A Role for TASK-1 (KCNK3) Channels in the Chemosensory Control of Breathing
https://doi.org/10.1523/JNEUROSCI.1810-08.2008
Abstract
Acid-sensitive K+ channels of the tandem P-domain K+-channel family (TASK-1 and TASK-3) have been implicated in peripheral and central respiratory chemosensitivity; however, because of the lack of decisive pharmacological agents, the final proof of the role of the TASK channel in the chemosensory control of breathing has been missing. In the mouse, TASK-1 and TASK-3 channels are dispensable for central respiratory chemosensitivity (Mulkey et al., 2007). Here, we have used knock-out animals to determine whether TASK-1 and TASK-3 channels play a role in the carotid body function and chemosensory control of breathing exerted by the carotid body chemoreceptors. Ventilatory responses to hypoxia (10% O2 in inspired air) and moderate normoxic hypercapnia (3–6% CO2 in inspired air) were significantly reduced in TASK-1 knock-out mice. In contrast, TASK-3-deficient mice showed responses to both stimuli that were similar to those developed by their wild-type counterparts. TASK-1 channel deficiency resulted in a marked reduction of the hypoxia (by 49%)- and CO2 (by 68%)-evoked increases in the carotid sinus nerve chemoafferent discharge recorded in the in vitro superfused carotid body/carotid sinus nerve preparations. Deficiency in both TASK-1 and TASK-3 channels increased baseline chemoafferent activity but did not cause a further reduction of the carotid body chemosensory responses. These observations provide direct evidence that TASK-1 channels contribute significantly to the increases in the carotid body chemoafferent discharge in response to a decrease in arterial PO2 or an increase in PCO2/[H+]. TASK-1 channels therefore play a key role in the control of ventilation by peripheral chemoreceptors.
Introduction
Describes the basic rhythm of breathing and how it is generated and modified.
Mentions the role of the carotid bodies and central chemoreceptors in the brainstem in regulating breathing.
Presents the TASK family of K+ channels and their potential role in chemosensory control of breathing.
Highlights the lack of specific inhibitors for TASK channels and the need for further research.
The basic rhythm of breathing is generated within the pre-Bötzinger complex of the medulla oblongata and then is subsequently shaped, modified, and transmitted to the bulbospinal premotor neurons, which relay the resulting respiratory pattern to the spinal motor neurons controlling respiratory muscles. The brainstem respiratory network continuously receives chemoafferent information about the arterial levels of PO2, PCO2, and pH and adjusts respiratory motor output, ensuring appropriate ventilation of the lungs in various environmental and physiological conditions.
In mammals, respiratory chemoafferent inputs originate primarily from the receptors in the carotid bodies and from the central chemoreceptors in the brainstem. Type I (glomus) cells of the carotid body are the principal peripheral chemosensitive elements which rapidly detect alterations in arterial levels of PO2, PCO2, and pH, and transmit this information to the chemoafferent fibers of the carotid sinus nerve which, in turn, relays to the brainstem respiratory centers to evoke adaptive changes in ventilation. PCO2 and pH are also monitored by the chemoreceptors localized within the brainstem, primarily at, or in close proximity to, the ventral surface of the medulla oblongata, and possibly in several other distinct brainstem regions.
Acid-sensitive K+ channels of the tandem P-domain K+-channel family (TASKs) have been proposed to contribute significantly to various aspects of the chemosensory control of breathing. TASK currents are inhibited by external acidic pH, activated by alkali, and reduced by low O2. TASK-1 (KCNK3) homodimers go from open to shut within 0.5 pH unit around pH 7.4 , whereas TASK-3 (KCNK9) channels shut under more acid conditions. TASK-1 and TASK-3 can form homodimeric or heterodimeric channels.
Type I cells of the carotid body express a prominent background K+ conductance that displays some TASK-like properties [including weak outward rectification, inhibition by low pH, and activation by halothane] and is inhibited by hypoxia. In addition, the TASK genes are expressed in all central CO2-chemosensitive regions, including areas of the ventrolateral medulla, raphe nuclei, and locus ceruleus. However, in mice, TASK-1 and TASK-3 channels appear to be dispensable for central respiratory chemosensitivity.
Primarily because of the lack of specific inhibitors for these channels, it is still unknown whether and how TASK-1 and TASK-3 channels contribute to the carotid body function and the control of ventilation exerted by these peripheral chemoreceptors. Here, using knock-out mice, we directly confirm that TASK-1 channel does not indeed contribute to the central respiratory chemosensitivity but appears to be essential for the carotid body CO2/pH sensitivity and also contributes significantly to the mechanism of oxygen sensing in the carotid body.
Results
Presents the impaired ventilatory response to hypoxia in TASK-1 knockout mice.
Shows the impaired ventilatory response to CO2 in TASK-1 knockout mice.
Describes the impaired carotid body function in TASK-1 knockout mice.
Discusses the impaired carotid body function in TASK-1/TASK-3 double knockout mice.
Impaired ventilatory response to hypoxia in TASK-1−/− mice
The resting ventilation in normoxia/normocapnia was similar in TASK-1−/− mice (1.58 ± 0.09 ml min−1 g−1, n = 8) and their wild-type counterparts (1.79 ± 0.09 ml min−1 g−1, n = 7; p = 0.12). When challenged with hypoxia (10% O2 in the inspired air), wild-type mice showed an increased rate (fR) and depth (VT) of breathing and, therefore, an increased minute ventilation (VE) (Fig. 1A). This hypoxia-induced increase in ventilation was markedly reduced in TASK-1−/− animals (Fig. 1A). Thus, in the air containing 10% O2, increases in tidal volume were smaller in TASK-1−/− mice, resulting in VE of 2.04 ± 0.14 ml min−1 g−1 (n = 8), whereas in the wild-type animals, VE was 2.94 ± 0.19 ml min−1 g−1 (n = 7) (p < 0.05). The hypoxia-induced decrease in core body temperature in TASK-1−/− mice was not significantly different from that in TASK+/+ animals (−0.7 ± 0.1°C, n = 3 vs −0.8 ± 0.3°C, n = 4; p = 0.7). The resting ventilation and the ventilatory response of TASK-3−/− and TASK+/+ mice to hypoxic stimulation were similar (Fig. 2A).
Impaired ventilatory response to CO2 in TASK-1−/− mice
Mice lacking TASK-1 or TASK-3 channels were exposed to graded levels of normoxic hypercapnia, which induced profound increases in ventilation in all groups of animals (Figs. 1B, 2B). TASK-1−/− mice displayed significantly smaller ventilatory responses to moderate levels of inspired CO2 (Fig. 1B). In an atmosphere of 3% and 6% CO2, VE in TASK-1−/− mice increased to 2.56 ± 0.20 ml min−1 g−1 and 4.66 ± 0.27 ml min−1 g−1 (n = 7), whereas in the wild-type animals in the same conditions, VE was elevated to 3.94 ± 0.32 ml min−1 g−1 (p < 0.05) and 5.97 ± 0.34 ml min−1 g−1 (p < 0.05) (n = 7), respectively (Fig. 1B). Separate groups of TASK-1−/− (n = 3) and wild-type (n = 4) mice were also challenged with a high level of hypercapnia (10% CO2 in the inspired air). In these conditions, the difference in minute ventilation between TASK-1−/− and TASK+/+ mice was no longer observed (6.9 ± 0.91 ml min−1 g−1 vs 6.17 ± 0.83 ml min−1 g−1, respectively; p = 0.57), although the increase in the rate of breathing was significantly smaller in TASK-1−/− animals (345 ± 8 vs 394 ± 5 breaths min−1, p < 0.05). Respiratory responses to increases in PCO2/[H+] of the in situ brainstem–spinal cord preparations of TASK-1−/− and TASK+/+ mice with denervated peripheral chemoreceptors were similar (Fig. 1C). No significant differences in any measures of ventilation were detected between TASK-3−/− and the wild-type control mice when concentration of CO2 in the inspired air increased to 3% or 6% (Fig. 2B).
Impaired carotid body function in TASK-1−/− mice
The results of the whole-body plethysmography experiments presented above strongly suggested that carotid body function is compromised in TASK-1-deficient mice. To test this hypothesis, we recorded activity of the carotid sinus nerve in the in vitro superfused carotid body/carotid sinus nerve preparations taken from wild-type and TASK-1−/− mice. Hypoxia- and CO2-evoked increases in the carotid sinus nerve chemoafferent discharge were recorded, and the effect of TASK-1 deficiency on these responses was determined. In preparations taken from TASK+/+ animals, hypoxic stimulation evoked a dramatic increase in the carotid sinus nerve discharge: the whole-nerve chemoafferent activity increased from 50 ± 9 spikes s−1 to a peak of 414 ± 37 spikes s−1 (n = 8) (Fig. 3A,C). In preparations taken from TASK-1−/− mice, hypoxia-induced increases in the carotid sinus nerve peaked at 221 ± 31 spikes s−1 (n = 10) (Fig. 3A,C), representing some 49% reduction in the whole-nerve response to a decrease in PO2 (p < 0.05). Accordingly, the average hypoxia-induced peak firing rate of single chemoafferent fibers was significantly reduced in the carotid body/sinus nerve preparations from TASK-1−/− mice (5.4 ± 0.8, n = 27 vs 14.6 ± 1.5, n = 31 in TASK+/+ mice; p < 0.05) (Fig. 4B).
Genetic ablation of TASK-1 not only reduced the peak of the response to hypoxia, but also made it more transient. Consequently, the area under the curve of the frequency versus time plot was calculated to compare the responses (Figs. 3E, 4B).
In preparations taken from wild-type mice, carotid sinus nerve chemoafferent discharge also significantly increased (peak, 102 ± 15 spikes s−1, n = 8) in response to respiratory acidosis (increase in PCO2/[H+]), albeit to a lesser degree compared with that during hypoxia (Fig. 3B,D). Hypercapnia-evoked increase in the carotid sinus nerve discharge was considerably reduced in preparations taken from TASK-1−/− mice (peak, 49 ± 10 spikes s−1, n = 10; p < 0.05). This represents a 68% reduction of the response in the knock-out animals (Fig. 3B,D). Moreover, in TASK-1−/− mice, the increase of discharge in response to CO2 failed to reach significance. Similarly, the average CO2-induced peak increase in the activity of single-sinus nerve chemoafferent fibers was significantly lower in preparations taken from the TASK-1−/− mice (1.2 ± 0.2 spikes s−1, n = 27 vs 3.7 ± 0.7 spikes s−1, n = 31 in TASK+/+; p < 0.05) (Fig. 4C).
Impaired carotid body function in TASK-1−/−:TASK-3−/− double knock-out mice
The results presented above demonstrated a blunted but not abolished carotid body response to hypoxia in mice deficient in TASK-1 channels. To test whether the remaining response to hypoxic stimulation might be attributable to TASK-3 channels, experiments were conducted using the carotid body/carotid sinus nerve preparations taken from TASK-1/TASK-3 double knock-out mice. It was found that in these animals the average basal firing rate of single chemoafferent fibers (4.1 ± 0.7 spikes s−1, n = 31) (Fig. 4B,C) and, accordingly, the activity of the whole carotid sinus nerve (Fig. 3A–D) were significantly (p < 0.05) higher compared with both TASK+/+ and TASK-1−/− mice (basal firing of single units, 0.9 ± 0.2 and 0.6 ± 0.1 spikes s−1, respectively). However, the absolute increases in discharge frequency in response to hypoxia or CO2 were significantly reduced compared with the responses in TASK+/+ mice and very similar to those observed in preparations taken from TASK-1−/− mice, both at the whole-nerve and single chemoafferent fiber levels (Figs. 3C–F, 4B,C).
Discussion
Discusses the role of TASK-1 channels in central CO2 chemoreception and their dispensability for this process.
Presents the role of TASK-1 channels in chemoreception in the carotid body, highlighting their importance in mediating responses to hypoxia and hypercapnia.
Explains the compensatory mechanisms in TASK-1 knockout mice and the potential role of TASK-3 channels.
Our observations provide the first direct evidence that TASK-1 channels contribute significantly to the increases in the carotid body chemoafferent discharge in response to a decrease in arterial PO2 or an increase in PCO2/[H+] and, therefore, play an important role in the control of ventilation by the peripheral chemoreceptors. Another member of the tandem P-domain K+-channel family, TASK-3, is not essential for mediating changes in breathing in response to chemosensory stimulation.
TASK-1 channels and central CO2 chemoreception
TASK channels are expressed in various central CO2-chemosensitive regions including ventrolateral medulla (Washburn et al., 2003), medullary dorsal and caudal raphe (Washburn et al., 2002), and pontine locus ceruleus (Bayliss et al., 2001), and considering their unique sensitivity to small changes in external pH, they have been proposed to participate in central CO2/pH chemoreception (Mulkey et al., 2004; Putnam et al., 2004). However, Mulkey et al. (2007) demonstrated recently that mouse TASK-1 and TASK-3 channels are nonessential for central respiratory chemosensitivity. Indeed, although increases in the carotid chemoafferent discharge evoked by rising levels of PCO2/[H+] were reduced in our TASK-1−/− mice, these animals still developed vigorous ventilatory responses to CO2. This is not surprising considering that the brainstem chemosensitive sites account for up to 80% of the overall CO2-evoked ventilatory response when peripheral chemoafferent input is interrupted experimentally (Heeringa et al., 1979).
Our data agree with the evidence of Mulkey et al. (2007), who demonstrated, using independently generated mice, that TASK-1, TASK-3, and TASK-1/TASK-3 double knock-out animals develop normal ventilatory responses to hyperoxic hypercapnia. Considering that peripheral chemoreceptors can still discharge even at high levels of PO2, we conducted experiments using in situ working brainstem–spinal cord preparations from TASK-1−/− and TASK+/+ mice in which peripheral chemoreceptors were surgically denervated. No difference was observed in CO2-induced respiratory responses between preparations taken from TASK-1−/− and TASK+/+ mice, confirming the conclusions of Mulkey et al. (2007) that TASK-1 channel is indeed dispensable for central respiratory chemosensitivity.
In our study, mice were also challenged with normoxic hypercapnia, and the differences in the ventilatory responses between TASK-1−/− and wild-type mice were only observed at moderate levels of hypercapnia (3 or 6% CO2 in inspired air), reflecting impairment of the carotid body function. It was also found that TASK-1−/− and wild-type mice mounted similar ventilatory responses to severe levels of hypercapnia (10% CO2 in the inspired air), confirming that intact central CO2 chemoreceptors can fully compensate for the loss of the peripheral chemoafferent input under these conditions. These data also indicate that TASK channel deficiency does not impair the function of the medullary respiratory rhythmogenic neurons as well as respiratory premotor and motor neurons, all of which were found previously to express TASK channels (Washburn et al., 2003) implicated in the control of motoneuronal excitability (Bayliss et al., 2003).
TASK-1 channels and chemoreception in the carotid body
Hypoxia-induced inhibition of K+ channels in type I cells, first demonstrated almost two decades ago (López-Barneo et al., 1988), is believed to constitute the key event in the carotid body chemosensory transduction mechanism (for recent reviews, see Kemp, 2006; Buckler, 2007; Kumar, 2007). Inhibition of K+ channels leads to depolarization (Buckler, 1997), Ca2+ entry through voltage-gated Ca2+ channels (Buckler and Vaughan-Jones, 1994a,b), and subsequent activation of the carotid sinus nerve chemoafferent fibers via release of ATP and acetylcholine (Zhang et al., 2000; Rong et al., 2003). The exact mechanisms leading to inhibition of K+ channels are unresolved (Kemp, 2006; Kumar, 2007), but rat type I cells express background K+ channels that display some TASK-like properties, showing greatest similarity to TASK-1 and TASK-3 (for review, see Buckler, 2007).
The proof of the functional role played by these TASK channels in the carotid body chemoreception has been missing. Both TASK-1 and TASK-3 immunoreactivities have been demonstrated in the rat carotid body (Yamamoto et al., 2002; Yamamoto and Taniguchi, 2006); however, some of these antibodies still bind to knock-out brain tissue (Aller et al., 2005; Brickley et al., 2007). Here, using knock-out mice, we demonstrated that TASK-1 channel deficiency abolished the carotid sinus nerve responses to hypercapnia. However, loss of a “background” potassium conductance would be expected to cause an increase in baseline activity, which we did not observe. Similar to this, Aller et al. (2005) reported that the resting membrane potential of cerebellar granule cells was not reduced in TASK-1−/− mice. They demonstrated that this was the result of a replacement of TASK-1 channels, or TASK-1/TASK-3 heterodimeric channels, by TASK-3 channels. This could also explain our present observations. TASK-3 channels would replace the TASK-1 homodimers or TASK-1/TASK-3 heterodimers in the carotid body type I cells, thus preventing depolarizing shift of the membrane potential. Furthermore, the acid-shifted pH sensitivity of TASK-3 homodimeric channels (for review, see Duprat et al., 2007) would explain why no response was observed during hypercapnia, which was accompanied by a moderate decrease in pH from 7.4 to 7.2.
These conclusions were further supported by the results obtained using TASK-1/TASK-3 double knock-out mice. These animals displayed the same reduced carotid chemoafferent response to hypoxia and hypercapnia as the TASK-1−/− mice, evident from both a smaller increase in frequency for the peak response and a smaller area under the curve for the frequency versus time plot (Figs. 3, 4). However, these blunted responses were developing from an increased level of baseline activity compared with both wild-type and TASK-1−/− preparations. This would be expected because in these animals, both TASK-1 and TASK-3 channels are lost and the depolarizing shift of the membrane potential cannot be prevented, which is reflected in the increased baseline firing rate of the carotid sinus nerve. The pH sensitivity is similarly lost, specifically because TASK-1 is not present.
Similarly to these results, Mulkey et al. (2007) noted a significant hyperventilation during hypoxia (10% O2) in TASK-1/TASK-3 double knock-out mice. In their case, ventilation during hypoxia in TASK-1−/−:TASK-3−/− mice was not significantly different from that in the controls, most likely reflecting this increased level of the peripheral chemoafferent activity in the double knock-out mice.
In summary, TASK-1 channels (but not TASK-3 channels) indeed play an important role in the mechanisms leading to an increase in the carotid sinus nerve chemoafferent discharge during hypoxia and hypercapnia. The response to an increase in PCO2/[H+] was abolished in both TASK-1−/− and TASK-1/TASK-3 double knock-out carotid body preparations. Also, the hypoxia-induced responses were significantly attenuated, although not abolished, by TASK-1 deficiency. This suggests the existence of either a parallel mechanism of hypoxic chemotransduction, which works in synergy with the one involving TASK-1 channels, or a mechanism that can partially compensate for the loss of the latter in the knock-out animals, or both. Likewise, whereas TASK-3 channels were not essential for the expression of the hypoxic ventilatory response, we cannot exclude that they still play a role in normal conditions, but TASK-1 (or other K+ channels) can fully compensate for their loss in the knock-out mice.
Because hypoxia does not stimulate respiration centrally, it is unsurprising that the reduced responsiveness of the carotid bodies to hypoxia in TASK-1−/− mice resulted in a roughly similar reduction of the overall ventilatory response. In contrast, the attenuation of the CO2-evoked ventilatory response was smaller than the attenuation of the CO2-evoked increases in the carotid sinus nerve chemoafferent discharge in the TASK-1−/− animals. These data indicate that in the carotid body, TASK-1 channels play an even more significant role in sensing alterations in PCO2/[H+]. Indeed, in our experimental conditions, an increase in PCO2/[H+] failed to evoke significant increases in the carotid sinus nerve discharge in preparations taken from either TASK-1 or TASK-1/TASK-3 double knock-out mice. TASK-1 channels are uniquely sensitive to changes in external pH within the physiological range 7.3–7.4 (Duprat et al., 1997). Because changes in external pH that follow changes in PCO2 represent the adequate and main stimuli for the carotid body chemoreceptors (Gray, 1968), TASK-1 channels are ideally suited to act as the primary PCO2/[H+] chemosensors of type I cells. Other acid-sensing ion channels (Tan et al., 2007) may work in synergy with TASK-1; however, their relative contribution to PCO2/[H+] sensitivity in the carotid body appears to be insignificant.
Perspectives
Summarizes the key findings of the study, emphasizing the role of TASK-1 channels in carotid body chemoreception.
Highlights the need for further research to identify the oxygen sensor and biochemical pathways involved in TASK-1 channel inhibition during hypoxia.
Mentions the need to investigate the parallel or compensatory mechanisms of oxygen sensitivity that do not involve TASK channels.
The data obtained in the present study indicate that in the carotid body, TASK-1 channels account for at least half of the increases in the chemoafferent discharge in response to hypoxia, mediate CO2/pH sensitivity, and, therefore, play a key role in the control of ventilation exerted by the peripheral respiratory chemoreceptors. This function alone would be expected to maintain a high selection pressure for the TASK-1 gene. Although decreases in extracellular pH, which follow increases in PCO2, could directly inhibit TASK-1 channels expressed by chemosensitive type I cells of the carotid body, the actual oxygen sensor as well as biochemical pathways leading from the oxygen sensor to inhibition of these channels during hypoxia have not been definitely identified. The parallel (or compensatory) mechanism(s) of oxygen sensitivity not involving TASK channels and responsible for the residual chemoafferent responses observed in the TASK-1 knock-out mice also remain to be determined.
Figures
Figure 1 - TASK-1 channel is essential for the development of normal ventilatory responses to hypoxia and CO2 in mice.
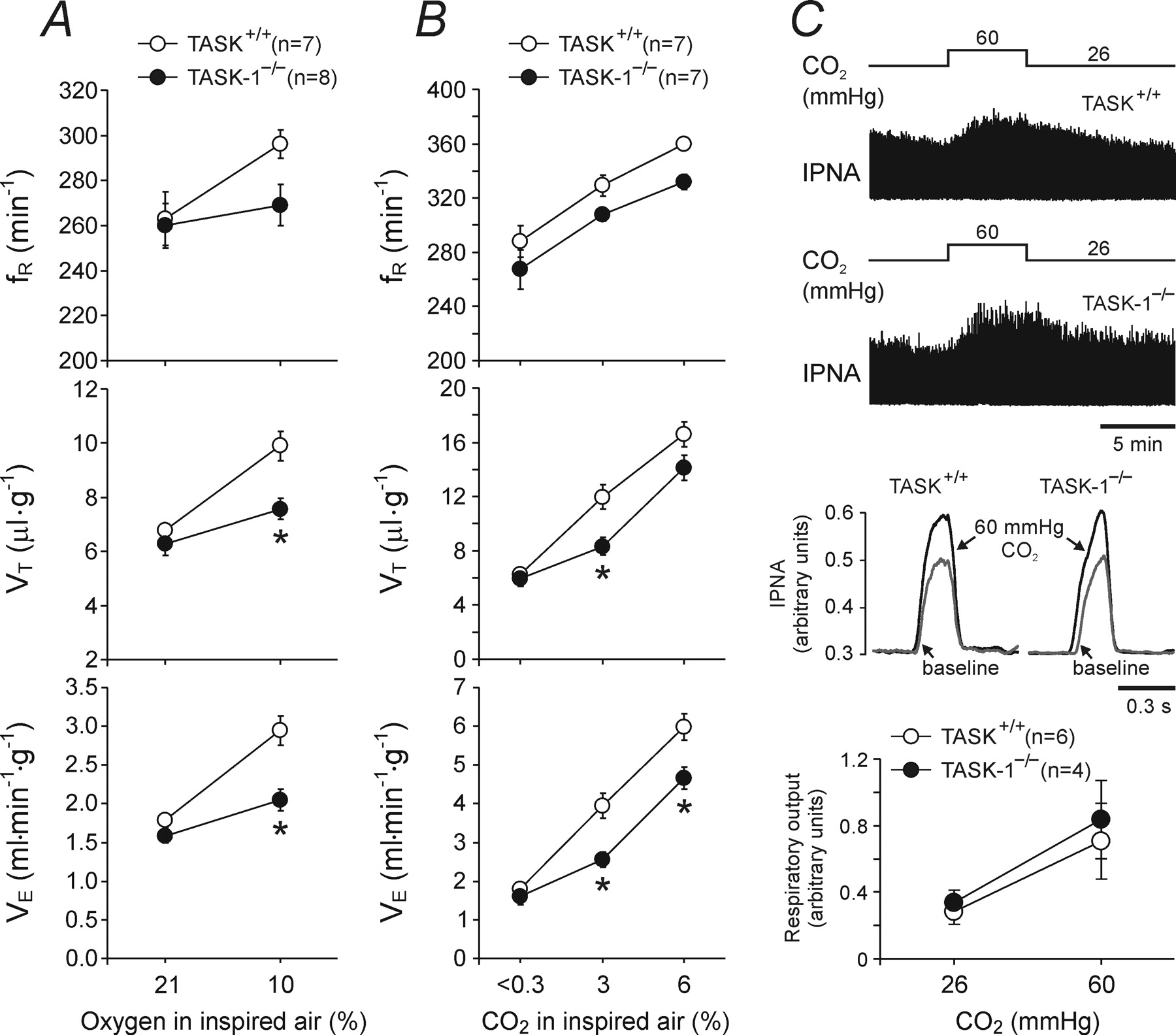
A, Ventilatory responses to hypoxia (10% O2 in the inspired air) in conscious TASK-1-deficient mice (TASK-1−/−) and their wild-type counterparts (TASK+/+).
B, Ventilatory responses to varying levels of normoxic hypercapnia in TASK-1−/− and TASK+/+ mice.
C, Phrenic nerve responses to an increase in PCO2/[H+] in in situ brainstem–spinal cord preparations with denervated peripheral chemoreceptors from TASK-1−/− and TASK+/+ mice.
Top, Raw data showing time-condensed records of integrated phrenic nerve activity (IPNA) in basal conditions (PCO2 26 mmHg, pH 7.52) and during respiratory acidosis (PCO2 60 mmHg, pH 7.24).
Middle, Results are presented as waveform averages of the integrated phrenic nerve activity for 60 respiratory cycles at basal conditions and during respiratory acidosis.
Bottom, Summary data of changes in minute respiratory output (phrenic amplitude × respiratory rate) in response to increases in PCO2/[H+].
Data are presented as means ± SE. Numbers in parentheses indicate sample sizes. *p < 0.05, significantly different from TASK+/+ response.
"When challenged with hypoxia (10% O₂ in the inspired air), wild-type mice showed an increased rate (fR) and depth (VT) of breathing and, therefore, an increased minute ventilation (VE) (Fig. 1A)."
"This hypoxia-induced increase in ventilation was markedly reduced in TASK-1−/− animals (Fig. 1A)."
"Mice lacking TASK-1 or TASK-3 channels were exposed to graded levels of normoxic hypercapnia, which induced profound increases in ventilation in all groups of animals (Figs. 1B, 2B)."
"TASK-1−/− mice displayed significantly smaller ventilatory responses to moderate levels of inspired CO₂ (Fig. 1B)."
"Respiratory responses to increases in PCO₂/[H⁺] of the in situ brainstem–spinal cord preparations of TASK-1−/− and TASK+/+ mice with denervated peripheral chemoreceptors were similar (Fig. 1C)."
Figure 2 - TASK-3 channel is not required for the development of the ventilatory responses to hypoxia and CO2 in mice.
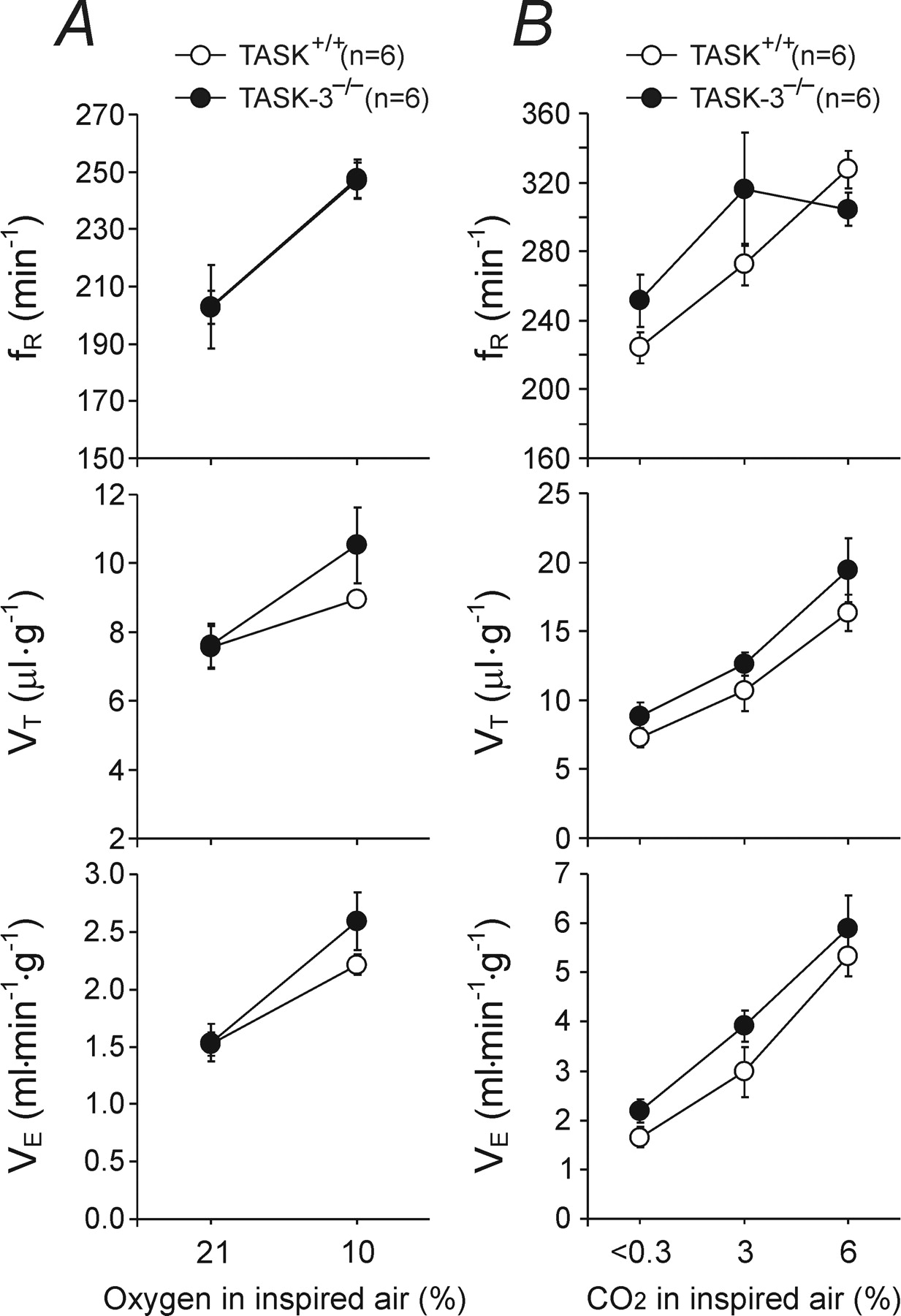
A, Ventilatory responses to hypoxia (10% O2 in the inspired air) in conscious TASK-3-deficient mice (TASK-3−/−) and their wild-type counterparts (TASK+/+).
B, Ventilatory responses to varying levels of normoxic hypercapnia in TASK-3−/− and TASK+/+ mice.
Data are presented as means ± SE. Numbers in parentheses indicate sample sizes.
"The resting ventilation and the ventilatory response of TASK-3−/− and TASK+/+ mice to hypoxic stimulation were similar (Fig. 2A)."
"No significant differences in any measures of ventilation were detected between TASK-3−/− and the wild-type control mice when concentration of CO₂ in the inspired air increased to 3% or 6% (Fig. 2B)."
"Mice lacking TASK-1 or TASK-3 channels were exposed to graded levels of normoxic hypercapnia, which induced profound increases in ventilation in all groups of animals (Figs. 1B, 2B)."
Figure 3 - Impaired carotid body function in TASK-deficient mice
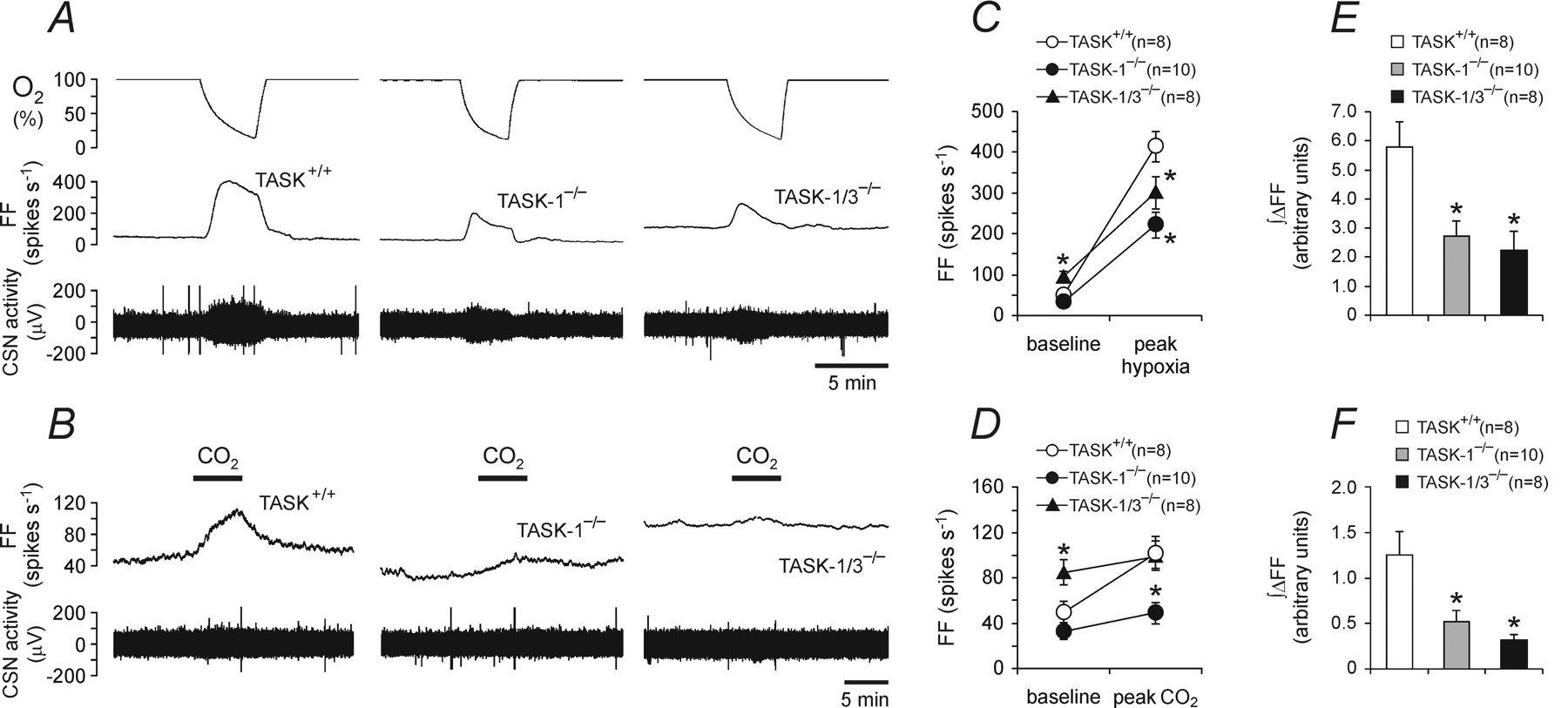
A, Representative raw data showing hypoxia-evoked increases in the carotid sinus nerve (CSN) chemoafferent discharge recorded in the in vitro superfused carotid body/carotid sinus nerve preparations taken from the TASK-1-deficient mice (TASK-1−/−), TASK-1- and TASK-3-deficient mice (TASK-1/3−/−), and their wild-type counterparts (TASK+/+).
B, Raw data illustrating CO2-evoked increases in the carotid sinus nerve chemoafferent discharge recorded in the in vitro superfused carotid body/carotid sinus nerve preparations taken from TASK-1−/−, TASK-1/3−/−, and TASK+/+ mice. C, D, Summary data of the mean peak hypoxia (C)- and CO2 (D)-induced increases in discharge frequency of the carotid sinus nerve in preparations taken from TASK-1−/−, TASK-1/3−/−, and TASK+/+ mice.
E, F, Summary data of the mean integral of hypoxia (E)- and CO2 (F)-induced increases in discharge frequency (∫ΔFF) of the carotid sinus nerve in preparations taken from TASK-1−/−, TASK-1/3−/−, and TASK+/+ mice.
Data are presented as means ± SE. Numbers in parentheses indicate sample sizes.
FF, Discharge frequency. *p < 0.05, significantly different from TASK+/+ under the same conditions.
"Hypoxia- and CO₂-evoked increases in the carotid sinus nerve chemoafferent discharge were recorded, and the effect of TASK-1 deficiency on these responses was determined (Fig. 3A, C)."
"In preparations taken from TASK+/+ animals, hypoxic stimulation evoked a dramatic increase in the carotid sinus nerve discharge: the whole-nerve chemoafferent activity increased from 50 ± 9 spikes s⁻¹ to a peak of 414 ± 37 spikes s⁻¹ (n = 8) (Fig. 3A, C)."
"In preparations taken from TASK-1−/− mice, hypoxia-induced increases in the carotid sinus nerve peaked at 221 ± 31 spikes s⁻¹ (n = 10) (Fig. 3A, C), representing some 49% reduction in the whole-nerve response to a decrease in PO₂ (p < 0.05)."
"In preparations taken from wild-type mice, carotid sinus nerve chemoafferent discharge also significantly increased (peak, 102 ± 15 spikes s⁻¹, n = 8) in response to respiratory acidosis (increase in PCO₂/[H⁺]), albeit to a lesser degree compared with that during hypoxia (Fig. 3B, D)."
"Hypercapnia-evoked increase in the carotid sinus nerve discharge was considerably reduced in preparations taken from TASK-1−/− mice (peak, 49 ± 10 spikes s⁻¹, n = 10; p < 0.05) (Fig. 3B, D)."
"The response to an increase in PCO₂/[H⁺] was abolished in both TASK-1−/− and TASK-1/TASK-3 double knock-out carotid body preparations (Fig. 3D, F)."
Figure 4 - Single-unit analysis of the carotid sinus nerve responses to chemosensory stimulation in TASK-deficient mice
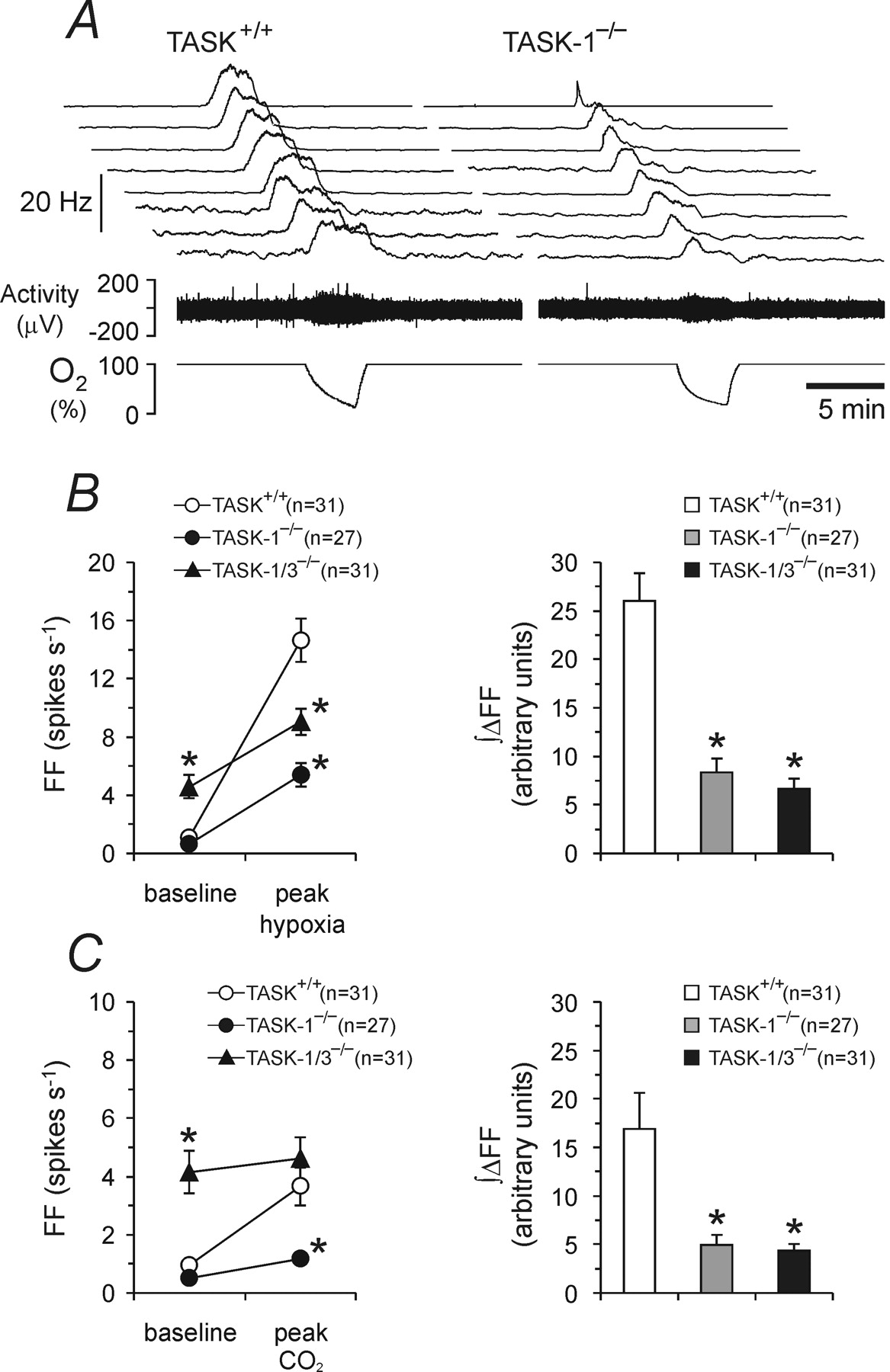
A, Representative raw data of the discharge frequency profiles of eight carotid sinus nerve single chemoafferent fibers recorded under basal conditions and during hypoxic stimulation in the in vitro superfused carotid body/carotid sinus nerve preparations taken from the TASK-1-deficient mice (TASK-1−/−; right) and their wild-type counterparts (TASK+/+; left).
B, Summary data of the mean peak hypoxia-induced increases in discharge frequency (left) and mean integral of the hypoxia-induced increase in discharge frequency (∫ΔFF; right) of the carotid sinus nerve chemoafferent fibers in preparations taken from TASK-1−/−, TASK-1/3−/−, and TASK+/+ mice.
C, Summary data of the mean peak CO2-induced increases in discharge frequency and mean integral of CO2-induced increase in discharge frequency (∫ΔFF) of the carotid sinus nerve chemoafferent fibers in preparations taken from TASK-1−/−, TASK-1/3−/−, and TASK+/+ mice.
Data are presented as means ± SE. Numbers in parentheses indicate sample sizes.
FF, Discharge frequency. *p < 0.05, significantly different from TASK+/+ under the same conditions.
"Accordingly, the average hypoxia-induced peak firing rate of single chemoafferent fibers was significantly reduced in the carotid body/sinus nerve preparations from TASK-1−/− mice (5.4 ± 0.8, n = 27 vs 14.6 ± 1.5, n = 31 in TASK+/+ mice; p < 0.05) (Fig. 4B)."
"Similarly, the average CO₂-induced peak increase in the activity of single-sinus nerve chemoafferent fibers was significantly lower in preparations taken from the TASK-1−/− mice (1.2 ± 0.2 spikes s⁻¹, n = 27 vs 3.7 ± 0.7 spikes s⁻¹, n = 31 in TASK+/+; p < 0.05) (Fig. 4C)."
"These animals displayed the same reduced carotid chemoafferent response to hypoxia and hypercapnia as the TASK-1−/− mice, evident from both a smaller increase in frequency for the peak response and a smaller area under the curve for the frequency versus time plot (Figs. 3C–F, 4B, C)."
Materials and Methods
Lists the animal models used: TASK-1 knockout mice, TASK-3 knockout mice, and double knockout mice.
Briefly describes the methods used: whole-body plethysmography, in situ brainstem-spinal cord preparation, and in vitro sinus nerve recording.
Notes the data analysis techniques employed.
Animals
The TASK-1−/− and TASK-3−/− mice used in this study have been described in detail previously (Aller et al., 2005; Brickley et al., 2007). In both lines, the first coding exon of the respective gene is destroyed and the mutant allele is not transcribed. TASK-1−/− and TASK-3−/− mice were mainly of the C57BL/6 background. We used adult (3–4 months) TASK-1−/− and TASK-3−/− mice and their respective wild-type counterparts. Double knock-out mice (TASK-1−/−:TASK-3−/−) were produced by interbreeding the individual knock-out strains. Genotypes were confirmed by PCR using genomic DNA from ear biopsies as template. Double knock-out mice appeared overtly healthy, and could be bred with each other. All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act, 1986.
Whole-body plethysmography.
Respiratory rate (fR, breaths min−1) and tidal volume (VT, μl g−1) in conscious, freely moving mice were measured by whole-body plethysmography as described in detail previously (Onodera et al., 1997; Rong et al., 2003). All experiments were performed at room temperature (22–24°C). In brief, the mouse was placed in a Plexiglas recording chamber (∼400 ml) that was flushed continuously with a mixture of 79% nitrogen and 21% oxygen (unless otherwise required by the protocol) at a rate of ∼1 L min−1. Concentrations of O2 and CO2 in the chamber were monitored on-line using a fast-response O2/CO2 monitor (Morgan Medical). The animals were allowed at least 30 min to acclimatize to the chamber environment at normoxia/normocapnia (21% O2, 79% N2, and <0.3% CO2) before measurements of baseline ventilation were taken. Hypoxia was induced by lowering the O2 concentration in the inspired air down to a level of 10% for 5 min. In separate experiments, normoxic hypercapnia was induced by titrating CO2 into the respiratory mixture up to a level of 3, 6, or 10% (lowering N2 accordingly) for 5 min at each CO2 level. The pressure signal was amplified, filtered, recorded, and analyzed off-line using Spike 2 software (Cambridge Electronic Design). The measurements of fR and VT were taken during the last 2 min before exposure to the stimulus and during the 2 min period at the end of each stimulus, when breathing stabilized. Hypoxia- or hypercapnia-induced changes in the fR, VT, and minute ventilation (VE) (fR × VT; ml min−1 kg−1) were averaged and expressed as means ± SE.
In situ brainstem–spinal cord preparation.
A separate experiment was conducted using in situ brainstem–spinal cord preparations described in detail previously (Paton, 1996). In brief, TASK+/+ and TASK-1−/− mice were given heparin (500 U, i.p.), anesthetized deeply with halothane until loss of paw withdrawal reflex, bisected under the diaphragm, immersed in cold carbogenated Ringer solution, and decerebrated precollicularly. Preparations were then transferred to a recording chamber, and a double-lumen cannula was placed into the descending aorta for retrograde perfusion with carbogenated (saturated with 95% O2/5% CO2) solution containing the following (in mm): 124 NaCl, 26 NaHCO3, 3 KCl, 2 CaCl2, 1.25 MgSO4, 1.25 KH2PO4, and 10 dextrose (PCO2 40 mmHg, pH 7.4, 32°C). Ficoll 70 (1.25%) was added as an oncotic agent, and vecuronium bromide (4 μg ml−1) was added to block neuromuscular transmission. Aortic perfusion pressure was monitored via the second lumen of the cannula. Both vagi and carotid sinus nerves were cut to eliminate inputs from the peripheral chemoreceptors. Activity of the phrenic nerve was recorded using a suction electrode. Nerve activity was amplified, filtered (0.1–3 kHz), rectified, and integrated (50 ms time constant), relayed to a computer, and recorded using a 1401 interface and Spike 2 software (Cambridge Electronic Design).
In a preliminary study using rats, we found relatively weak respiratory responses of the preparations with denervated peripheral chemoreceptors when extra CO2 was applied (saturating the perfusate with 90% O2/10% CO2). Therefore, in this study, to assess the central respiratory chemosensitivity, the amount of CO2 bubbled through the solution was lowered to 3% (resulting in a solution with PCO2 of 26 mmHg and pH of 7.52) and then increased to 8% (PCO2 60 mmHg, pH 7.24), leading to significant and reproducible increases in the amplitude of the phrenic nerve discharge (see Fig. 1C). This protocol was used in the current study.
In vitro sinus nerve recording.
To assess carotid body function, superfused preparations of the carotid body/carotid sinus nerve were used (Rong et al., 2003). Mice were terminally anesthetized with halothane (6% in air mixture) and were decapitated at the lower cervical level. The head was placed in a chamber with circulating ice-cold Krebs solution saturated with 95% O2/5% CO2. The region of the carotid bifurcation containing the carotid body and the attached sinus nerve was dissected under a microscope and was placed into a recording chamber (1 ml). The preparation was superfused with carbogenated (saturated with 95% O2/5% CO2) solution containing the following (in mm): 124 NaCl, 3 KCl, 2 CaCl2, 26 NaHCO3, 1.25 NaH2PO4, 1 Mg(SO4)2, 10 d-glucose (PCO2 40 mmHg, pH 7.4). Perfusion rate was 6 ml min−1, and the temperature in the chamber was kept constant at 37°C. The sinus nerve was desheathed, and recordings were made using a suction electrode. The chemoafferent activity was amplified, filtered (0.2–3 kHz), relayed to a computer, and recorded using a 1401 interface and Spike 2 software (Cambridge Electronic Design).
Hypoxia was induced for 3 min by perfusing the chamber with the above solution in which O2 had been replaced by bubbling it with 95% N2/5% CO2. Changes in the PO2 of the perfusate were monitored on-line using an oxygen meter (model ISO2; World Precision Instruments). The analog of hypercapnia (respiratory acidosis) was induced for 5 min by perfusing the chamber with solution in which extra CO2 had been added to increase PCO2 from its normal value of 40 mmHg to 65 mmHg, which is accompanied by a reduction in pH from 7.4 to 7.2 (PCO2; pH values were measured using a Siemens Blood Gas Analyzer).
Data analysis
Recordings were processed using a 1401 interface and analyzed using Spike 2 software (Cambridge Electronic Design). Discharge frequency of the whole carotid sinus nerve was determined after discrimination of activity with a window discriminator (Digitimer D130 Spike Processor). The level of background noise was determined before each experiment by placing a recording electrode outside the preparation. Analysis of single chemoafferent fiber discharge was performed using the spike-sorting function of the Spike 2 program (Cambridge Electronic Design) as described in detail previously (Rong et al., 2003). Changes in the whole-nerve and single chemoafferent fiber activities are presented as peaks (the highest level of activity during the period of stimulation, in spikes s−1) and integral (frequency vs time, ∫ΔFF) increases in discharge. Integral increases in activity during the same time periods were determined by measuring area under the curve relative to a straight line joining the level of discharge before and after the stimulus.
All of the data are reported as means ± SE. Comparisons between experimental groups were made using Student's t test or ANOVA followed by Tukey-Kramer's post hoc test, as appropriate. A value of p < 0.05 was considered to be significant.
Definitions
TASK (Tandem P-domain K⁺ channel)
TASK channels are a subset of potassium (K⁺) channels within the tandem P-domain family. They are background or "leak" K⁺ channels that regulate cell membrane potential and are modulated by pH, oxygen levels, and other factors. TASK-1 and TASK-3 are the specific subtypes discussed in this study.
TASK-1 (KCNK3)
TASK-1 is a type of tandem P-domain K⁺ channel encoded by the KCNK3 gene. It is sensitive to changes in pH and oxygen levels, playing a significant role in regulating respiratory responses by modulating the activity of carotid body chemoreceptors.
TASK-3 (KCNK9)
TASK-3 is another tandem P-domain K⁺ channel encoded by the KCNK9 gene. It is less sensitive to pH changes in physiological conditions compared to TASK-1. Its role in respiratory chemoreception is minimal compared to TASK-1.
Carotid Body
The carotid body is a small organ located at the bifurcation of the carotid artery. It contains type I (glomus) cells that sense changes in arterial PO₂ (oxygen), PCO₂ (carbon dioxide), and pH, triggering responses to regulate breathing.
Chemosensory Control of Breathing
This refers to the physiological mechanisms by which sensory receptors detect changes in blood gases (PO₂ and PCO₂) and pH, and adjust respiratory patterns accordingly.
Hypoxia
A condition characterized by reduced oxygen levels (PO₂) in the blood or inspired air. Hypoxia stimulates the carotid body to increase ventilation.
Hypercapnia
A condition characterized by elevated carbon dioxide levels (PCO₂) in the blood. It is a potent stimulant of breathing, primarily through central and peripheral chemoreceptors.
Peripheral Chemoreceptors
These are specialized sensory cells located in the carotid and aortic bodies that detect changes in blood oxygen, carbon dioxide, and pH levels.
Central Chemoreceptors
Receptors located in the brainstem that primarily sense changes in PCO₂ and pH in the cerebrospinal fluid, contributing to the control of breathing.
Carotid Sinus Nerve
A nerve that transmits sensory information from the carotid body to the brainstem, influencing respiratory responses.
Minute Ventilation (VE)
The total volume of air inhaled or exhaled from the lungs per minute, calculated as the product of tidal volume (VT) and respiratory rate (fR).
Phrenic Nerve
A nerve that controls the diaphragm, playing a critical role in the mechanics of breathing.
Whole-body Plethysmography
A non-invasive technique to measure respiratory parameters such as tidal volume and respiratory rate in freely moving animals.
In Situ Brainstem-Spinal Cord Preparation
An experimental model that isolates the brainstem and spinal cord while maintaining functional respiratory circuits, used to study respiratory control mechanisms.
In Vitro Sinus Nerve Recording
A technique that records electrical activity from the carotid sinus nerve in isolated preparations, allowing detailed analysis of chemosensory responses.
Chemoafferent Discharge
The electrical activity transmitted by sensory nerves (e.g., the carotid sinus nerve) in response to chemical stimuli such as changes in PO₂, PCO₂, or pH.
Spike 2 Software
A data acquisition and analysis software used to process electrophysiological recordings.
∫ΔFF (Integral of Frequency vs. Time)
A quantitative measure of the total activity of a nerve or cell during a given stimulus, calculated as the area under the frequency versus time curve.
Hyperoxic Hypercapnia
A condition where oxygen levels are high while carbon dioxide levels are elevated, used experimentally to isolate specific chemoreceptive responses.
Krebs Solution
A physiological buffer solution used in laboratory experiments to maintain the viability of biological tissues during recordings.
Vecuronium Bromide
A neuromuscular blocking agent used in experiments to prevent muscle movements that could interfere with recordings.
Halothane
A volatile anesthetic agent used to induce anesthesia in experimental animals.
C57BL/6
A common inbred strain of laboratory mouse used in genetic and physiological research.
Genotype
The genetic constitution of an organism, typically referring to specific mutations or gene knockouts in experimental studies.
ANOVA (Analysis of Variance)
A statistical test used to compare means among multiple groups to identify significant differences.
Tukey-Kramer Post Hoc Test
A statistical method used after ANOVA to determine which specific groups differ from each other.