Describe the ENDOCRINE regulation of testosterone and estrogen production
The endocrine regulation of testosterone and estrogen production occurs through the hypothalamic-pituitary-gonadal ( HPG ) axis
The hypothalamus releases gonadotropin-releasing hormone ( GnRH ) in a pulsatile manner.
GnRH stimulates the anterior pituitary to release luteinizing hormone ( LH ) and follicle-stimulating hormone ( FSH )
LH stimulates Leydig cells in testes ( in males ) and theca cells in ovaries ( in females ) to produce testosterone.
FSH works synergistically with LH to stimulate Sertoli cells in males ( spermatogenesis ) and granulosa cells in females ( estrogen synthesis )
Feedback Mechanisms :
Testosterone and estrogen exert negative feedback on the hypothalamus and pituitary to reduce GnRH , LH , and FSH secretion
In females , estradiol has a dual role :
low levels provide negative feedback
but high levels during the late follicular phase cause positive feedback , triggering the LH surge and ovulation
Describe the Cell Signalling MOLECULAR mechanisms that regulate testosterone and estrogen production
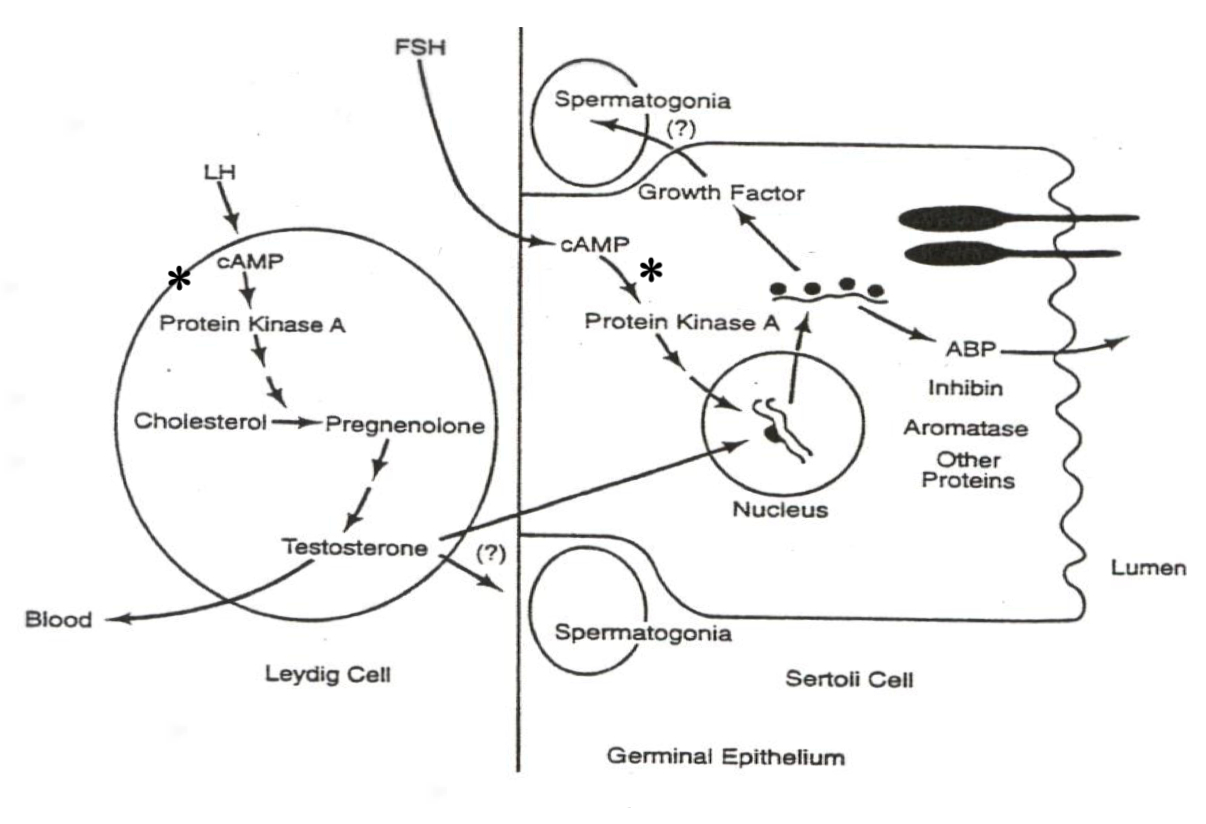
Male System ( Spermatogenesis ) :
LH in Leydig Cells :
LH ➡️ cAMP ➡️ PKA ➡️ Cholesterol ➡️ Pregnenolone ➡️ TestosteroneExplanation : LH activates cAMP and PKA , driving gene transcription of enzymes that convert cholesterol to testosterone in Leydig cells.
FSH in Sertoli Cells :
xxxxxxxxxxFSH ➡️ cAMP ➡️ PKA ➡️ Gene Transcription ➡️ Androgen-Binding Protein ( ABP )Explanation : FSH activates gene transcription for ABP , aiding sperm maturation and transport.
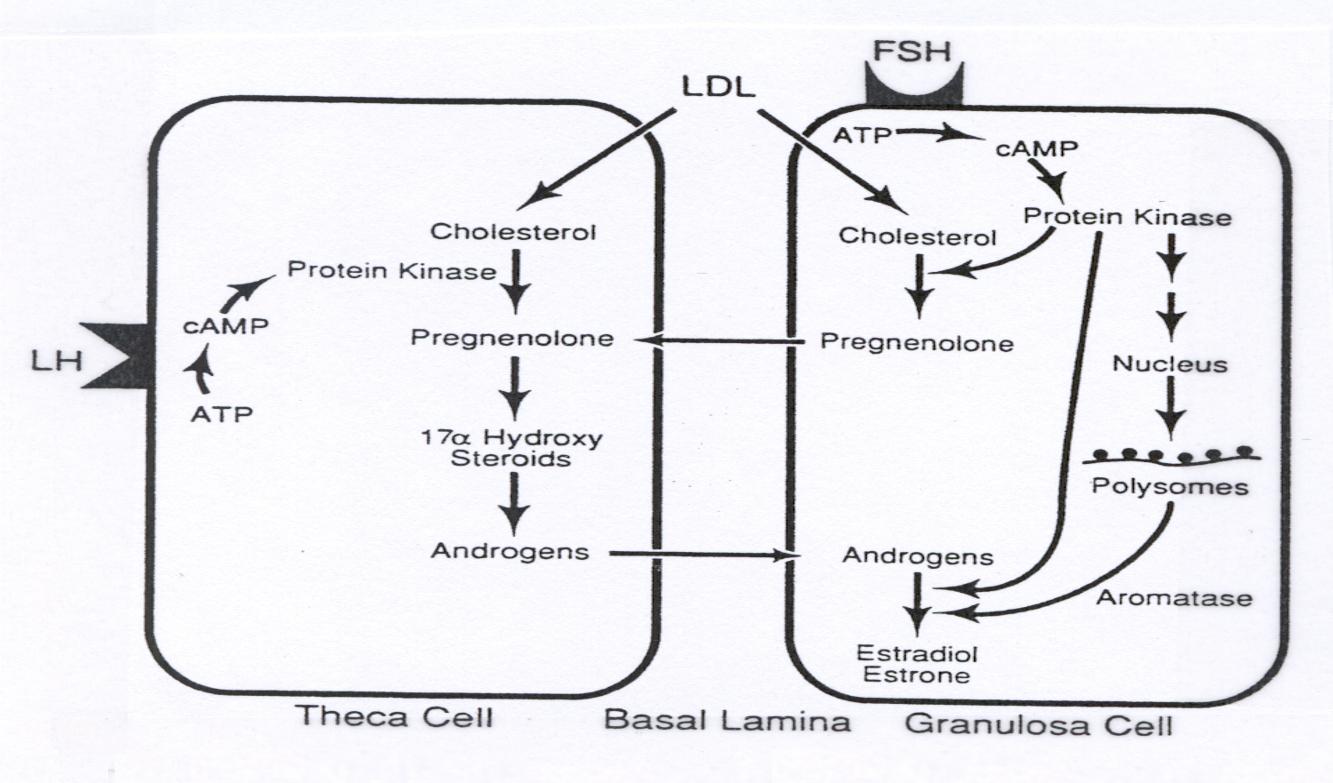
Female System ( Oogenesis and Follicle Development ) :
LH in Theca Cells :
xxxxxxxxxxLH ➡️ cAMP ➡️ PKA ➡️ Cholesterol ➡️ Pregnenolone ➡️ AndrogensExplanation : LH activates gene transcription for enzymes that convert cholesterol to androgens in theca cells.
FSH in Granulosa Cells :
xxxxxxxxxxFSH ➡️ cAMP ➡️ PKA ➡️ Gene Transcription ➡️ Aromatase ➡️ Androgens ➡️ EstrogensExplanation : FSH in granulosa cells transcribes aromatase , converting androgens ( from theca cells ) to estrogens , completing estrogen production.
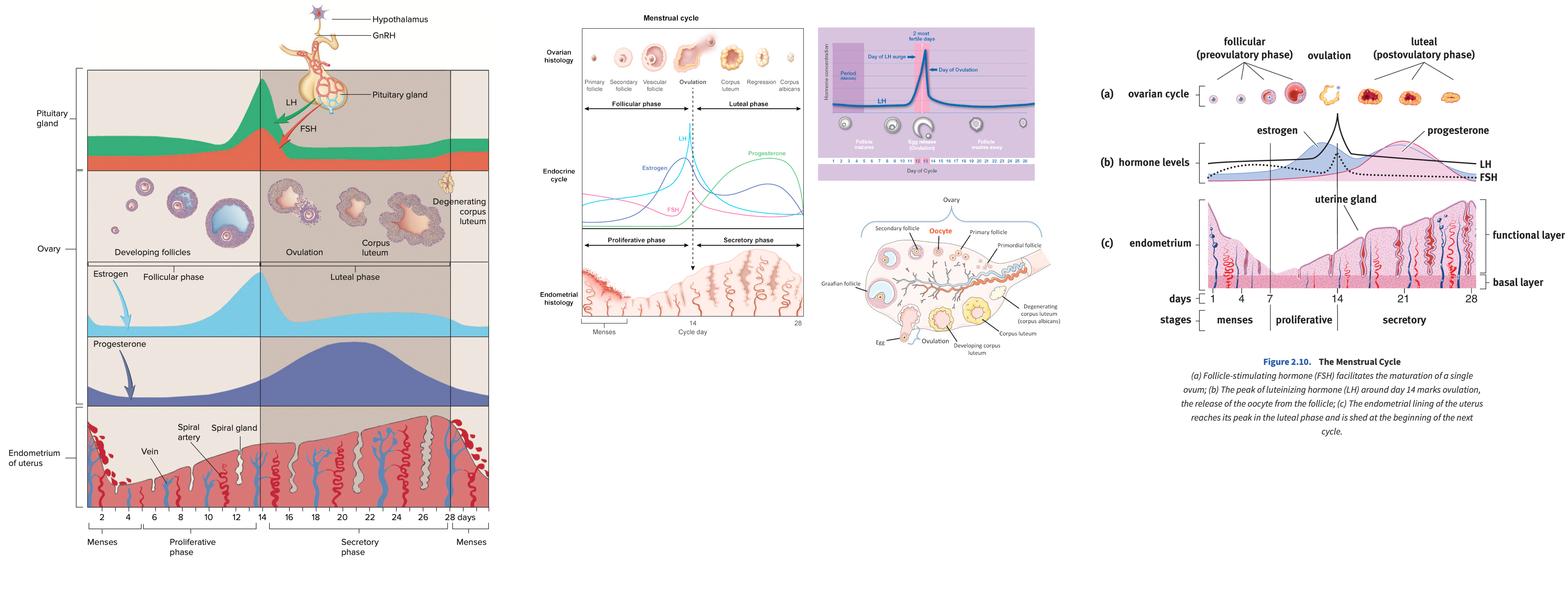
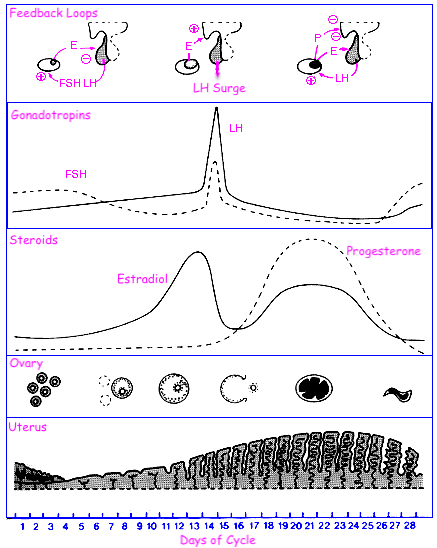
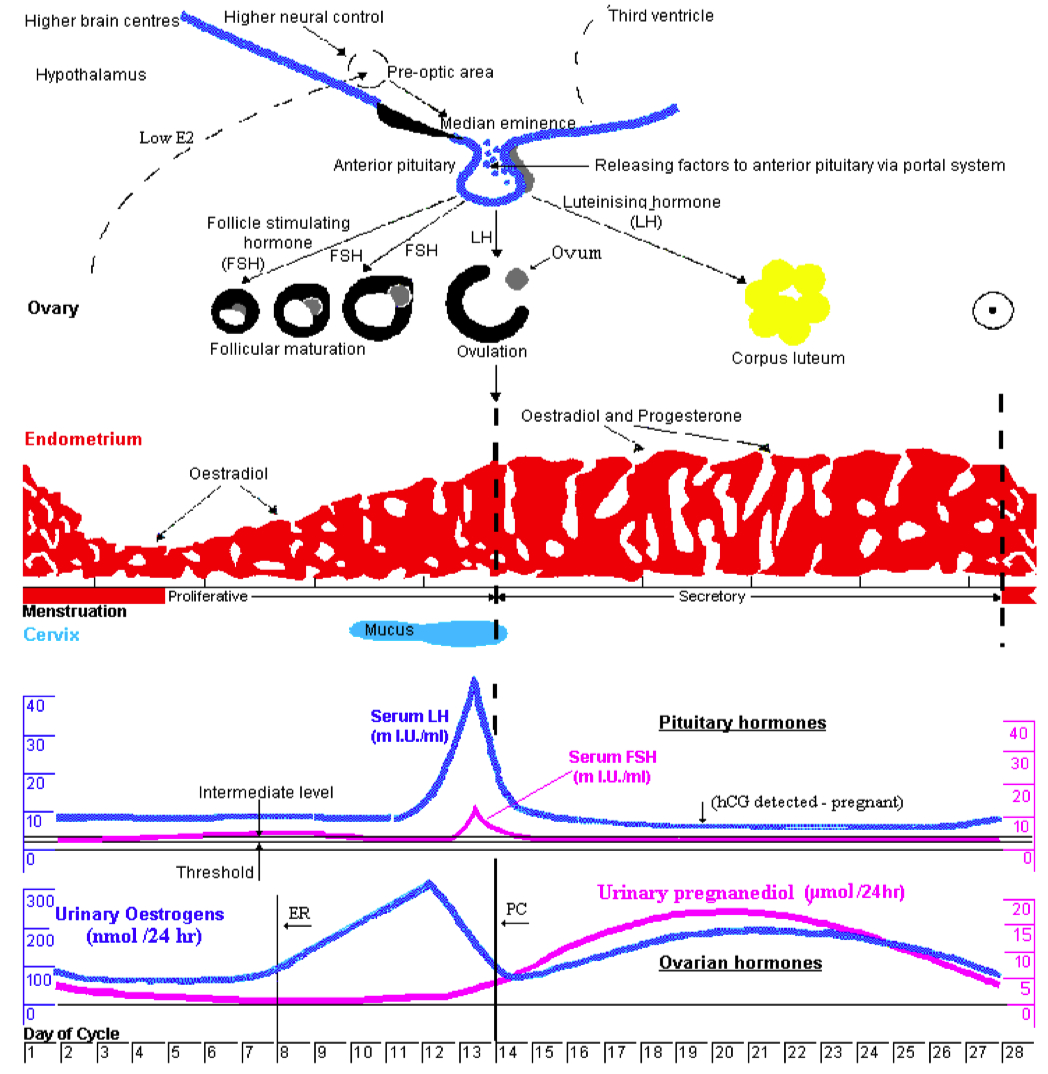
Describe the menstrual cycle in detail in relation to the ovary and uterus
Hormones :
FSH ( Follicle-Stimulating Hormone ) : Stimulates follicle development in the ovaries.
LH ( Luteinizing Hormone ) : Triggers ovulation through the LH surge.
Estrogen : Rises during the follicular phase, supporting uterine lining growth; drops after ovulation.
Progesterone : Rises post-ovulation during the luteal phase to support the endometrium for potential implantation.
Sequence of Hormone Events :
Follicular Phase ( Days 1-14 ) : FSH and estrogen levels gradually increase , supporting follicle development.
Ovulation ( Day 14 ) : Estrogen hits a threshold, causing a positive feedback loop that triggers the LH surge. This surge leads to ovulation.
Luteal Phase ( Days 14-28 ) : Progesterone rises as the corpus luteum forms , maintaining the uterine lining. If no fertilization occurs, progesterone drops , leading to menstruation.
Describe what constitutes an embryo - what is the makeup
Cellular Composition :
Zygote : The single-cell stage resulting from the fusion of a sperm and an egg, marking the beginning of the embryo.
Blastomeres : As the zygote undergoes mitotic divisions ( cleavage ) , it forms smaller, undifferentiated cells called blastomeres.
Embryonic Stem Cells : These are pluripotent cells that arise during the early stages ( e.g., inner cell mass of the blastocyst ) and have the potential to differentiate into various cell types.
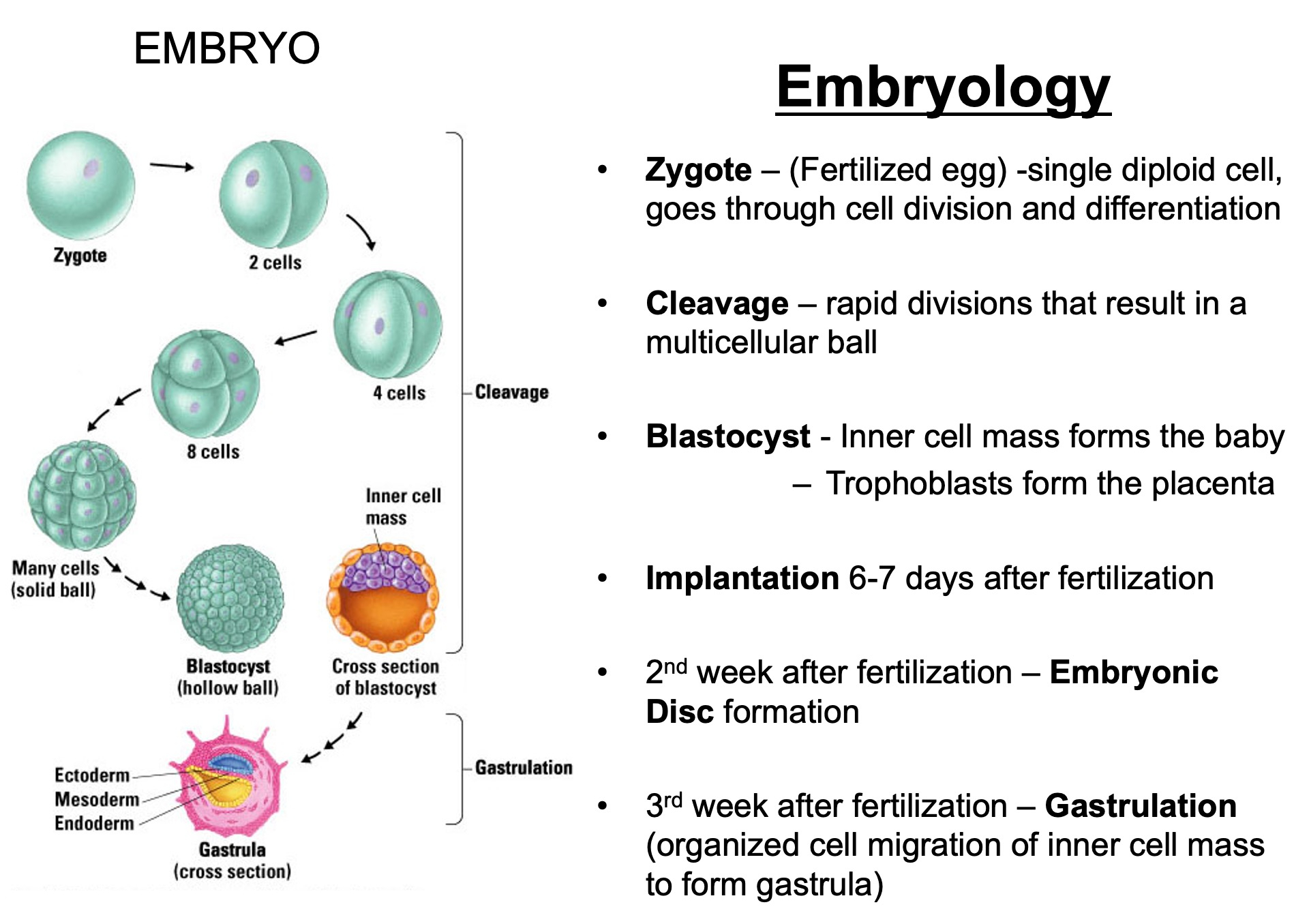
Zygote : Day 0 ( fertilization )
Blastomeres : Day 1-3 ( cleavage )
Morula : Day 3-4 ( solid ball of cells )
Blastocyst : Day 5-6 ( hollow structure , first differentiation )
Bilaminar Disc : Week 2 ( epiblast and hypoblast form )
Trilaminar Disc : Week 3 ( gastrulation creates germ layers )
Organogenesis : Weeks 4-8 ( development of organs and systems )
Fetal Stage : Week 9 onward ( growth and maturation )
By Week 9 , it is no longer referred to as an embryo but as a fetus
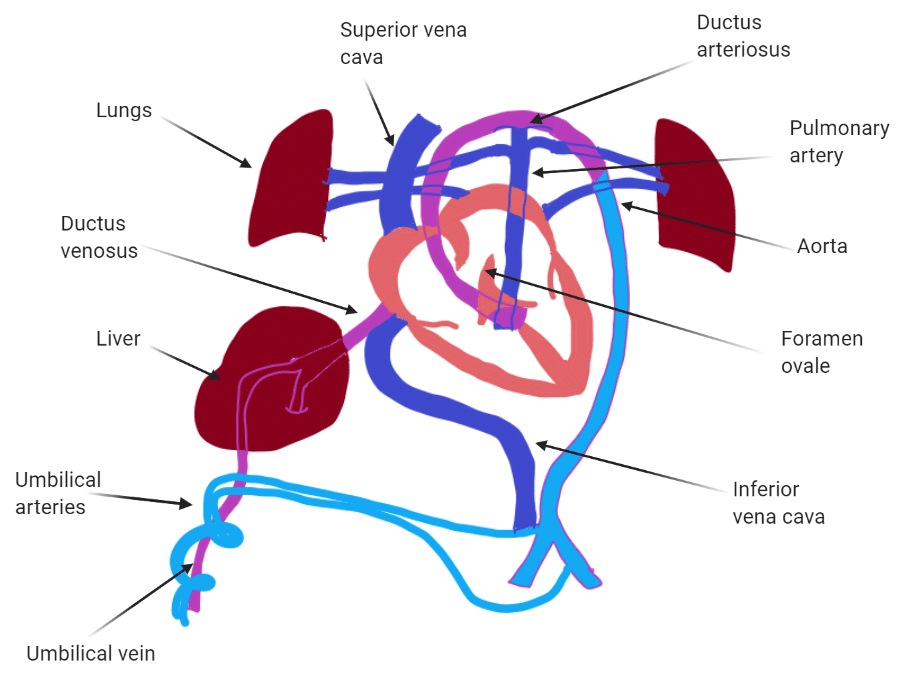
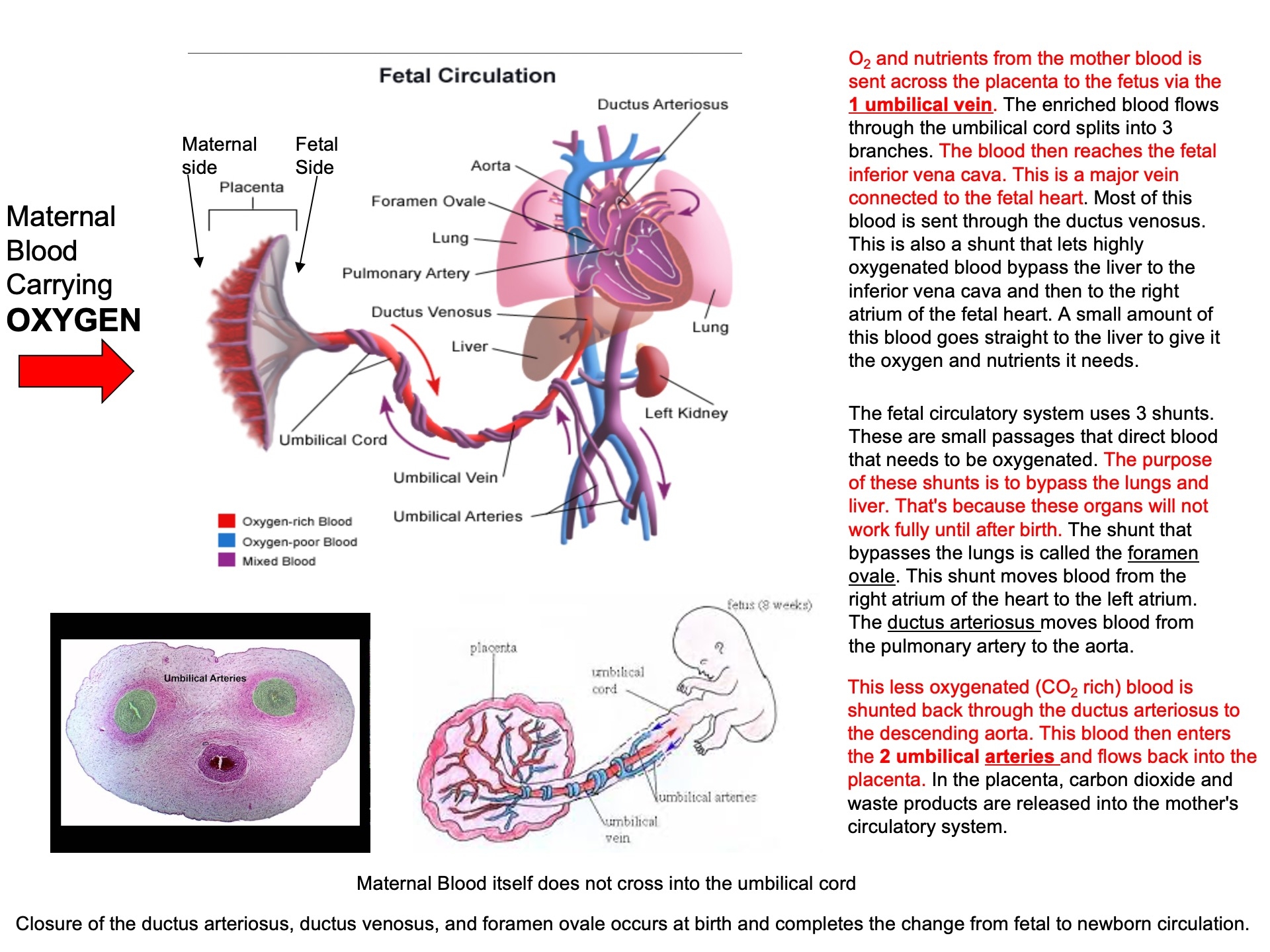
Describe why the umbilical cord is important and how it works
Acts as the conduit connecting the embryo/fetus to the placenta.
Directly connects to the fetal liver and subsequently to the fetal heart via the umbilical vein.
Carries oxygenated blood from the placenta to the fetus.
Carries deoxygenated blood and waste from the fetus to the placenta via the umbilical arteries.
Umbilical Cord Stem Cells :
Multipotent
you bank them at birth and then don't have to worry about donor rejection problems
expensive over time
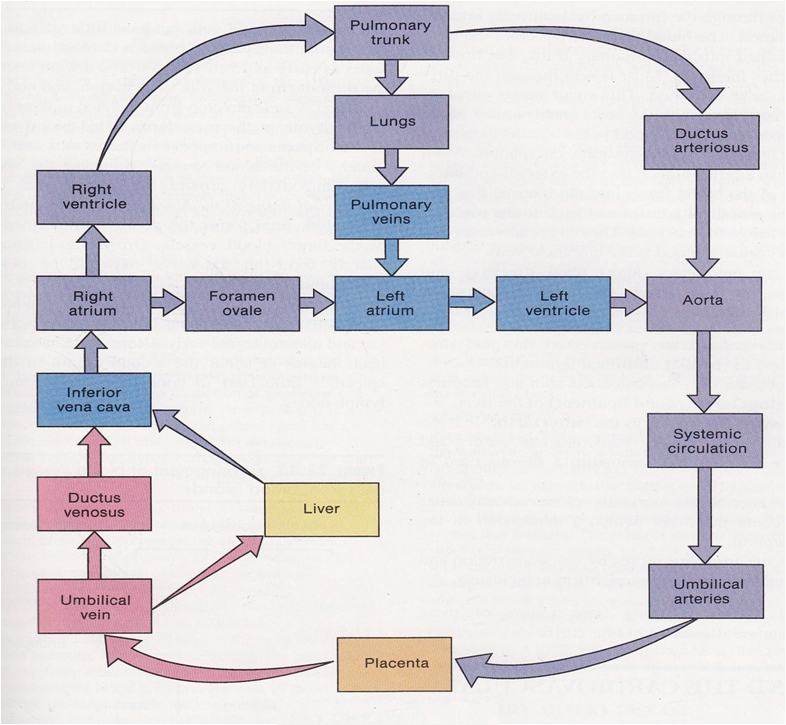
Route of Maternal Blood Oxygen to Baby
Maternal Lungs → Pulmonary veins → Left atrium → Left ventricle → Aorta → Uterine arteries → Placenta ( intervillous space )
Placenta → Oxygen diffuses into fetal capillaries → Umbilical vein :
Liver Branch : A portion of oxygenated blood enters the fetal liver via smaller branches of the umbilical vein
Bypass Liver ( Ductus Venosus ) : The rest bypasses the liver through the ductus venosus , merging with the inferior vena cava ( IVC )
IVC → Right atrium → Foramen ovale → Left atrium → Left ventricle → Aorta → Fetal tissues
Route of CO₂ from Baby Back to Mother
Fetal Tissues ( CO₂ produced ) → Fetal veins → Right atrium → Right ventricle → Pulmonary artery :
Bypass Lungs ( Ductus Arteriosus ) : Most blood bypasses the lungs via the ductus arteriosus , joining the descending aorta
Descending Aorta → Umbilical arteries → Placenta
Placenta ( CO₂ diffuses into maternal blood ) → Uterine veins → Inferior vena cava → Right atrium → Right ventricle → Pulmonary arteries → Maternal lungs ( CO₂ exhaled )
G Proteins
Signal Molecule binds and Activates GPCR (
binds and inhibits adenylyl cyclase
reduces PKA activation
reduces cell response ( gene transcription )
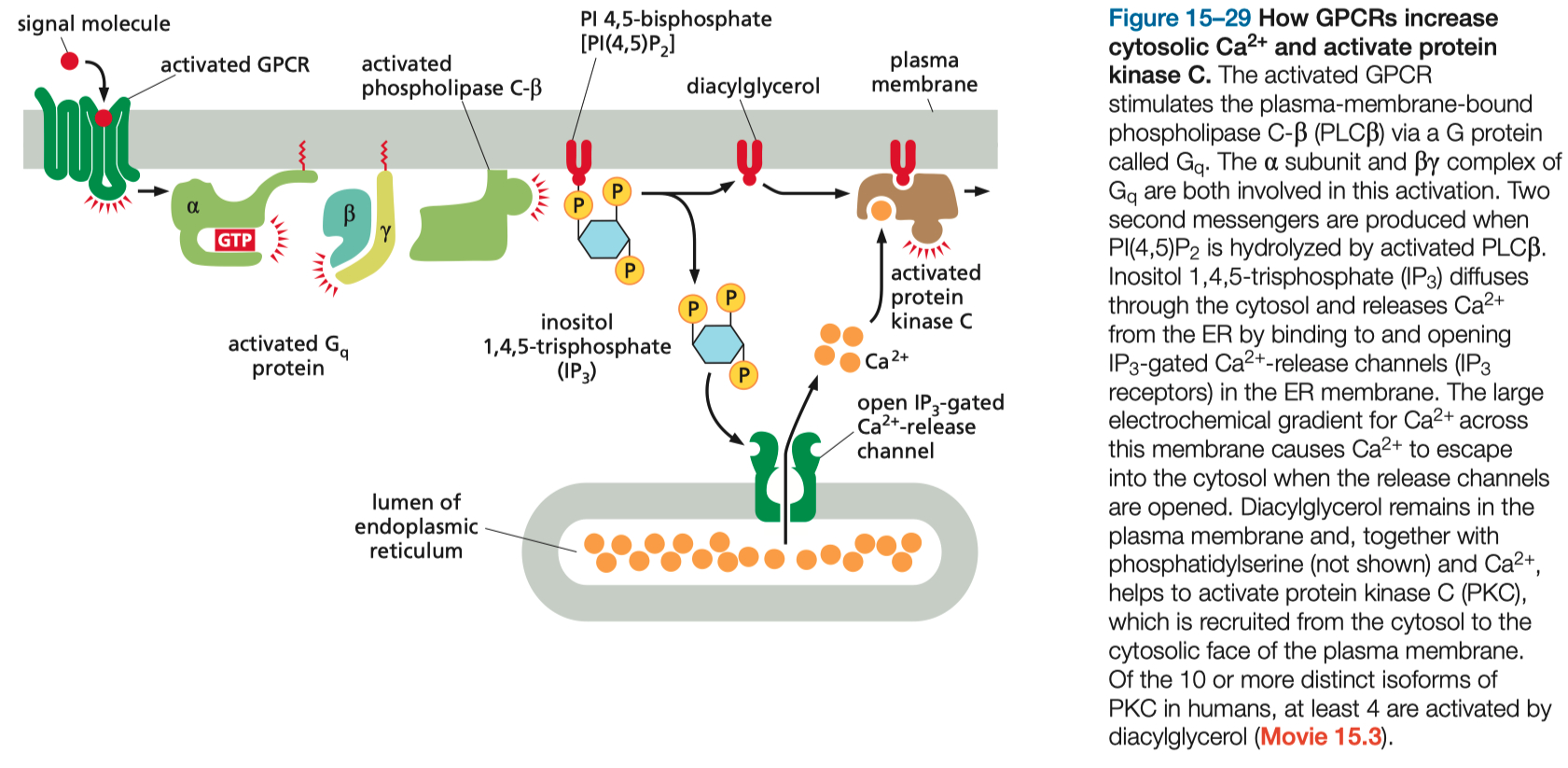
Signal Molecule binds and Activates GPCR (
binds and stimulates adenylyl cyclase
increases PKA activation
increases cell response ( gene transcription )
Signal Molecule binds and Activates GPCR (
this depletes levels of PIP2 in the membrane
but voltage gated M-channels ( potassium channels ) need PIP2 in order to open
so M-channels close
this is a SIGNALING event !
the removal of PIP2 closed potassium channels , depolarizes the membrane ,
makes it very easy to fire action potential now
only a little bit of sodium is need to reach the action potential threshold
DAG then activates PKC
IP3 causes calcium to be released from endoplasmic reticulum
the calcium can also reinforce / enhance signaling of PKC
3 Different Ways
Depletion of
Liberation of
Libration of DAG and hence activation of PKC
Describe one alternative pathway that G-protein activation may signal via
Conventional Way = producing signaling molecules
Alternative Way = reduction ! of
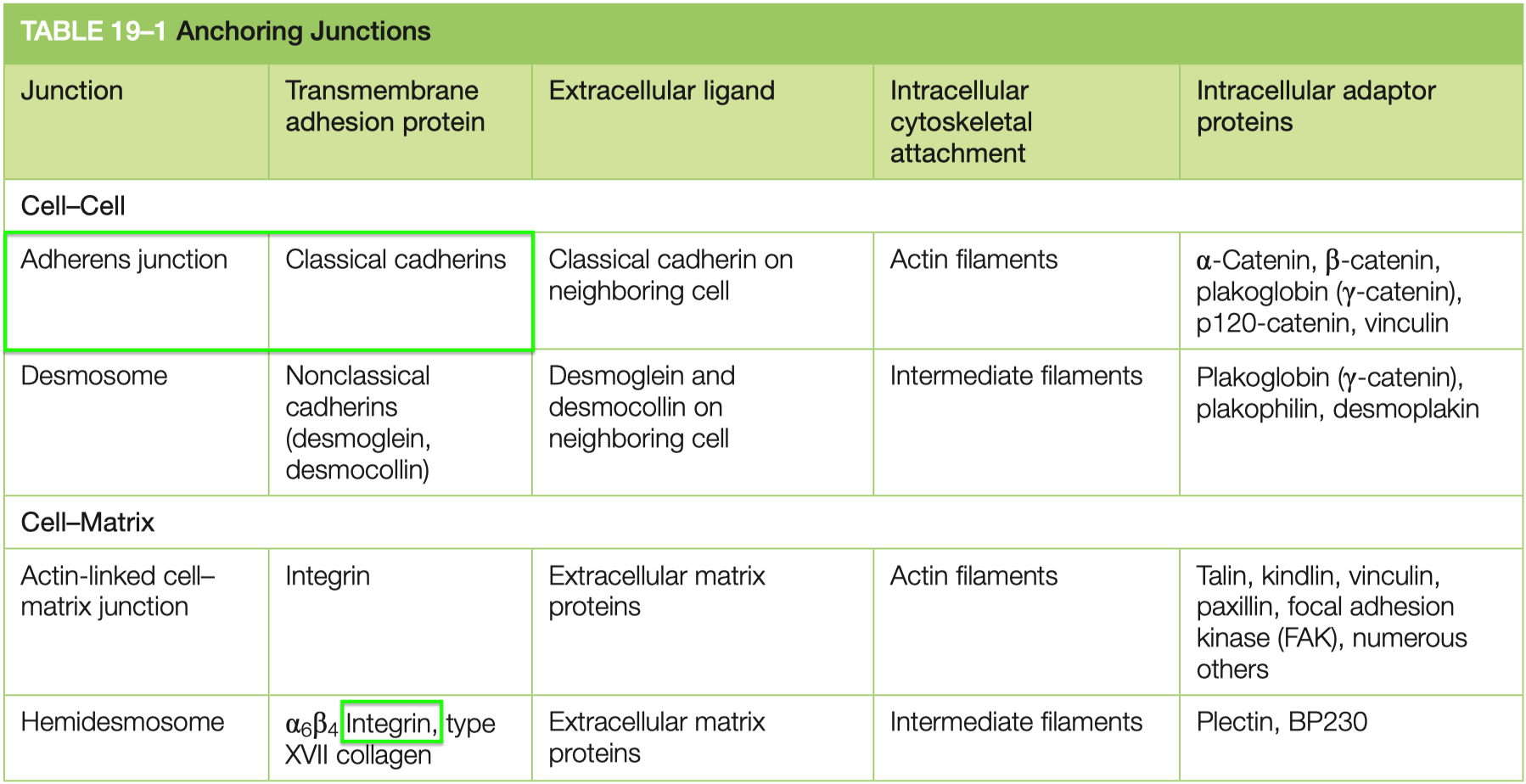
Cell Adhesion
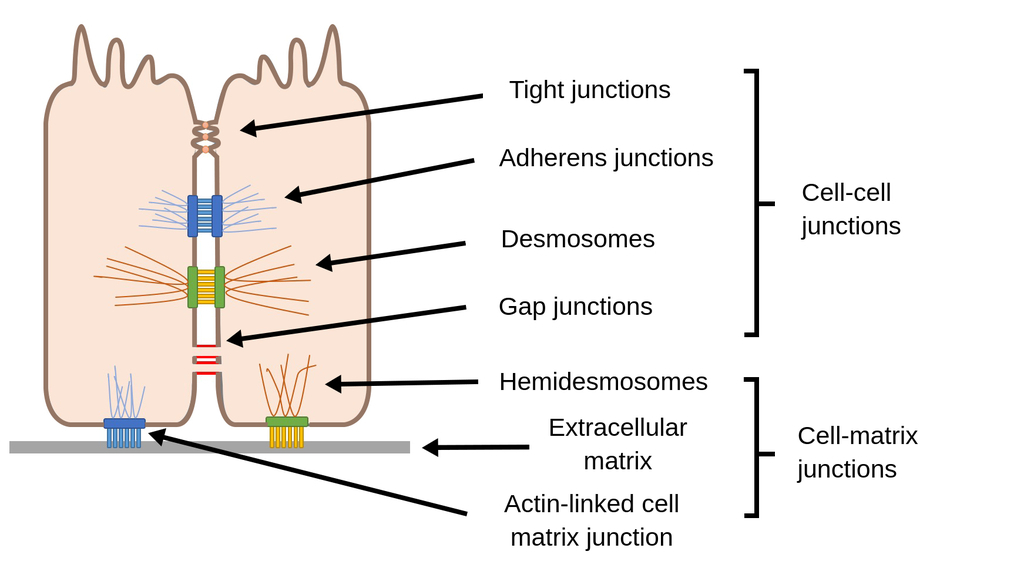
Cell Junctions
Tight Junctions :
contain strands of cloudin and occludin
example = blood-brain-barrier
Adherens :
strong mechanical
peristalsis
uses classical cadherins to link cytoskeleton ➡️ actin
Desmosomes :
stronger than adherens
tissue rigidity
uses non-classical cadherins to link cytoskeleton ➡️ intermediate filaments
cardiac muscle , skin
Hemidesmosomes :
uses integrins to link cytoskeleton ➡️ basal lamina intermediate filaments
Gap Junctions :
facillitate communication
synchronized heart beat
allow electricity , ATP , misc things to pass
6 trans membrane domains
connected by connexins
Protein Transport
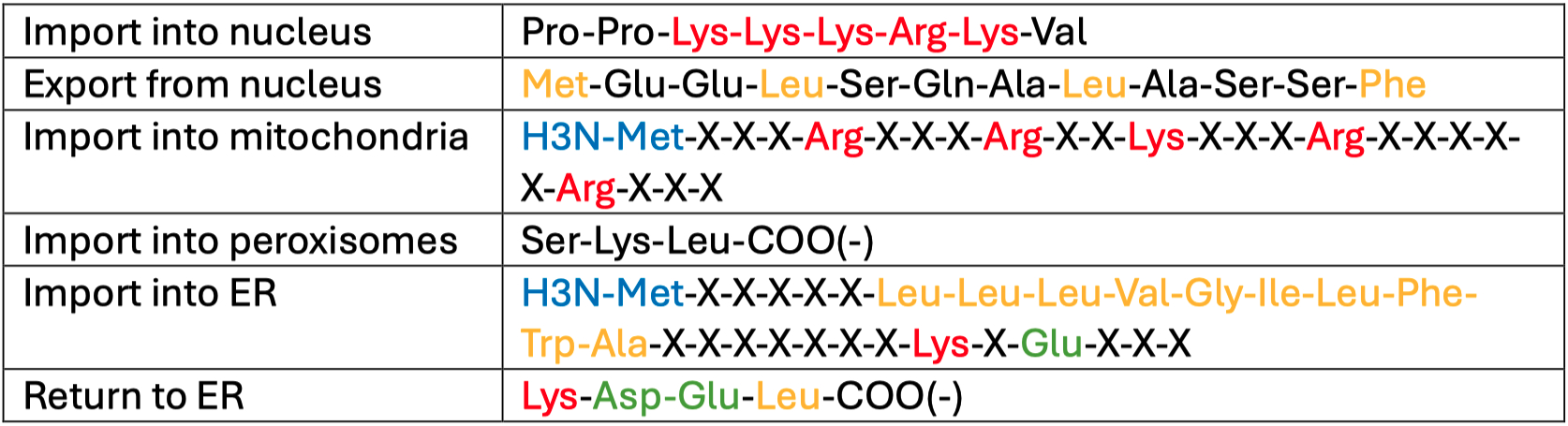
Nuclear Localization Sequences ( NLS ) :
General Trend :
Imports = basic : lysine ( K ) , arginine ( R ) , histidine ( H )
Exports = aliphatic : isoleucine ( I ) , leucine ( L )
LxxxLxxLxL
Specific :
Nuclear Import = continuous basic
Mitochondria Import = spaced basic
ER Import = continuous hydrophobic
Nuclear Import Process :
cargo-NLS is complexed with an importin
the complex travels through the Nuclear Pore Complex ( NPC ) from the cytosol , into the nucleus
Ran-GTP binds to the complex , allowing the cargo to be released from the importin
Ran-GTP bound to the important exits the nucleus back into the cytosol
Ran-GTP is hydrolyzed into Ran-GDP and the importin is released
Nuclear Localization Signals use the adapters like importins to facilitate movement into the nucleus
Mitochondria also use signal localization sequences
instead of importins , they use TOM / TIM pore complexes
the localization sequence gets cleaved once its inside
depends on heat shock proteins as chaperones
Peroxisomes also use short signal sequences to get proteins from ER ➡️ peroxisome
Signal sequences are also used to direct partially started ribosomal complexes to the ER wall itself
here it can stabilize , and continue translation , dumping the contents into the ER lumen
N-terminal domain enters into the ER lumen first
Glycosylation is very common for rough ER synthesized proteins
the tags mark the state of protein folding
Improperly folded proteins get ubiquitinated , and then exported into the cytosol , to the be broken down in peroxisomes
Accumulation of Improperly folded proteins ,
triggers gene transcription of chaperones to assist in folding
Vesicle Coatings :
Clathrin = normal endocytosis
COPI = golgi ➡️ ER
retrograde , backward direction
COPII = ER ➡️ golgi
anterograde , forward direction , for export
Dynamin = GTPase that pinches off vesicles
uncoats clathrin
Rab = used to guide endosomes cargo to its target location in the cell
SNARES mediate membrane fusion
they need to be separated after
used in exocytosis
Endocytosis :
clathrin = normal
Process : Cargo binds to receptors, adaptors recruit clathrin, and vesicle formation begins. Dynamin constricts the vesicle neck, enabling release.
Uncoating : ATP-driven disassembly of the clathrin coat for vesicle integration with endosomes.
pinocytosis = water
LDL fats = also use clathrin
Exocytosis :
Continuous : Supplies membrane and secretory proteins without regulation
Stimulus-Dependent : Triggered by external signals, often involving calcium influx binding to synaptotagmin, facilitating vesicle fusion with the plasma membrane.
SNARE Complex :
Components: Synaptobrevin ( V-SNARE ) , Syntaxin , and SNAP-25 ( T-SNAREs )
Parkinson's Disease :
can't release or reuptake dopamine
Parkin 1 = accumulates and prevents synaptic vesicle release
𝛼-synuclein = blocks SNARE complexes , aka vesicle fusion
Golgi = sorting device :
lysosomes
secretory vesicles ( signal mediated )
secretory vesicles ( constitutive secretion )
Pathways to a Lysosome :
enocytose , phagocytose , micropinocytose , or autophagy of mitochondrion
Mannose-6-Phosphate Receptors = on golgi , used in early endosome formation
Neurons :
golgi ➡️ synaptic vesicle ( endosome ) ➡️ fuses , offloads contents into synaptic cleft ➡️ contents "re-uptook" / endocytosed back into pre-synaptic terminal
Post Translational Modifications
Hydroxylation
Definition : Adds hydroxyl ( -OH ) groups , primarily to proline or lysine residues.
What Occurs :
Example: Hypoxia-Inducible Factor ( HIF-α )
Under normal oxygen levels ( normoxia ) :
Prolyl hydroxylase enzymes hydroxylate HIF-α.
Hydroxylation enables binding to von Hippel-Lindau ( VHL ) protein
HIF-α is ubiquitinated and degraded by the proteasome.
Under low oxygen ( hypoxia ) :
Hydroxylation does not occur.
HIF-α stabilizes , binds with HIF-β , and activates gene transcription for hypoxia response.
Effect on Protein Function :
Regulates protein stability.
Hydroxylation under normoxia ensures degradation of HIF-α , preventing hypoxia response.
Lack of hydroxylation under hypoxia allows HIF-α to activate survival genes.
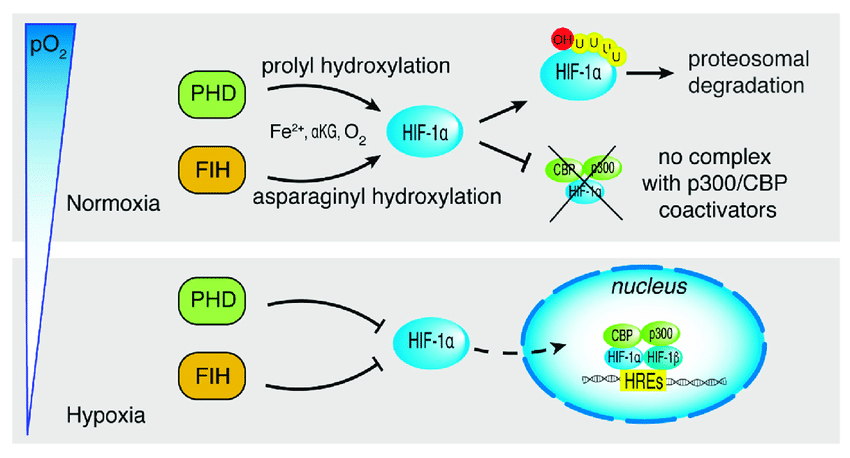
Normoxia = HIF gets hydroxylated ➡️ becomes ubiquinated ➡️ proteosomal degradation
Hypoxia = HIF doesn't get hydroxylated ➡️ becomes nuclear transcription factor ➡️ hypoxia response elements ( HREs ) are transcribed
Ubiquitination
Definition : Attaches ubiquitin proteins to lysine residues on target proteins.
What Occurs :
Marks proteins for degradation by the 26S proteasome.
Example : HIF-α ubiquitination under normoxia.
Ubiquitination is triggered by hydroxylation and VHL protein binding.
Effect on Protein Function :
Regulates protein turnover by targeting them for degradation.
Maintains cellular homeostasis by removing damaged or unnecessary proteins.
Lipidation
Definition : Adds lipid groups ( e.g., palmitate, myristate ) to cysteine residues.
What Occurs :
Anchors proteins to membranes, altering localization and function.
Examples :
AMPA Receptor ( GluA1-4 ) :
Lipidation at TM2 ➡️ Golgi retention.
Lipidation at TM4 ➡️ Membrane insertion; enables PKC phosphorylation.
Potassium Channel ( Kcnma1 ) :
Lipidation at S0-S1 loop ➡️ ER and Golgi exit.
Lipidation at STREX site ➡️ Regulates kinase activity ( e.g., PKA inhibition )
Effect on Protein Function :
Alters membrane localization , which impacts protein interactions and signaling.
Specific lipidation sites determine whether proteins are retained in organelles or inserted into membranes , influencing downstream activity.
Phosphorylation
Definition : Adds phosphate groups to serine, threonine, or tyrosine residues.
What Occurs :
Carried out by kinases and reversed by phosphatases.
Example: AMP-Activated Protein Kinase ( AMPK ) :
Activated under stress ( e.g., low ATP/high AMP )
Phosphorylates glucose transporters to increase glucose uptake
Energy regulation :
AMP rises during low ATP ➡️ Activates AMPK.
Effect on Protein Function :
Alters protein activity , stability , and interactions.
Phosphorylation of glucose transporters enhances glucose uptake , helping maintain energy balance under stress.
Low ATP / High AMP ➡️ Activates AMPK ➡️ phosphorylates serine and threonine residues on glucose transporters ➡️ increases glucose import
Glycosylation
Definition : Addition of sugar groups ( glycans ) to proteins , primarily on extracellular domains.
What Occurs :
Facilitates cell-cell adhesion and protein-protein interactions.
Example - Sialic acid :
Negative charges repel but allow connections with positively charged glycans.
Example in podocytes: Glycosylation maintains podocyte-endothelial cell linkages.
Effect on Protein Function :
Enhances structural integrity and adhesion in cellular networks.
Modulates interactions with other proteins or cells , crucial for processes like immune responses and tissue stability.
Disulfide Bond Formation
Definition : Covalent linkage between two cysteine residues , forming disulfide bonds ( S-S )
What Occurs :
Stabilizes protein conformation by maintaining structural integrity.
Influenced by cellular redox states , which are regulated by reactive oxygen species ( ROS ) and antioxidants like glutathione.
Complex I and III of the mitochondrial electron transport chain can leak ROS , altering the redox environment and influencing disulfide bond formation.
This can lead to oxidative stress and misfolding if the redox balance is disrupted.
Effect on Protein Function :
Ensures correct protein folding and structural stability.
Misregulation can lead to misfolded proteins, resulting in dysfunction and disease.
Role of Glutathione : Glutathione maintains the proper redox environment for forming and breaking disulfide bonds.
It ensures that disulfide bonds are formed correctly by reducing incorrect linkages and preventing oxidative damage to cysteine residues.
SUMOylation
Definition : Addition of SUMO ( Small Ubiquitin-like Modifier ) proteins to target proteins.
What Occurs :
Modulates nuclear transport , transcriptional regulation , and DNA repair.
SUMO is attached to lysine residues , similar to ubiquitin.
Effect on Protein Function :
Alters protein localization and interactions.
Enhances or suppresses transcriptional activity , depending on the target.
Methylation
Definition: Adds methyl groups , primarily to lysine or arginine residues on histones.
What Occurs :
Regulates chromatin structure and gene expression.
Example: Histone methylation :
Specific methylation patterns can activate or repress gene transcription.
Effect on Protein Function :
Modulates gene expression by altering chromatin accessibility.
Plays a role in epigenetic regulation , influencing long-term cellular behavior.
Kinases
Pyruvate Kinase Deficiency in Red Blood Cells
RBCs don't have mitochondria
They rely on anaerobic glycolysis for energy
no TCA or ETC
Pyruvate Kinase Deficiency ➡️
Slow Glycolysis ➡️
Loss of ATP ➡️
Can't Power/Run Sodium-Potasium-ATPase ( pump ) ➡️
Sodium ions accumulate in cytosol ➡️
Osmotic pressure increases ➡️
cell looses shape here , becomes "spiculated"
Cell lyses ➡️
Anemia
Build up of 2,3-BPG
Major Protein Kinase Families
Serine / Threonine
example = AMPK
Tyrosine
example = insulin receptor
Serine / Threonine and Tyrosine ( dual-specificity )
can phosphorylate both serine/threonine and tyrosine residues
example = MEK1 / MEK2 ( Mitogen-Activated Protein Kinase Kinase )
Histidine
example = CheA ( involved in bacterial chemotaxis )
Know 3 Receptors that Act via Tyrosine Kinase Signaling
RTKs are generally membrane-anchored to facilitate ligand binding and signal transduction
Insulin
stimulates carbohydrate utilization and protein synthesis
rate = seconds to minutes
direct ligand to receptor binding
immediate release of pre-stored granules
but also has longer transcriptional effects
Platelet Derived Growth Factor
stimulates survival , growth , proliferation , and migration of various cell types
rate = minutes to hours
needs time for secondary signaling cascades to occur
Nerve Growth Factor
stimulates survival and growth of some neurons
rate = hours to days
transcriptional changes take a while