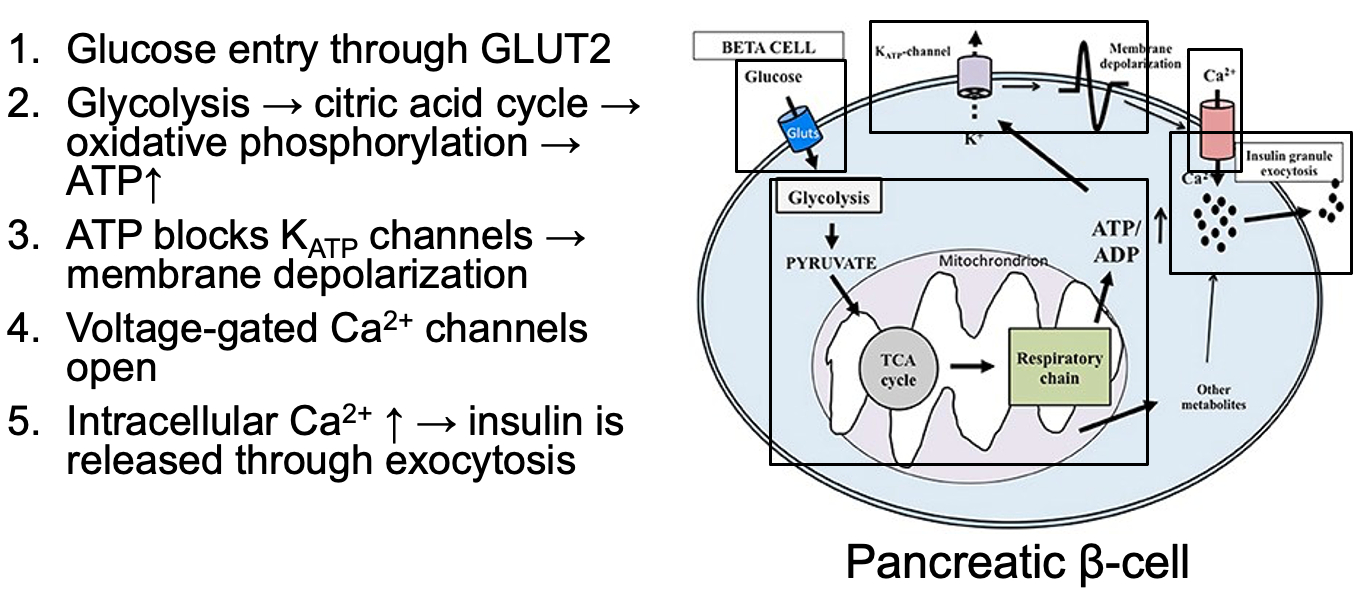
Second Phase: Delayed Response
Sustained release of insulin that is synthesized and secreted by the β-cells in response to prolonged high blood glucose. This phase helps maintain blood glucose levels within the normal range.
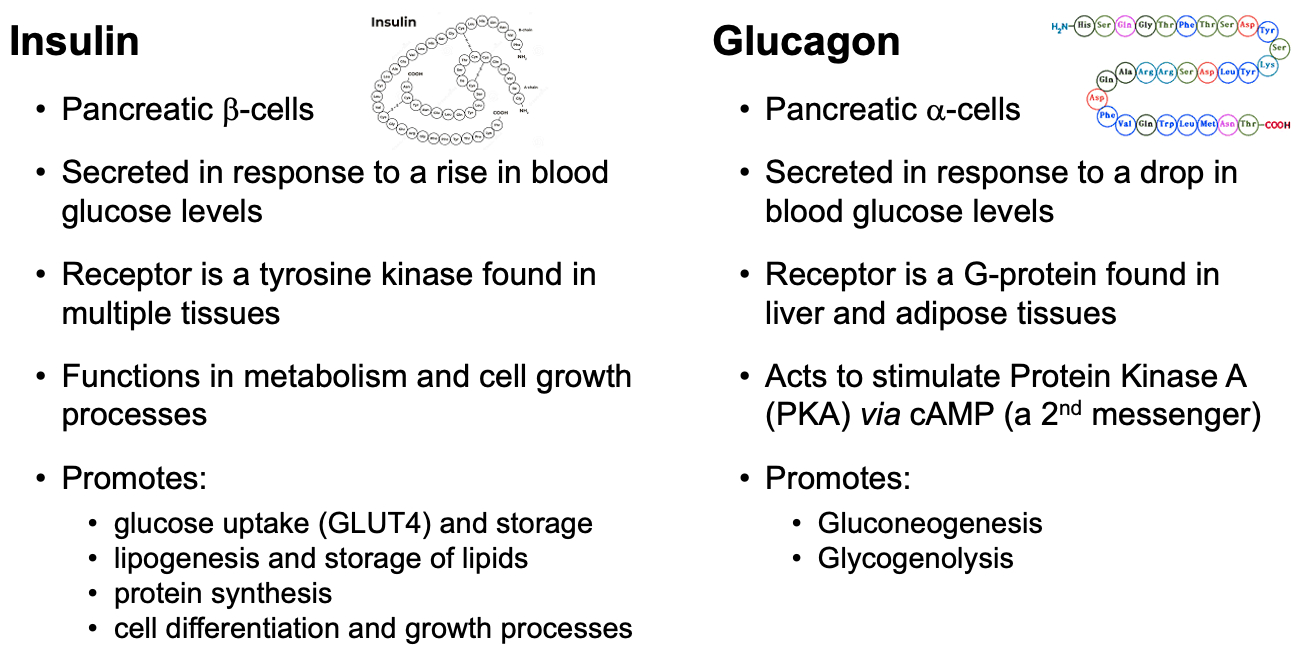
Describe and compare insulin and glucagon signaling and describe how they regulate hepatic glucose production
Insulin:
Signaling: Insulin binds to its receptor, triggering a signaling cascade that promotes glucose uptake, glycogen synthesis, and fat storage. It inhibits gluconeogenesis and glycogenolysis in the liver.
Regulation: Insulin reduces hepatic glucose production by promoting the storage of glucose as glycogen and inhibiting the enzymes involved in gluconeogenesis and glycogenolysis.
Glucagon:
Signaling: Glucagon binds to its receptor, activating adenylate cyclase and increasing cAMP levels, which activates protein kinase A (PKA). This leads to the activation of enzymes involved in glycogenolysis and gluconeogenesis.
Regulation: Glucagon increases hepatic glucose production by promoting the breakdown of glycogen (glycogenolysis) and the production of glucose from non-carbohydrate sources (gluconeogenesis).
What is the role of the molecule fructose-2,6-bisphosphate and the enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase ( 6PFK2 / FBPase2 ) in regulating hepatic glucose production?
Fasting State :
Glucagon binds to its hepatic receptor.
cAMP activates Protein Kinase A (PKA).
PKA phosphorylates 6PFK2/FBPase2.
Phosphorylation decreases the kinase activity and increases the phosphatase activity of 6PFK2/FBPase2.
Fructose-2,6-bisphosphate levels decrease.
Reduced Fructose-2,6-bisphosphate levels lead to a decrease in Phosphofructokinase-1 (6PFK1) activity.
As a result, glycolysis decreases, and gluconeogenesis increases.
Feeding State :
Insulin binds to its hepatic receptor.
Insulin Receptor Substrate 2 (IRS-2) is activated.
IRS-2 activation triggers Phosphatidylinositol 3-Kinase (PI 3-Kinase) activation.
PI 3-Kinase activates phosphoprotein phosphatase.
Phosphoprotein phosphatase dephosphorylates 6PFK2/FBPase2, increasing its kinase activity and decreasing its phosphatase activity.
Fructose-2,6-bisphosphate levels increase.
Increased Fructose-2,6-bisphosphate levels enhance Phosphofructokinase-1 (6PFK1) activity.
Additionally, Protein Phosphatase 2A (PP2A) is activated through an independent pathway, which acts synergistically with insulin signaling.
As a result, glycolysis increases.
HLO
The more negative the reduction potential , the more likely an electron is to move towards that negative reduction potential
Metabolism Stages :
1 : hydrolyze macromolecules to building blocks
2 : further metabolize building blocks to common units like acetyl-CoA
3 : convert acetyl-CoA into ATP
Glycolysis Regulation :
Step 1 - traps glucose in the cell
inhibitors = G-6-P
activators = AMP , ADP
Step 3 - rate limiting
inhibitors = citrate , ATP
activators = AMP , ADP , F-2,6-BP
Step 10 - pyruvate kinase
inhibitors = alanine , ATP , acetyl-CoA
activators = AMP , ADP
ETC makes 15 times for ATP than glycolysis , but glycolysis is way faster
GLUT and SGLT receptors.
insulin upregulates GLUT4 expression in muscle and adipose
Pyruvate Dehydrogenase Complex ( PDHC ) :
inactive = kinase phosphorylated
promoted by = ATP , NADH , acetyl-CoA
active = phosphatase de-phosphorylated
promoted by = Calcium , Magnesium , Insulin
TCA Regulation :
Pre-Cycle - PDHC
Step 1 :
inhibitors = citrate , NADH , succinyl-CoA , ATP
Step 3 :
inhibitors = ATP
Step 4 :
inhibitors = succinyl-CoA , NADH
Cataplerotic TCA Reactions = decrease efficiency
Anaplerotic TCA Reactions = help beta cells in pancreas sustain TCA cycle
also by-products of these reactions help trigger exocytosis of insulin granules
Acute Phase of Insulin Release = pre-stored insulin granules released
glycolysis causes high ATP levels from glycolysis
ATP blocks potassium channels
cell depolarizes
calcium enters via voltage-gated calcium channels
causes insulin release from pre-stored insulin granules
lasts a few minutes
Latent Phase of Insulin Release = glucose enters beta cells of pancreas
by-products of anapleorotic pathways trigger exocytosis of insulin-containing granules
produces delayed but amplified insulin release
Bi-Functional Enzyme :
Fasting :
Glucagon ➡️ PKA ➡️ Phosphorylates Enzyme ➡️ Less Kinase Activity ➡️ Less F-2,6-BP ➡️ Less PFK1 Activation ➡️ Less Glycolysis
Fed :
Insulin ➡️ PI 3-Kinase ➡️ De-phosphorylates Enzyme ➡️ More Kinase Activity ➡️ More F-2,6-BP ➡️ More PFK1 Activation ➡️ More Glycolysis