Cellular localization of mitochondria contributes to Kv channel-mediated regulation of cellular excitability in pulmonary but not mesenteric circulation
The mechanisms involved in this response are complex and currently incompletely understood ( 14 , 48 , 52 ) . Such responses do not occur in the systemic circulation, which dilates when oxygen tension is reduced to maximize blood supply to all parts of the body.
14 :
part of the O2 sensing mechanism resides in the endothelium, to regulate the release of agents that influence the function of pulmonary vascular muscle.
The smooth muscle cells of the pulmonary vasculature may be the primary sensors of O2 that initiate or terminate the contractile response to hypoxia, but their ability to influence tone may be highly dependent on the presence of factors released from the endothelium
seems likely that the final vasoconstriction produced by hypoxia results from a number of O2 sensing pathways acting in concert
O2 sensing is such an important property of the pulmonary vasculature, the presence of multiple sensors could provide a protective mechanism, whereby loss of function in one pathway may be compensated by another
48 :
the role of pretone, the precise sensor mechanisms that result in :
K+ channel inhibition
Ca2+ release from stores
synthesis/release of the putative EDCF from the endothelium
and of course the identity and mechanism of the EDCF itself
52 :
Mechanistic Background : HPV occurs when low oxygen levels in alveoli trigger constriction in nearby pulmonary arteries, shunting blood away from poorly ventilated areas and enhancing gas exchange efficiency in oxygen-rich regions. Historically traced to von Euler and Liljestrand in 1946, HPV has since been studied intensely but remains incompletely understood, particularly regarding the precise molecular pathways involved.
Biological Mechanisms :
Phasic Responses : HPV is classified into three phases based on hypoxia duration:
Acute response (seconds) for immediate perfusion matching.
Prolonged hypoxia response (minutes to hours), potentially altering vascular structure.
Chronic hypoxia response that may lead to pulmonary hypertension and vascular remodeling.
Ion Channels and Ca²⁺ Dynamics : Pulmonary arterial smooth muscle cells are both sensors and effectors in HPV. Hypoxia inhibits voltage-gated K⁺ (KV) channels, reducing K⁺ current, leading to membrane depolarization, increased intracellular Ca²⁺, and contraction.
Sarcoplasmic Reticulum (SR) Role : Dipp et al. observed that SR Ca²⁺ release is crucial for HPV, suggesting Ca²⁺ stores within cells are significant in this response, though extracellular Ca²⁺ plays a role in prolonged phases.
Hypoxia Sensing Theories : Five leading theories are explored to elucidate oxygen sensing:
KV Channels : Hypoxia may inhibit KV channels, causing depolarization and activating L-type Ca²⁺ channels.
NADH Oxidoreductase : Generates H₂O₂, which influences vasodilation, but in hypoxia, a reduction in H₂O₂ could lead to vasoconstriction.
NADPH Oxidase : Activated paradoxically during hypoxia, increasing reactive oxygen species and triggering vasoconstriction.
Mitochondrial Sensors: Hypoxia blocks electron transport, raising superoxide levels.
Cytochrome P-450 Pathway : Involves arachidonic acid metabolites in the vasoconstrictive response.
Challenges and Future Directions : Variations in experimental outcomes stem from differences in animal models, species, pretone in vessel walls, and methodological limits in reactive oxygen species detection. The need for a unified mechanism remains. Advancements in transgenic models and technology may clarify these processes further, providing insights to treat conditions like pulmonary hypertension without compromising perfusion efficiency.
Figure 1 - Spatial organization of mitochondria relative to sarcoplasmic reticulum ( SR ) in pulmonary arterial smooth muscle cells ( PASMCs )
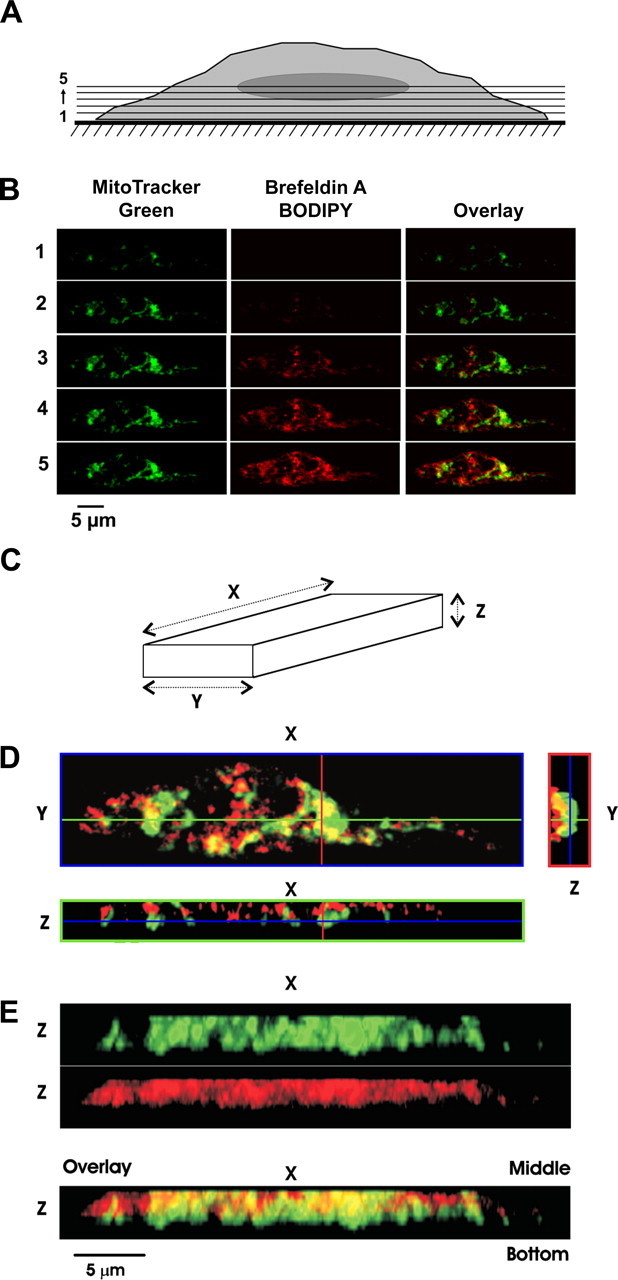
A: schematic diagram indicating sectional imaging from the bottom to the center of the representative PASMC. For statistical analysis, the 1st optical slice (from the bottom of the cell) that revealed the fluorescence of either of the dyes was referred to as slice 1. PASMC mitochondria were stained with 1 μM MitoTracker Green FM, SR was stained with 2 μM brefeldin A BODIPY 558/568, and the confocal z-sectioning protocol was applied (see materials and methods).
B: gallery of sequential x-y optical slices from slice 1 to slice 5 (from bottom to center of cell depth as indicated) shows images of mitochondria (green), SR (red) and their overlay (as indicated).
C: schematic indicating cell orientation for images in D and E.
D: 3 individual optical slices: x-y image (blue box) was taken at z position depicted by blue line in x-z and y-z images, x-z image (green box) was taken at y position depicted by green line in x-y and y-z images, and y-z image (red box) was taken at x position depicted by red line in x-y and x-z images. Note that in x-z image cell bottom is toward bottom of image while in y-z image cell bottom is toward right of image.
E: 3-dimensional (3D) images of mitochondria (green), SR (red), and their overlay (from top to bottom, respectively) were reconstructed from 1st 10 optical slices and are presented after rotation by 90° around x-axis. Note that in 3D images cell bottom is at bottom of image.
"This approach revealed that in PASMCs, on movement from the cell bottom toward the middle of the myocyte depth (Fig. 1A), the fluorescent signal from MitoTracker Green appeared in the x-y confocal optical slices (Fig. 1C) before that of brefeldin A BODIPY (Fig. 1B)."
"In PASMCs, in individual x-z and y-z optical cross sections of the myocyte, MitoTracker Green fluorescence (mitochondria) was detected distinctly closer to the cell periphery than that of brefeldin A BODIPY (SR) (Fig. 1, D and E)."
This image appears to be a series of fluorescence microscopy images and diagrams depicting the localization and co-localization of cellular structures, likely mitochondria and the Golgi apparatus, within a cell. Here’s a breakdown of the results based on the panels:
Panel A :
This shows a cross-sectional schematic of a cell, with numbered horizontal lines (1-5) indicating different focal planes, suggesting that the cell was imaged at multiple depths (Z-stacks) to capture 3D information. This provides context for the images shown in Panel B, where each numbered row corresponds to a different Z-plane.
Panel B : Each row (1-5) corresponds to a different Z-plane through the cell, stained with:
MitoTracker Green: Labels mitochondria, appearing as green fluorescence.
Brefeldin A BODIPY: Likely labels Golgi-related structures, shown as red fluorescence.
Overlay: Combines both channels to show co-localization, which would appear yellow where both signals overlap.
Results :
The overlay images suggest regions of co-localization between mitochondria and Golgi structures, particularly in certain Z-planes, indicated by yellow areas. This could imply interactions or close proximity between mitochondria and Golgi at specific cellular depths.
Panel C :
This is a schematic of the coordinate system (X, Y, Z axes), likely indicating how the 3D imaging was conducted. It provides a reference for interpreting the images in Panels D and E.
Panel D :
A 3D reconstruction showing different views along the YZ and XZ planes, giving a more complete spatial relationship between the green (mitochondria) and red (Golgi) signals. The overlap in colors in certain regions indicates co-localization. The orthogonal views (XY, YZ, XZ) allow for visualization of how these organelles are distributed in the cell.
Panel E :
Shows the Z-axis views at different positions within the cell. The first two images display separate green and red channels along the Z-axis, and the third row shows an overlay. The middle and bottom slices show differences in distribution and overlap, indicating that co-localization may vary along the Z-axis.
Summary
Mitochondria and Golgi Apparatus Localization: The mitochondria and Golgi structures are closely associated in certain regions of the cell, as shown by the yellow overlap in the fluorescence images.
3D Spatial Distribution: This co-localization is not uniform across all Z-planes, suggesting that mitochondria and Golgi are more closely associated in some cellular regions or depths than others.
Potential Interaction Sites: The co-localization could suggest potential sites of functional interaction or spatial organization relevant to cellular processes involving both organelles, such as energy production and lipid or protein trafficking.
These observations might be part of a study investigating the spatial relationship and functional interactions between mitochondria and the Golgi apparatus in the cell.
Figure 2 - Spatial organization of mitochondria relative to SR in mesenteric arterial smooth muscle cells (MASMCs).
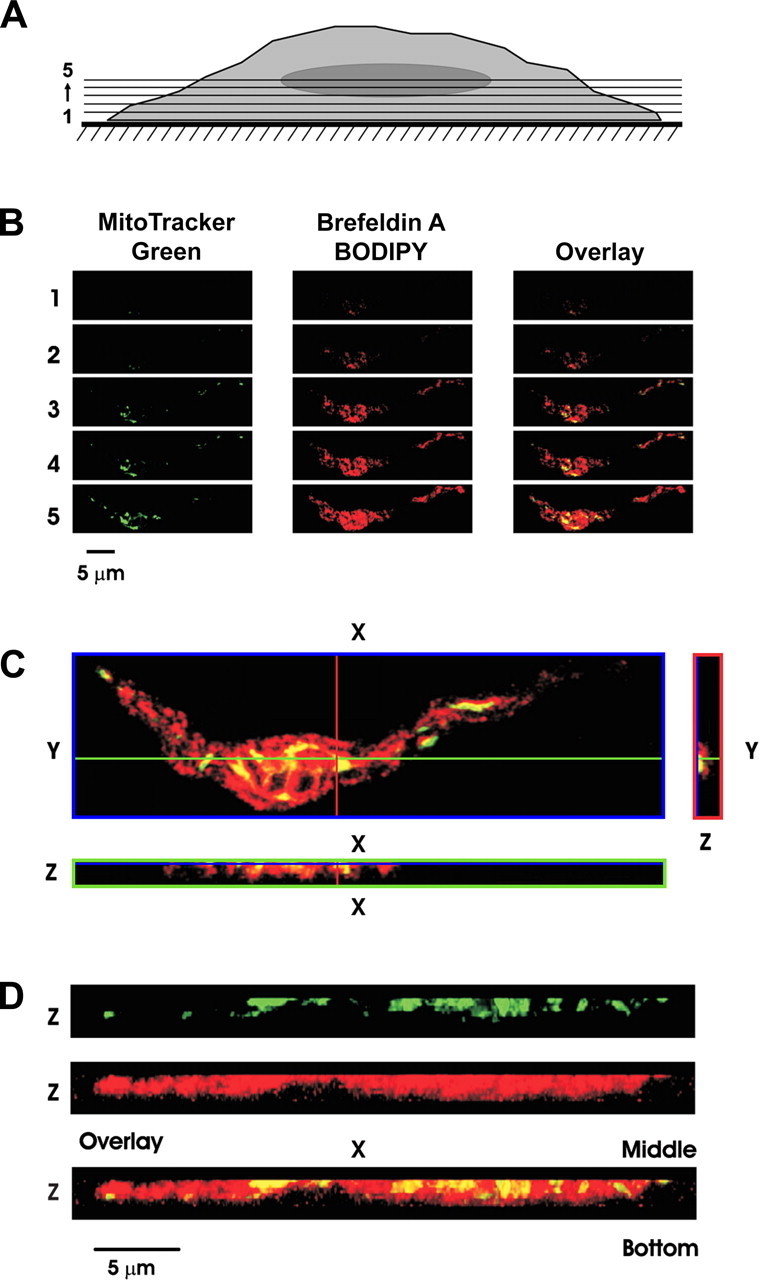
A: schematic diagram indicating sectional imaging from bottom to center of representative MASMC. For statistical analysis, the 1st optical slice (from the bottom of the cell) that revealed the fluorescence of either of the dyes was referred to as slice 1.
B: gallery of the sequential x-y optical slices from slice 1 to slice 5 (from bottom to center of cell depth) shows images of MitoTracker Green fluorescence, brefeldin A BODIPY fluorescence, and their overlay (as indicated).
C: 3 individual optical slices: x-y image (blue box) was taken at z position depicted by blue line in x-z and y-z images, x-z image (green box) was taken at y position depicted by green line in x-y and y-z images, and y-z image (red box) was taken at x position depicted by red line in x-y and x-z images. Note that in x-z image cell bottom is toward bottom of image, while in y-z image cell bottom is toward right of image.
D: 3D images of mitochondria (green), SR (red), and their overlay (reconstructed from the 1st 10 optical slices and presented after rotation by 90° around x-axis). Note that in 3D images cell bottom is at bottom of image.
"In MASMCs, however, brefeldin A BODIPY fluorescence staining the SR appeared before or simultaneously with MitoTracker Green fluorescence (Fig. 2B)."
"Such opposing juxtaposition between the SR and mitochondria in MASMCs compared with PASMCs becomes more evident from the comparison of individual x-z and y-z optical cross sections of the myocyte MitoTracker Green (mitochondria) and brefeldin A BODIPY (SR) fluorescent signals (Fig. 2, C and D)."
Panel A: This schematic diagram illustrates the imaging setup, showing a cross-sectional view through the center of a representative MASMC (Murine Aortic Smooth Muscle Cell). The sections (1-5) represent different optical slices from the bottom of the cell moving toward the center. For consistency in analysis, the authors define "slice 1" as the first optical section where fluorescence from either MitoTracker Green (mitochondria) or Brefeldin A BODIPY (Sarcoplasmic Reticulum, or SR) is detected.
Panel B: This gallery displays sequential x-y optical slices from the bottom of the cell (slice 1) through to the cell center (slice 5). The three columns represent:
MitoTracker Green fluorescence: Labels mitochondria, shown in green.
Brefeldin A BODIPY fluorescence: Marks the SR, shown in red.
Overlay: Combines the two channels to highlight regions of co-localization or proximity between mitochondria and the SR.
The key finding here is that Brefeldin A BODIPY fluorescence (SR) often appears before or simultaneously with MitoTracker Green (mitochondria) as the imaging progresses from the cell bottom upward. This suggests a unique spatial organization where SR structures are situated nearer the cell base, with mitochondria positioned just above or adjacent, reflecting an "opposing juxtaposition" between these organelles in MASMCs.
Panel C: This panel provides three different optical cross-sections (x-y, x-z, and y-z) to offer a detailed, 3D perspective on the relative positioning of mitochondria and SR in MASMCs. Here’s the layout:
x-y slice (blue box): A top-down view taken at a specific z-depth, as indicated by the blue line in the other images.
x-z slice (green box): A side view along the x-axis, with the cell bottom oriented at the bottom of the image, as depicted by the green line in the other views.
y-z slice (red box): A side view along the y-axis, with the cell bottom oriented to the right of the image, as shown by the red line in the other images.
This 3D perspective further emphasizes the juxtaposition between SR and mitochondria in MASMCs. In particular, SR structures consistently appear positioned in opposition to mitochondria, which contrasts with observations in PASMCs (Pulmonary Artery Smooth Muscle Cells) and suggests a distinctive arrangement in MASMCs.
Panel D: Here, we see reconstructed 3D images of the mitochondria (green) and SR (red), based on the first 10 optical slices. After a 90° rotation around the x-axis, the cell bottom is oriented at the bottom of each image, giving a side-on view. This reconstruction vividly demonstrates the spatial organization observed in the individual x-y, x-z, and y-z slices, reinforcing the notion of SR and mitochondria lying in opposing positions within the MASMC.
This configuration supports the hypothesis that mitochondria and SR in MASMCs are structurally organized in a way that facilitates functional compartmentalization, with each organelle occupying distinct but complementary regions.
Summary of Findings
Early Appearance of SR in MASMCs: In MASMCs, Brefeldin A BODIPY staining of the SR is detected before or at the same time as MitoTracker Green staining of mitochondria, suggesting SR structures are closer to the cell base.
Juxtaposition in MASMCs: Both the sequential x-y slices (Panel B) and the orthogonal views (Panel C) highlight a distinctive juxtaposition between mitochondria and SR in MASMCs that differs from PASMCs.
3D Organization: The 3D reconstruction (Panel D) visualizes this spatial separation, potentially indicating a functional relationship in MASMCs that might influence cellular processes such as calcium signaling or energy metabolism.
In essence, the unique positioning of SR and mitochondria in MASMCs may reflect specialized roles in smooth muscle cell function, distinct from the arrangement seen in PASMCs. This study adds to our understanding of organelle positioning in different smooth muscle cell types, potentially impacting their physiological and metabolic properties.
Figure 3 - Mitochondria are distributed peripherally in PASMCs but not in MASMCs
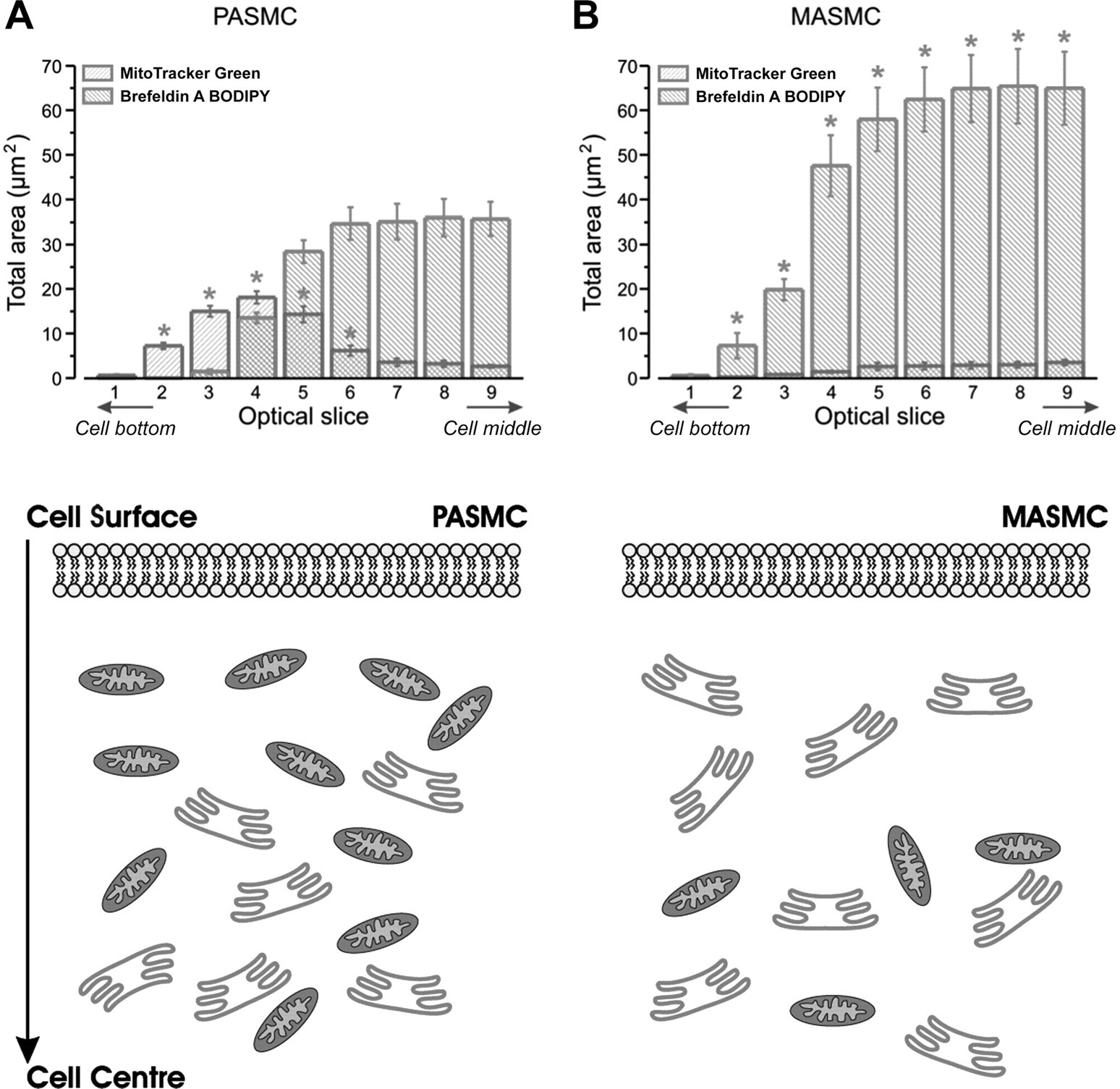
Total area occupied by mitochondria and SR plotted vs. corresponding optical slice number for 1st 9 optical slices taken from PASMCs (A and Fig. 1) and MASMCs (B and Fig. 2) upon movement from cell bottom toward middle of cell depth (n = 4).
Significant difference between areas occupied by mitochondria (P < 0.02) and SR (P < 0.04) in PASMCs and MASMCs, respectively.
Schematic diagrams, bottom, depict greater number and locality of mitochondria in relation to SR and plasma membrane in PASMCs (A) and MASMCs (B).
"To quantify these differences, the total area occupied by pixels showing MitoTracker Green and brefeldin A BODIPY fluorescence was analyzed in four PASMC and MASMCs and plotted against the optical slice number in Fig. 3."
"Figure 3 also shows diagrammatic representations of these areas, comparing PASMCs with MASMCs."
The image displays two graphs labeled A and B with bar plots, each representing data from two different cell types: PASMC (Pulmonary Artery Smooth Muscle Cells, left) and MASMC (Mesenteric Artery Smooth Muscle Cells, right).
Graph Analysis
Graph A (PASMC, Left)
The y-axis represents the "Total area (μm²)" of mitochondria and Golgi apparatus stained with MitoTracker Green and Brefeldin A BODIPY, respectively.
The x-axis represents different optical slices from the Cell Bottom to the Cell Middle.
In PASMC, the area stained by MitoTracker Green (indicating mitochondrial content) is relatively high, especially in the middle slices (6-9), showing substantial mitochondrial presence throughout the cell.
The Brefeldin A BODIPY staining (indicating the Golgi apparatus) is concentrated in the lower optical slices, suggesting that Golgi structures are denser closer to the cell bottom.
The asterisks (*) indicate statistically significant differences from a baseline or control level, with significant increases in mitochondrial content in certain slices.
Graph B (MASMC, Right)
MASMC shows less overall mitochondrial area compared to PASMC, as indicated by the lower MitoTracker Green-stained values across slices. The mitochondrial content is lower and less dense throughout the slices.
The Brefeldin A BODIPY staining remains relatively low across all slices, suggesting a more limited presence of Golgi structures in MASMC.
The asterisks (*) indicate significant differences in mitochondrial content at specific slices, but overall, MASMC has fewer mitochondria compared to PASMC.
Diagram (Bottom Panel)
The schematic below the graphs shows a cross-sectional view of PASMC and MASMC, depicting the relative positions of mitochondria (represented by ovals) and Golgi apparatus structures (represented by stacked, curved shapes).
In PASMC (left), mitochondria are densely packed throughout the cell, while Golgi structures are concentrated toward the cell bottom.
In MASMC (right), there are fewer mitochondria, and they are less densely distributed. Golgi structures are also sparse in MASMC compared to PASMC.
Updated Summary of Findings
Higher Mitochondrial Density in PASMC: PASMC exhibits a higher density of mitochondria throughout the cell, suggesting a greater energy requirement, likely linked to the pulmonary artery's metabolic needs.
Lower Mitochondrial and Golgi Density in MASMC: MASMC has fewer mitochondria and Golgi structures, implying lower metabolic and protein trafficking demands compared to PASMC.
Cellular Organization Differences: The contrasting distribution and density of mitochondria and Golgi between PASMC and MASMC highlight cell-type-specific structural adaptations, potentially reflecting their unique physiological roles in the pulmonary and mesenteric arteries.
Figure 4 - Close association of mitochondria with plasma membrane exists in PASMCs but not in MASMCs
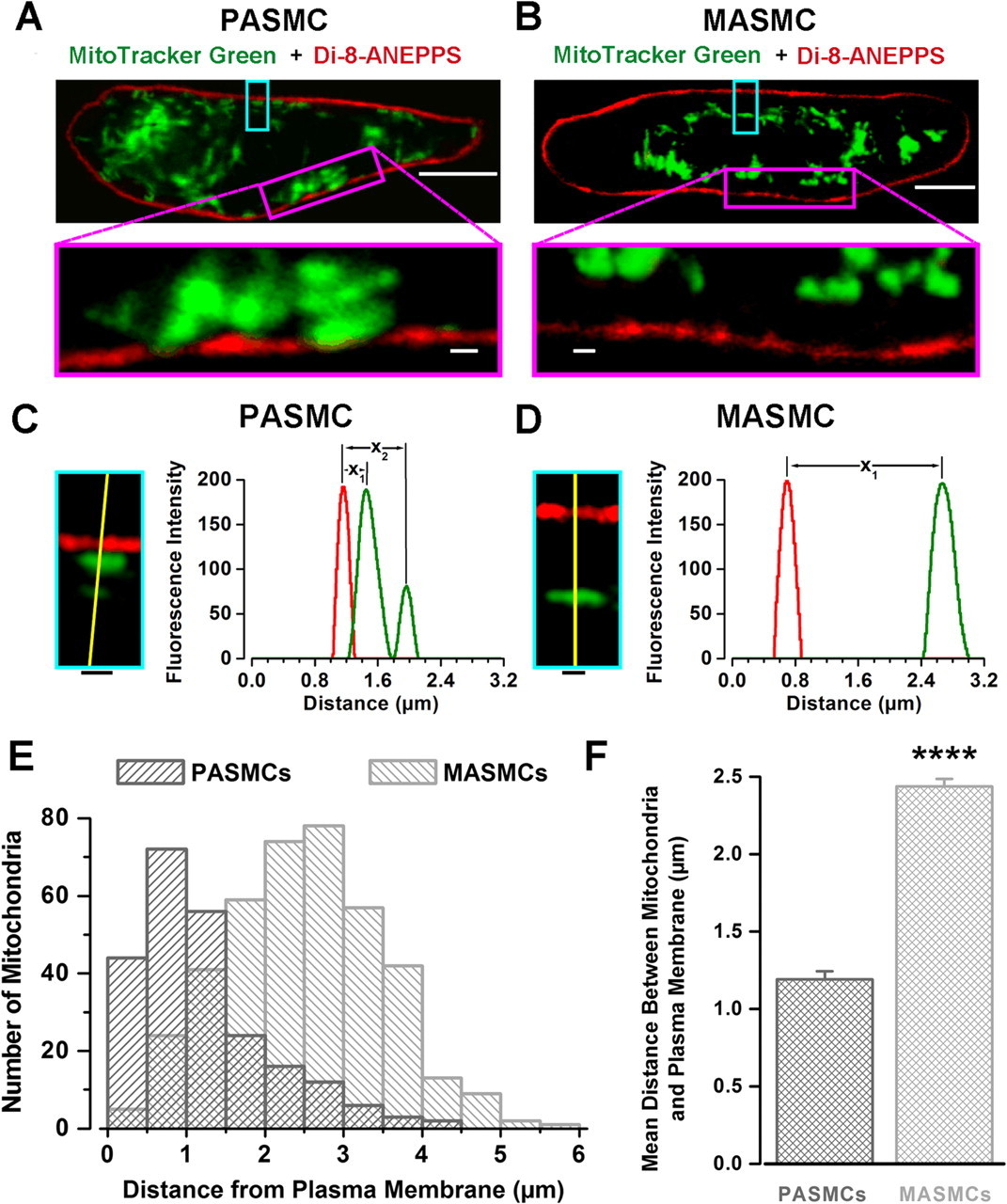
A and B: images of MitoTracker Green FM (green; mitochondria staining) and di-8-ANEPPS (red; plasma membrane staining) fluorescence acquired from representative PASMC and MASMC, respectively.
Top: confocal optical slice (<0.6 μm) at middle of cell depth. Calibration bars, 5 μm.
Bottom: comparison of relative distribution of peripheral mitochondria at enhanced magnification.
Calibration bars, 0.5 μm.
C and D: protocol of measurement of distance between plasma membrane and mitochondria in PASMC and MASMC, respectively. Insets, regions of PASMC and MASMC outlined by cyan boxes in A and B at higher magnification.
Calibration bars, 0.5 μm.
C and D: graphs show spatial profiles of fluorescent intensities (arbitrary units) of MitoTracker Green (mitochondria) and di-8-ANEPPS (plasma membrane) fluorescence measured along yellow lines (drawn across center of each peripheral mitochondrion to plasma membrane by shortest distance).
Distance between peaks in red and green fluorescence intensities denoted as x1 and x2 (for next adjacent mitochondrion) was used as a measure of distance between mitochondria and plasma membrane.
E: comparison of distribution of mitochondria in respect to their distance from the plasma membrane (as defined in C and D) for 6 PASMCs and 6 MASMCs.
F: comparison of mean distance between mitochondria and plasma membrane measured for 235 mitochondria in PASMCs and 405 mitochondria in MASMCs. P < 0.0001.
"The existence of the subplasmalemmal pool of mitochondria in PASMCs, but not in MASMCs, was further studied by double staining of both types of cells with MitoTracker Green and di-8-ANEPPS, selective fluorescent probes for mitochondria and the plasmalemmal membrane, respectively (Fig. 4)."
"Figure 4A shows x-y confocal images taken through the middle of a representative PASMC."
"As can be seen clearly in Fig. 4A, MitoTracker Green fluorescence often appeared to be 'fused' (within ∼200 nm, the limit of lateral resolution of our confocal microscope) with di-8-ANEPPS fluorescence shown in red (plasmalemma)."
"Conversely, in MASMCs, a clear gap between the two fluorescent signals was observed (Fig. 4B)."
"To quantitatively compare relative distances between mitochondria and the plasma membrane in the two cell types, the spatial profiles of fluorescent signals recorded for di-8-ANEPPS (red) and MitoTracker Green (green) were constructed across the shortest distance between the center of each mitochondrion and the plasma membrane (shown by yellow lines in Fig. 4, C and D, for PASMC and MASMC, respectively)."
"Histograms of distribution of mitochondria according to their distance from the plasma membrane measured in six PASMCs and six MASMCs are compared in Fig. 4E."
"On average, the mean distance between peripheral mitochondria and the plasma membrane calculated as described above was twofold greater in MASMCs (2.4 ± 0.05 μm, n = 405) than in PASMCs (1.2 ± 0.05 μm, n = 235) (P < 0.0001; Fig. 4F)."
This figure presents comparative analyses of mitochondrial distribution relative to the plasma membrane in pulmonary artery smooth muscle cells (PASMCs) and mesenteric artery smooth muscle cells (MASMCs) using MitoTracker Green and Di-8-ANEPPS for visualization. Here are the summarized results from each panel:
Panels A and B: Fluorescence images of PASMCs (A) and MASMCs (B) show mitochondria (green) and plasma membrane (red). Insets highlight closer views of the mitochondrial arrangement within the cells. PASMCs appear to have a denser clustering of mitochondria near the membrane compared to MASMCs.
Panels C and D: Fluorescence intensity profiles for PASMCs (C) and MASMCs (D) across the indicated yellow lines reveal the spatial separation between the plasma membrane and mitochondria. PASMCs show a closer proximity of mitochondria to the plasma membrane, indicated by shorter distances (labeled x1x_1x1) compared to MASMCs, where mitochondria are positioned further away (greater x1x_1x1).
Panel E: A histogram illustrates the distribution of mitochondria at various distances from the plasma membrane in PASMCs and MASMCs. PASMCs have a higher number of mitochondria close to the plasma membrane, while MASMCs display a broader distribution, with fewer mitochondria near the membrane and more at larger distances.
Panel F: This bar graph quantifies the mean distance between mitochondria and the plasma membrane in both cell types. The distance is significantly greater in MASMCs than in PASMCs, with MASMCs showing a mean distance nearly double that of PASMCs. The asterisks () indicate a highly significant statistical difference (p < 0.0001).
Overall Conclusion
The data suggest that mitochondria in PASMCs are positioned closer to the plasma membrane compared to MASMCs, potentially implying differences in mitochondrial function or metabolic requirements between these cell types.
Figure 5 - Differences in amplitude and steady-state activation of voltage-gated K+ channel current ( IKv ) in PASMCs and MASMCs
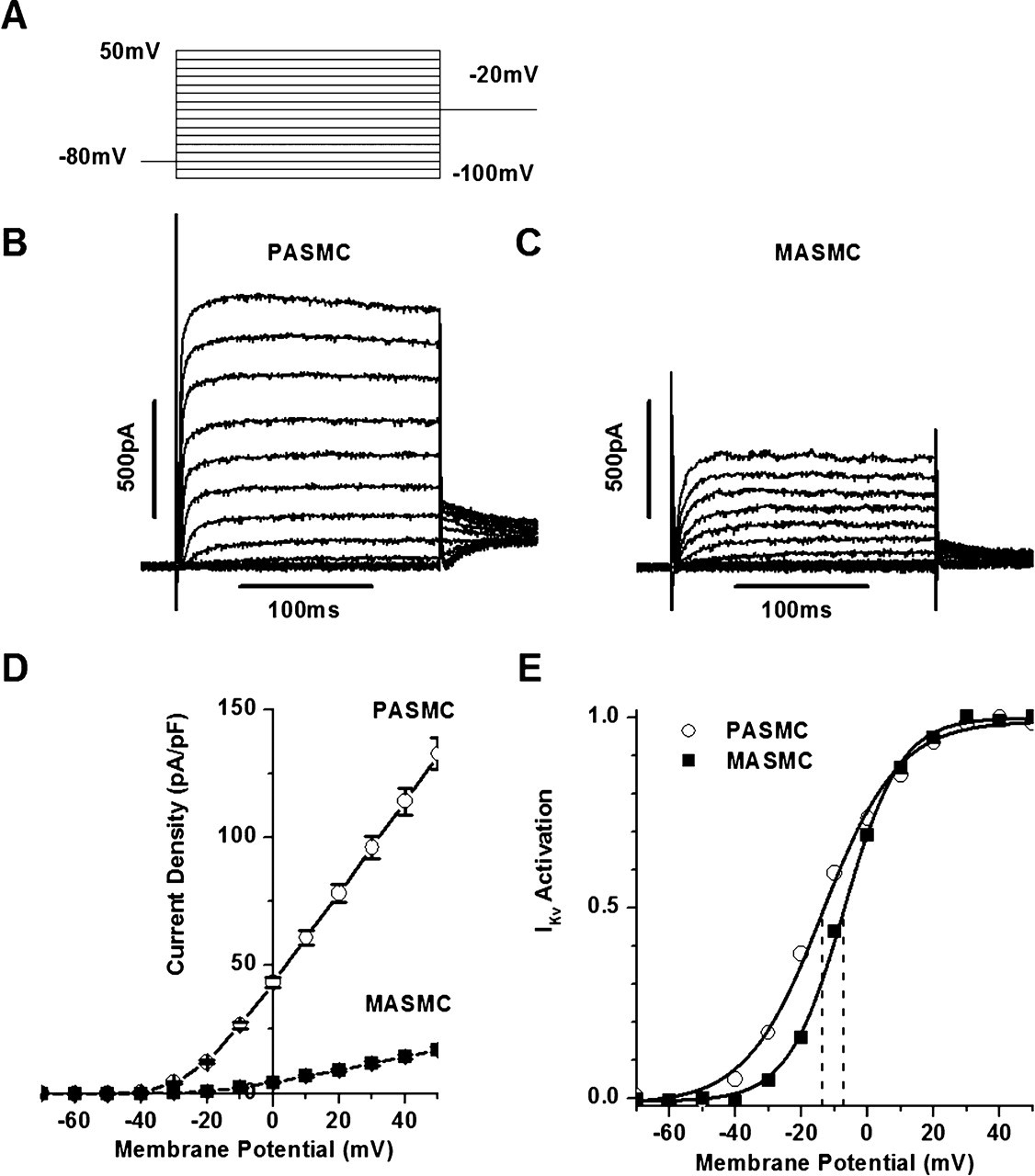
B and C: current traces from representative PASMC (B) and MASMC (C) using steady-state IKv activation protocol featured in A; cell membrane capacitance (Cm) = 11.1 and 16.7 pF, respectively.
D: comparison of mean IKv densities measured with protocol in A in 184 PASMCs and 60 MASMCs.
E: normalized current-volume (I-V) curves for IKv tail currents for representative PASMC and MASMC featured in B and C, respectively.
Solid lines represent fits to Boltzmann equation described in materials and methods with half-activation potential equal to −13.7 and −7.2 mV (dashed lines) and the slope factor of activation equal to 11.5 and 8.5 mV for PASMC and MASMC, respectively.
"With the IKv activation protocol featured in Fig. 5A, whole cell IKv was recorded in both PASMCs and MASMCs."
"The amplitude of the current recorded from a representative MASMC (Fig. 5C) was considerably smaller than in a PASMC (Fig. 5B)."
"The smaller IKv and the larger cell size in MASMCs result in significant differences in the whole cell current density in the two cell types (Fig. 5D), which was 10- to 18-fold greater between −30 and 0 mV and 8- to 9-fold greater between 0 and +50 mV in PASMCs compared with MASMCs."
"Comparison of the steady-state activation of IKv measured from the normalized I-V curves (which were derived from the IKv tail current measurements) from the representative PASMC and MASMC shown in Fig. 5, B and C, indicates that IKv activation in MASMC was shifted by ∼6 mV to more positive membrane voltages and was steeper than in PASMC (Fig. 5E)."
The image shows a set of electrophysiological data illustrating differences in current-voltage relationships and activation properties between two types of smooth muscle cells: Pulmonary Artery Smooth Muscle Cells (PASMC) and Mesenteric Artery Smooth Muscle Cells (MASMC). Here’s a breakdown of the panels and what each result shows:
Panel A :
This panel displays a voltage-clamp protocol. The cells were clamped at a series of potentials ranging from -100 mV to +50 mV, with the holding potential set at -80 mV. The goal of this protocol is to study the voltage-dependent properties of ionic currents in the cells.
Panels B and C :
These panels show representative current traces for PASMC (Panel B) and MASMC (Panel C) in response to the voltage-clamp protocol.
PASMC (Panel B): The traces indicate a substantial outward current as the membrane potential is stepped to more depolarized levels, suggesting the presence of active voltage-gated potassium (K+) channels. The currents increase with more positive voltages, showing the characteristic behavior of voltage-gated channels.
MASMC (Panel C): The outward currents appear much smaller compared to PASMC, suggesting that MASMC have fewer or less active K+ channels in response to depolarization under these conditions.
Panel D : This panel shows the current density-voltage (I-V) relationship for PASMC and MASMC.
Results: PASMC exhibit a significantly higher current density than MASMC at depolarized potentials, indicating that PASMC have a larger density of active voltage-gated K+ channels or that these channels have a greater conductance in PASMC compared to MASMC. The current density in MASMC remains relatively low across the voltage range tested.
E : This panel presents the activation curves for the voltage-gated K+ currents (IK) in PASMC and MASMC, derived from fitting the activation data.
Results: PASMC show a more negative voltage threshold for activation compared to MASMC, as indicated by the leftward shift in the activation curve. This means that K+^++ channels in PASMC activate at a lower (more negative) voltage compared to MASMC, suggesting a functional difference in channel properties between these cell types.
Summary
These results highlight that PASMC have a higher density of functional voltage-gated K+ channels compared to MASMC and that these channels activate at more negative membrane potentials in PASMC. This could imply that PASMC are more equipped for rapid repolarization in response to depolarizing stimuli, which might be relevant to the physiological demands of pulmonary arteries compared to mesenteric arteries.
Figure 6 - IKv inactivation parameters differ in PASMCs and MASMCs
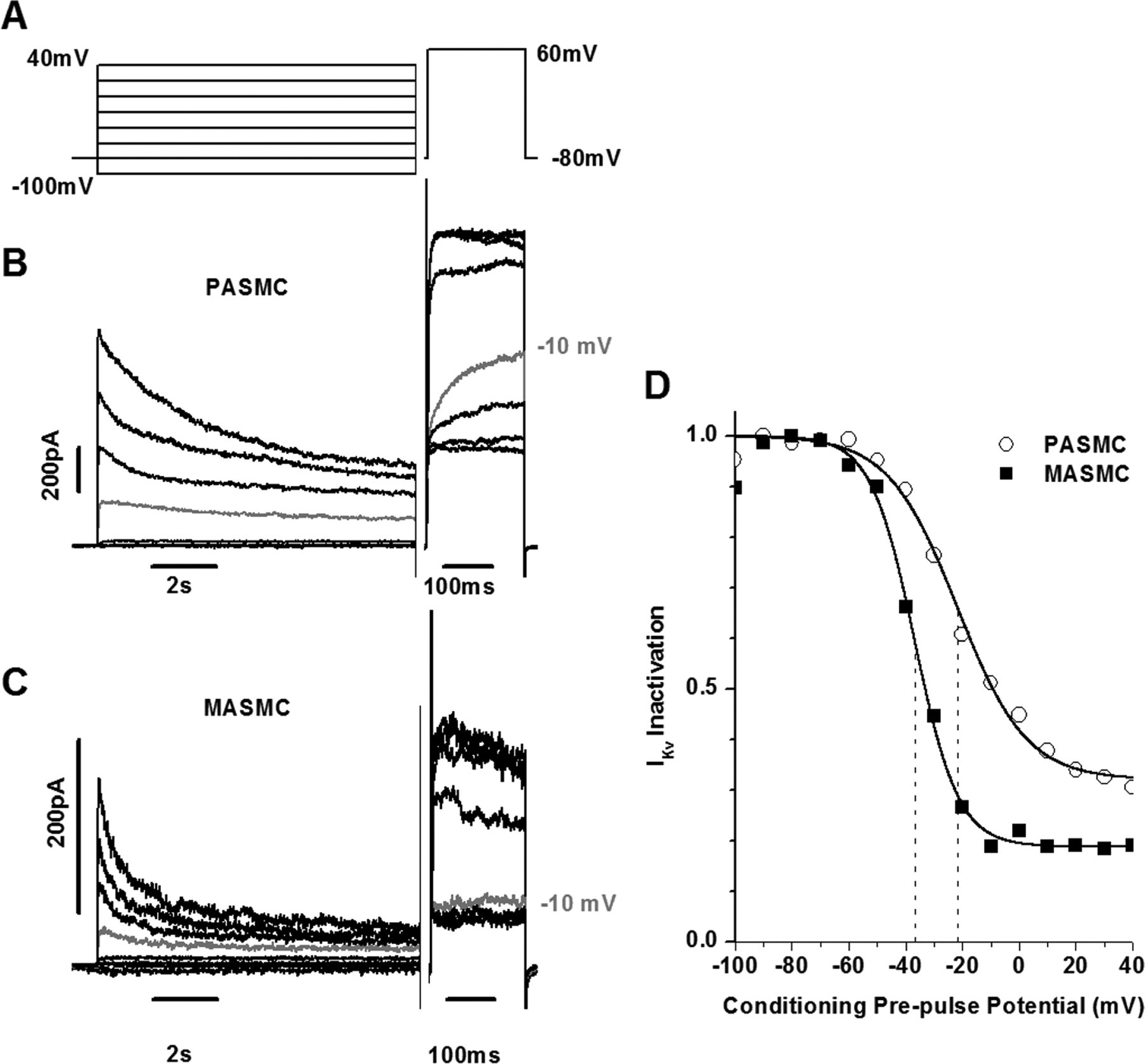
B and C: IKv traces from representative PASMC (B) and MASMC (C) using inactivation protocol shown in A.
Cm = 9.4 pF (B) and 10.4 pF (C).
Traces recorded with a prepulse to −10 mV are shown in gray for comparison.
Note that current traces in B and C are shown in 20-mV increments.
D: normalized IKv measured during a voltage step to +60 mV and plotted vs. conditioning prepulse potential.
Solid lines represent fits to equation described in materials and methods with half-inactivation potential equal to −21.6 and −36.7.2 mV (dashed lines), slope factor of inactivation equal to 12.2 and 7.7 mV, and noninactivating component A equal to 0.32 and 0.19 for PASMC and MASMC, respectively.
"Comparison of IKv inactivation measured with the voltage protocol shown in Fig. 6A from the representative PASMC (Fig. 6B) and MASMC (Fig. 6C) demonstrates that IKv inactivation in PASMCs is relatively slow and incomplete compared with that in MASMCs."
"The normalized IKv recorded during the test pulse was plotted against the prepulse potential, and resulting dependencies were fitted to the equation described in materials and methods, yielding the parameters of IKv inactivation, the half-inactivation potential Vh, the slope factor kh, and the noninactivating component A (Fig. 6D)."
This figure shows electrophysiological data assessing voltage-dependent potassium (Kv) channel inactivation in two types of smooth muscle cells: pulmonary artery smooth muscle cells (PASMC) and mesenteric artery smooth muscle cells (MASMC). Here’s a breakdown of the figure components:
Panel A shows the voltage protocol used in this experiment. Cells are subjected to a series of voltage steps from -100 mV to 40 mV and then held at 60 mV before returning to -80 mV.
Panel B displays the resulting current traces from PASMC in response to the voltage protocol. The progressive decay in current suggests inactivation of Kv channels at more depolarized voltages.
Panel C shows similar recordings from MASMC. The inactivation appears different compared to PASMC, as the decay pattern and amplitude response to voltage steps vary between the two cell types.
Panel D quantifies the inactivation as a function of the pre-pulse potential, with inactivation curves for both PASMC and MASMC. The data points are fitted with a Boltzmann curve, indicating a difference in inactivation kinetics between PASMC (open circles) and MASMC (filled squares). The rightward shift in the MASMC curve suggests that MASMC channels require a higher depolarizing pre-pulse to reach similar levels of inactivation compared to PASMC.
Key Findings:
Kv channels in PASMC inactivate more readily at lower pre-pulse potentials compared to those in MASMC.
This difference in Kv channel inactivation could reflect variations in the physiological roles of Kv channels in pulmonary versus mesenteric vascular tone regulation.
Figure 7 - Mitochondrial inhibition has significantly greater effects on IKv in PASMCs than in MASMCs
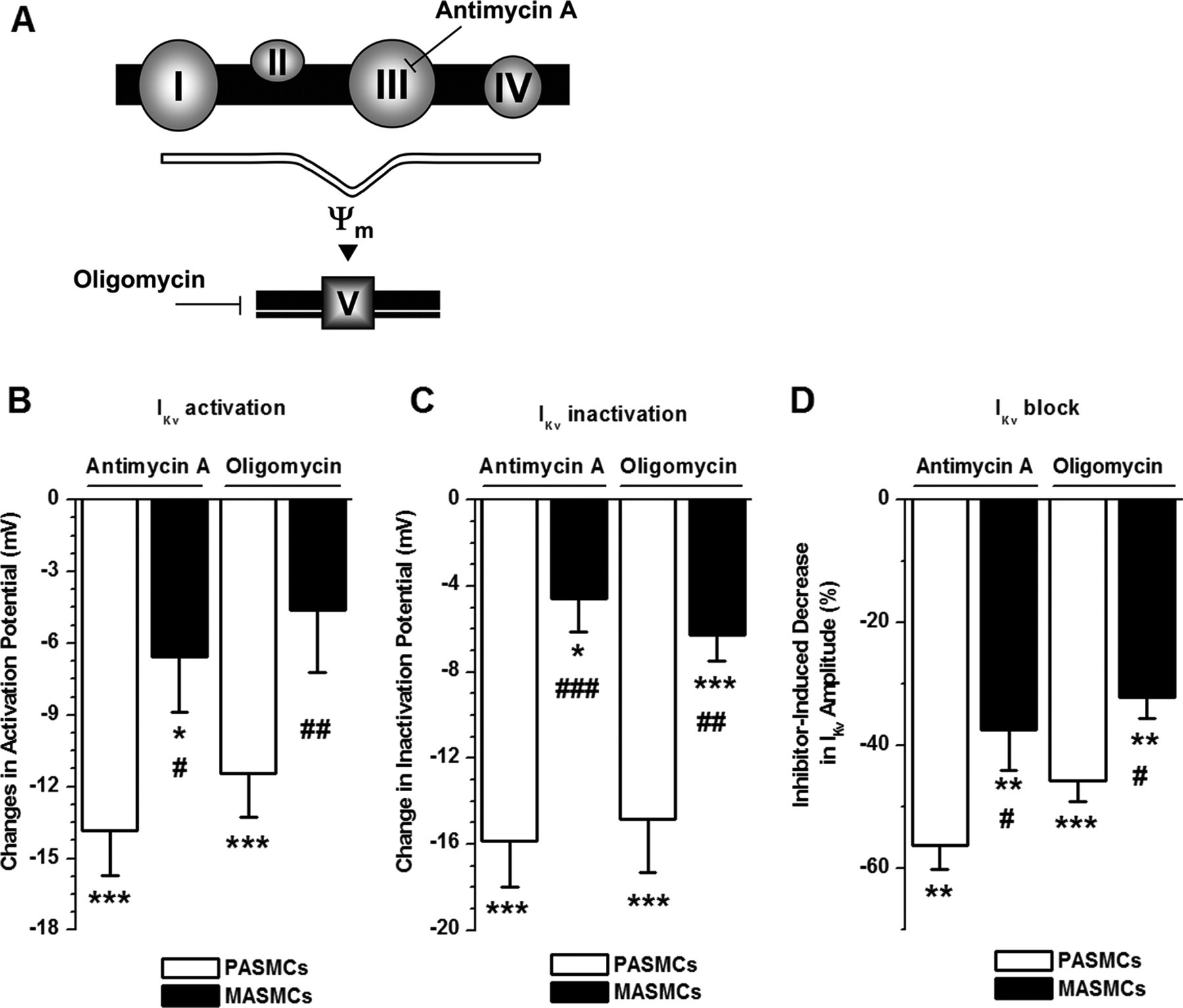
A: diagrammatic representation of sites of action of antimycin A and oligomycin in mitochondrial membrane.
B and C: comparison of antimycin A- and oligomycin-induced changes in half-activation and half-inactivation potentials. Relative changes in half-activation and half-inactivation potential in 8 and 12 (B) and 8 and 11 (C) PASMCs and in 11 and 9 (B) and 9 and 9 (C) MASMCs for antimycin A and oligomycin, respectively, are shown.
D: comparison of decrease in IKv amplitude in the presence of antimycin A or oligomycin for PASMCs (n = 9 and 12, respectively) and MASMCs (n = 11 and 9, respectively). P < 0.05, P < 0.01, P < 0.001, difference in parameters between control condition and presence of an inhibitor by paired statistical test. #P < 0.05, ##P < 0.01, ###P < 0.001, difference between 2 cell types by unpaired statistical test.
"The different sites of action of antimycin A and oligomycin in mitochondria are schematically depicted in Fig. 7A."
"The differences in the mean parameters for IKv activation and inactivation due to mitochondrial inhibition with antimycin A and oligomycin in PASMCs and MASMCs are comprehensively analyzed in Table 2, while Fig. 7, B–D, compares the relative changes in IKv activation, inactivation, and block in PASMCs to those in MASMCs."
"Although both inhibitors caused significant negative shifts in IKv inactivation (Fig. 7C) and significant decreases in IKv amplitude at +50 mV (Fig. 7D) in both cell types, all these effects were significantly smaller in MASMCs than in PASMCs."
"Similarly, antimycin A had a significantly greater effect in PASMCs than in MASMCs (Fig. 7B). Oligomycin, on the other hand, caused significant differences in IKv activation only in PASMCs and not in MASMCs (Fig. 7B and Table 2)."
The figure displays data examining the effects of Antimycin A and Oligomycin on the activation, inactivation, and block of potassium channels ($I_{Kv}$) in two cell types: Pulmonary Artery Smooth Muscle Cells (PASMCs) and Mesenteric Artery Smooth Muscle Cells (MASMCs).
Panel A
This schematic indicates that Antimycin A and Oligomycin inhibit components of the mitochondrial respiratory chain. Antimycin A acts on Complex III, and Oligomycin blocks ATP synthase (Complex V), leading to changes in mitochondrial membrane potential ($\Psi_m$).
Panel B - $I_{Kv}$ Activation
Result: Antimycin A and Oligomycin both decrease the activation potential of $I_{Kv}$ channels, indicated by the negative change in mV for both PASMCs and MASMCs.
Details:
In PASMCs, both treatments lead to a significant drop in activation potential, with Antimycin A showing a stronger effect (larger decrease).
In MASMCs, Oligomycin results in a slightly larger decrease in activation potential compared to Antimycin A.
Significance indicators: Asterisks (*) and hashtags (#) indicate statistically significant changes from baseline (with varying levels of significance).
Panel C - $I_{Kv}$ Inactivation
Result: Both inhibitors affect the inactivation potential of $I_{Kv}$ channels.
Details:
In PASMCs, both Antimycin A and Oligomycin induce a significant decrease in inactivation potential.
MASMCs show a similar trend, although the effects are less pronounced compared to PASMCs.
Multiple asterisks and hashtags denote levels of significance.
Panel D - $I_{Kv}$ Block (Amplitude Reduction)
Result: Antimycin A and Oligomycin significantly reduce the amplitude of $I_{Kv}$ currents, as indicated by the decrease in percentage for both PASMCs and MASMCs.
Details:
PASMCs experience a greater inhibitor-induced decrease in $I_{Kv}$ amplitude compared to MASMCs.
Statistical significance is noted with symbols (e.g., ** for high significance).
Summary
Overall, both Antimycin A and Oligomycin significantly alter the activation, inactivation, and amplitude of $I_{Kv}$ currents in PASMCs and MASMCs, with stronger effects typically observed in PASMCs. The inhibitors impact mitochondrial function, likely leading to downstream effects on potassium channel behavior, which varies between the two cell types.
Figure 8 - Cytoskeletal disruptor cytochalasin B alters mitochondrial distribution and attenuates antimycin A-induced changes in IKv voltage-dependent characteristics in PASMCs
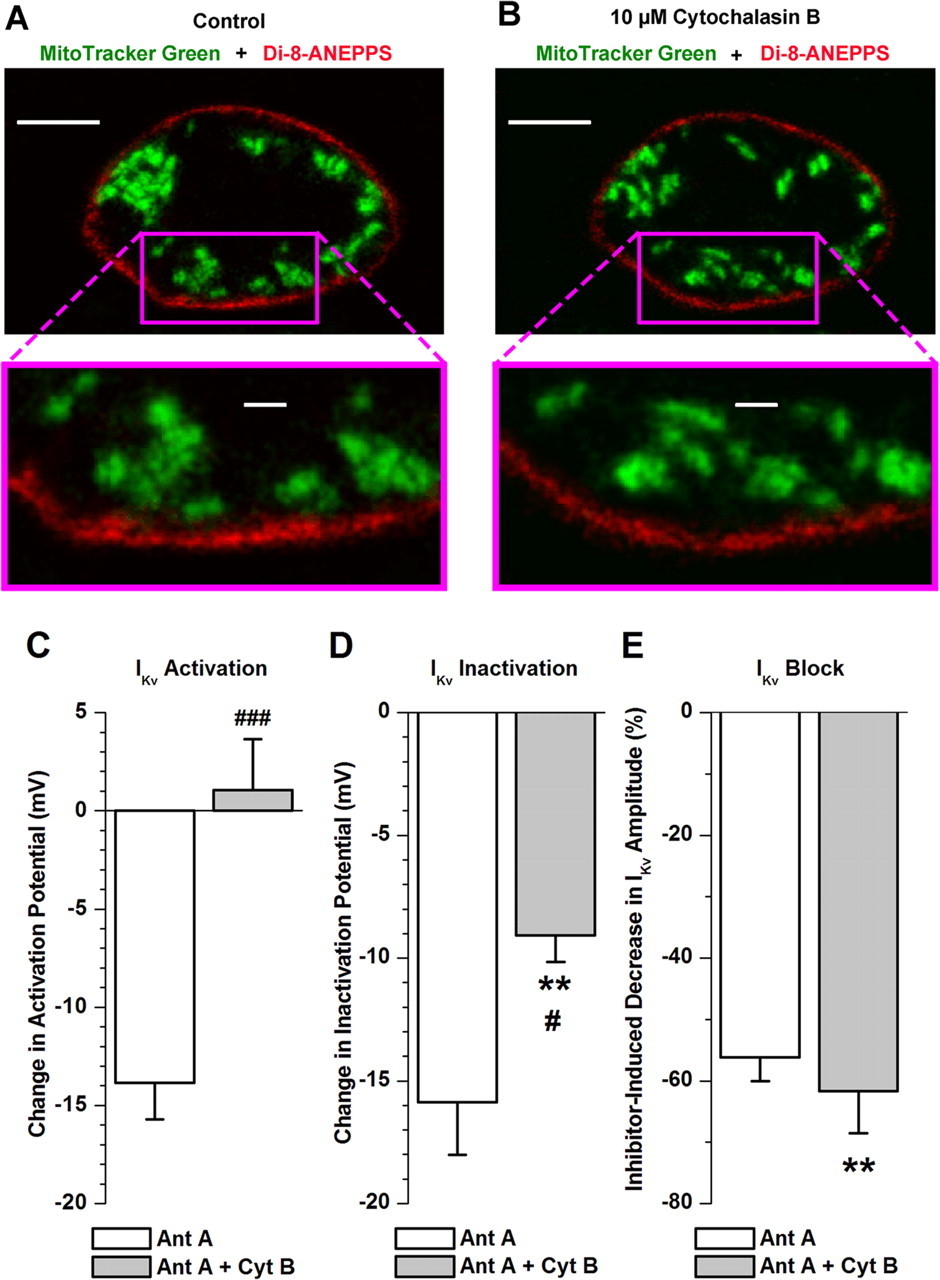
A and B: confocal fluorescence images of PASMC stained with MitoTracker Green FM (green, mitochondria) and di-8-ANEPPS (red, plasma membrane) recorded before (A) and 10 min after (B) incubation with 10 μM cytochalasin B.
Top: images acquired from a confocal optical slice (<0.6 μm) at middle of cell depth. Calibration bars, 5 μm.
Bottom: comparison of changes in distribution of peripheral mitochondria following cell treatment with cytochalasin B at high magnification. Calibration bars, 1 μm.
C–E: comparison of antimycin A (Ant A, 1 μM)-induced changes in steady-state activation (C, n = 5), inactivation (D, n = 7), and block (E, n = 5) of IKv in the absence and presence of 10 μM cytochalasin B (Cyt B). **P < 0.01, difference in parameters between control condition (in this case, presence of cytochalasin B) and in presence of antimycin A by paired statistical test.
#P < 0.05, ###P < 0.001, difference between effects of antimycin A in absence and presence of cytochalasin B by unpaired statistical test.
"Figure 8, A and B, compare distribution of mitochondria (shown in green) in relation to the plasma membrane (stained with di-8-ANEPPS and shown in red) in a single PASMC before and 10 min after incubation with cytochalasin B, respectively."
"As can be seen in Fig. 8, cytochalasin B did not significantly change the cell shape; however, the pattern of green fluorescence across the whole cell was altered, suggesting that the mitochondria are associated with F-actin filaments in PASMCs."
"Importantly, the distribution of mitochondria at the submembrane regions was altered, as can be seen at an increased magnification in Fig. 8, A and B, bottom."
"The above results suggest that if Kv channels in the plasma membrane and peripheral mitochondria are functionally coupled in PASMCs as the electrophysiological evidence indicates (Fig. 7; Ref. 9), then the cell treatment with cytochalasin B should also modulate the effect of the mitochondrial inhibitors on IKv characteristics."
"Importantly, the effect of antimycin A on IKv activation was completely abolished (Fig. 8C) and antimycin A-induced changes in IKv inactivation were significantly attenuated (Fig. 8D) in the presence of cytochalasin B."
"Pretreatment of cells with the cytoskeletal disruptor did not, however, significantly change the antimycin A-mediated inhibition of IKv (Fig. 8E), suggesting that other factors are likely to contribute to the IKv block caused by the mitochondrial inhibitors."
This figure presents the effects of the inhibitor Cytochalasin B (Cyt B) on mitochondrial distribution and Kv (voltage-gated potassium channel) activity. Here's a detailed interpretation of the results shown in panels A-E:
Panels A and B: Mitochondrial Distribution
A (Control): This panel shows cells stained with MitoTracker Green for mitochondria (green) and Di-8-ANEPPS for the plasma membrane (red) under control conditions (no Cyt B). Mitochondria appear as green structures distributed throughout the cell.
B (10 µM Cytochalasin B): After treatment with 10 µM Cyt B, mitochondrial distribution appears altered compared to the control, with mitochondria clustering more visibly in the central areas. This clustering suggests that Cyt B affects mitochondrial positioning, possibly due to disruption in cytoskeletal elements.
Panels C-E: Effects on Kv Channel Properties
C. Kv Activation Potential
Results: Cyt B significantly affects the activation potential of Kv channels. The bar labeled "Ant A + Cyt B" (grey) shows a positive change in activation potential compared to "Ant A" alone (white).
Interpretation: This shift indicates that Cyt B increases the activation threshold, requiring a more positive membrane potential for Kv channels to activate, which may affect cellular excitability.
D. Kv Inactivation Potential
Results: In the presence of Cyt B, there’s a more substantial reduction in inactivation potential (grey bar labeled "Ant A + Cyt B") compared to Ant A alone (white).
Interpretation: This suggests that Cyt B lowers the potential required for Kv channels to inactivate, making them more likely to enter an inactive state, which can alter the cell's ability to recover from depolarization.
E. Kv Block
Results: The inhibitor-induced decrease in Kv amplitude is more significant when both Ant A and Cyt B are present (grey bar).
Interpretation: Cyt B intensifies the blocking effect on Kv channels, further reducing their amplitude. This additional reduction in Kv current could lead to a decrease in cellular repolarization capacity, impacting overall cell function.
Summary
The results suggest that Cytochalasin B disrupts mitochondrial distribution within cells and modifies the electrical properties of Kv channels, including their activation, inactivation, and overall amplitude. These findings highlight the role of Cyt B in altering mitochondrial dynamics and cell excitability, likely through its effects on the cytoskeleton and channel behavior.
Figure 9 - Intracellular Mg2+ is responsible for changes in IKv inactivation induced by mitochondrial inhibitors in PASMCs.
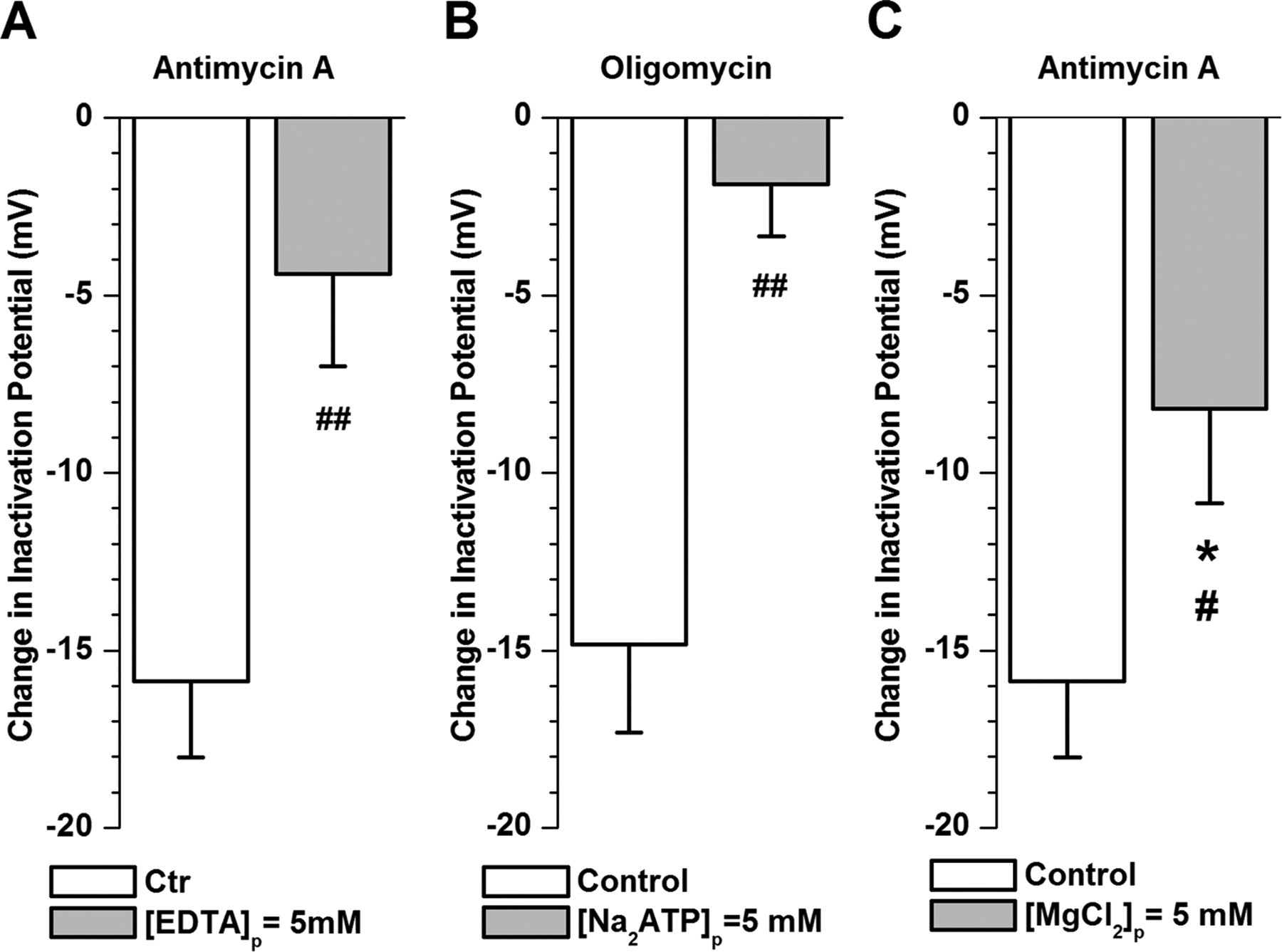
Gray bars demonstrate effect of antimycin A (A and C) and oligomycin (B) (both at 1 μM) investigated in PASMCs dialyzed with pipette solutions containing either 5 mM EDTA (equimolar substitution with EGTA; A, n = 8), 5 mM Na2ATP (B, n = 6), or increased MgCl2 concentration (5 mM instead of 0.5 mM; C, n = 6).
Open columns show changes in half-inactivation potential induced by each mitochondrial inhibitor in cells dialyzed with control pipette solution for comparison.
*P < 0.05, difference in change in half-inactivation parameter between control condition and presence of an inhibitor by paired statistical test.
#P < 0.05, ##P < 0.01, difference between effects of mitochondrial inhibitor in cells dialyzed with control and test pipette solutions by unpaired statistical test.
"Figure 9, A and B, clearly demonstrate that the relative changes in Vh caused by both inhibitors were significantly reduced under these conditions."
"Furthermore, antimycin A-induced changes in Vh were significantly attenuated (Fig. 9C), whereas changes in kh and A remained similar (compare Tables 2 and 4) under these conditions."
"The electrophysiological evidence presented in Fig. 9, demonstrating that addition of Mg²⁺-chelating agents into the pipette solution inhibits changes in Vh caused by antimycin A and oligomycin, clearly suggests that an increase in intracellular concentration of Mg²⁺ plays a key role in the modulation of IKv inactivation by mitochondrial inhibitors."
In this figure, each panel (A, B, C) represents changes in inactivation potential (in mV) under different experimental conditions with specific treatments compared to a control group:
Panel A (Antimycin A): This panel shows a comparison between the control group and a group treated with Antimycin A in the presence of EDTA at a concentration of 5 mM. The change in inactivation potential for the treatment group is less negative compared to the control group, with a significant difference indicated by
##.Panel B (Oligomycin): In this case, the experimental condition involves oligomycin treatment with 5 mM Na₂ATP. Again, the change in inactivation potential is less negative in the treatment group compared to the control, showing statistical significance (
##).Panel C (Antimycin A): This panel examines the effect of Antimycin A in the presence of 5 mM MgCl₂. The results indicate a less negative change in inactivation potential compared to the control, with statistical significance marked by
*and#.Interpretation:
Across all panels, the treatments (Antimycin A and Oligomycin) in the presence of various ions (EDTA, Na₂ATP, MgCl₂) consistently result in a less negative inactivation potential compared to the control. The symbols (
##,*,#) indicate statistical significance, suggesting that these treatments significantly alter inactivation potential under the given conditions.
Table 1 - Comparison of steady-state IKv parameters in MASMCs and PASMCs in control conditions
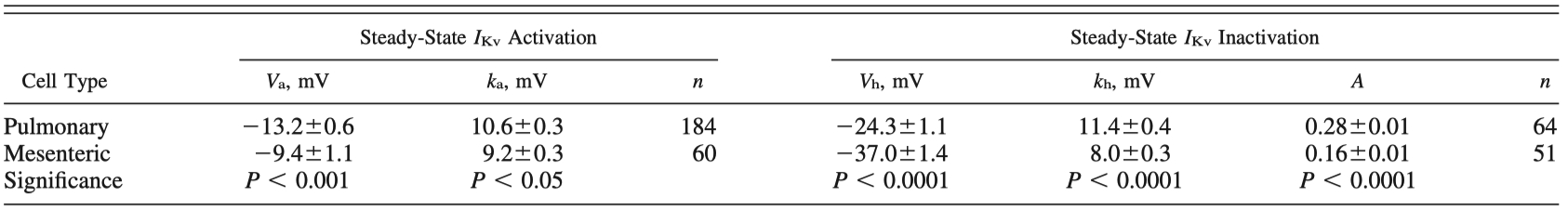
Values are means ± SE for n cells.
Comparison was performed by using all pooled data for voltage-gated K+ channel currents (IKv) recorded in pulmonary arterial smooth muscle cells (PASMCs) or mesenteric arterial smooth muscle cells (MASMCs) bathed in physiological saline solution containing 10 μM glibenclamide and 1 μM paxilline.
Va, half-activation potential; ka, e-fold steepness of activation dependence; Vh, half-inactivation potential; kh, e-fold steepness of inactivation dependence; A, noninactivating current component.
Student's unpaired t-test or nonparametric Mann-Whitney test was used as described in materials and methods.
"These differences in the activation parameters were significant (Table 1)."
"Statistical comparison of these parameters showed that IKv in MASMCs is inactivated at more negative membrane potentials, its dependence on the conditional prepulse potential is steeper, and it has less noninactivated current than IKv in PASMCs; these differences in inactivation characteristics were significant (Table 1)."
Table 2 - Comparison of changes in steady-state IKv characteristic kinetics due to mitochondrial inhibitors antimycin A and oligomycin
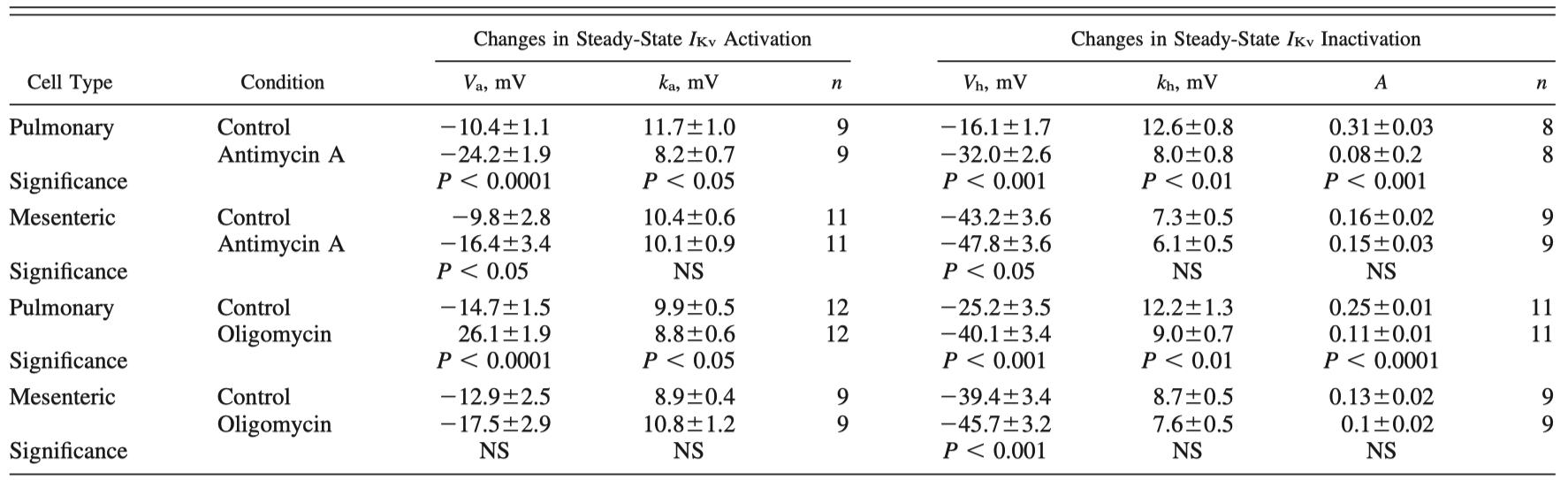
Values are means SE for n cells. Data compare mean IKv parameters measured in control condition and in presence of inhibitor (1 M) in 2 cell types. Student’s paired t-test or Wilcoxon test was used as defined in MATERIALS AND METHODS. NS, not significant
"The differences in the mean parameters for IKv activation and inactivation due to mitochondrial inhibition with antimycin A and oligomycin in PASMCs and MASMCs are comprehensively analyzed in Table 2, while Fig. 7, B–D, compares the relative changes in IKv activation, inactivation, and block in PASMCs to those in MASMCs."
"Similarly, antimycin A had a significantly greater effect in PASMCs than in MASMCs (Fig. 7B). Oligomycin, on the other hand, caused significant differences in IKv activation only in PASMCs and not in MASMCs (Fig. 7B and Table 2)."
"Furthermore, inhibitor-induced changes in other parameters, such as ka, kh, and A, were less pronounced and overall not significantly different in MASMCs from those in PASMCs (Table 2)."
"Furthermore, antimycin A-induced changes in Vh were significantly attenuated (Fig. 9C), whereas changes in kh and A remained similar (compare Tables 2 and 4) under these conditions."
Table 3 - Effect of cytoskeletal disrupter cytochalasin B on antimycin A-induced changes in steady-state IKv characteristic kinetics in PASMCs
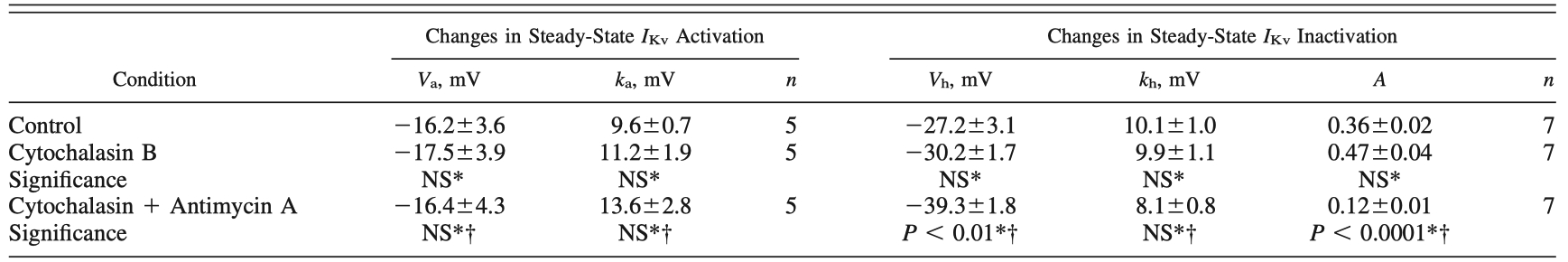
Values are means SE for n cells. *Data compare mean I Kv parameters measured in control condition and in presence of either cytoskeletal disrupter cytochalasin B (10 M) alone or cytochalasin B + mitochondrial inhibitor antimycin A (1 M) measured in the same PASMCs.
†Data compare mean IKv parameters measured in presence of cytochalasin B alone and after addition of mitochondrial inhibitor antimycin A (1 M). Student’s paired t-test or Wilcoxon test was used as described in MATERIALS AND METHODS.
"Cytochalasin B caused a decrease in IKv amplitude by 35.7 ± 6.1% (n = 5, P < 0.01) but itself did not have any significant effect on the steady-state activation or inactivation parameters of IKv (Table 3)."
Table 4 - Role of intracellular Mg2+ in antimycin A- and oligomycin-induced changes in IKv inactivation in PASMCs
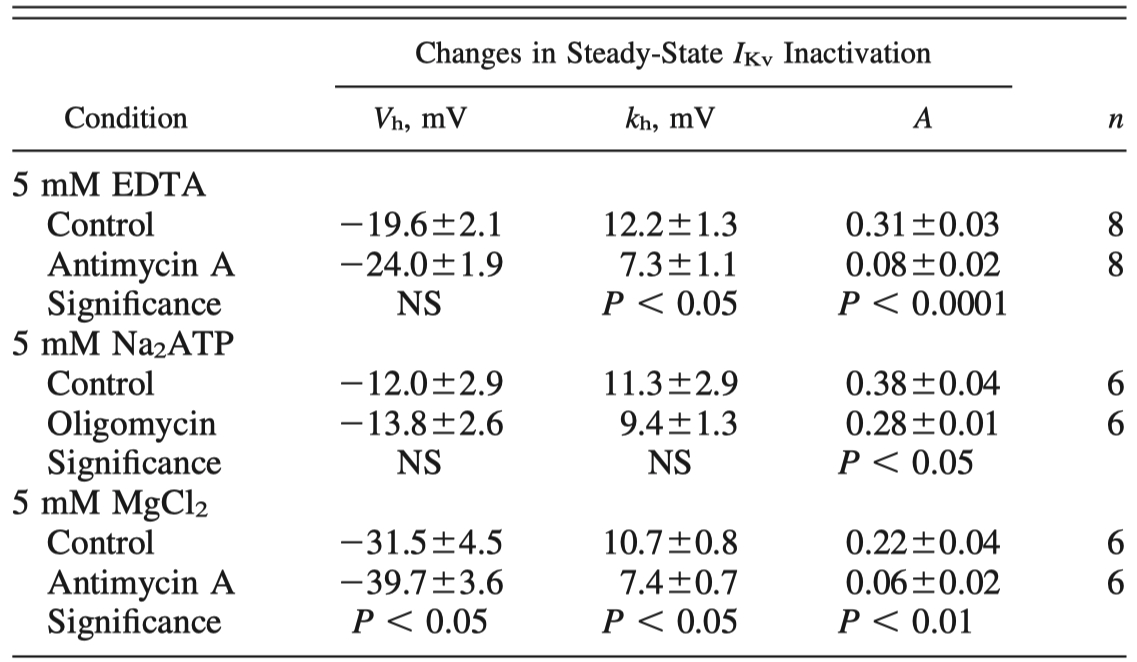
Values are means SE for n cells. Data compare mean IKv inactivation parameters measured in absence (control) and in presence of mitochondrial inhibitor antimycin A or oligomycin (both at 1 M) measured in the same PASMCs in different pipette solutions.
In EDTA-containing solution, 5 mM EGTA was replaced with 5 mM EDTA.
Student’s paired t-test or Wilcoxon test was used as described in MATERIALS AND METHODS.
"Furthermore, antimycin A-induced changes in Vh were significantly attenuated (Fig. 9C), whereas changes in kh and A remained similar (compare Tables 2 and 4) under these conditions."
"These two mETC inhibitors therefore were chosen to verify whether functional coupling between mitochondria and Kv channels in PASMCs is greater than in MASMCs, as would be predicted based on the difference in the distribution of mitochondria in the two cell types as demonstrated in Figs. 1–4."
"The above results suggest that if Kv channels in the plasma membrane and peripheral mitochondria are functionally coupled in PASMCs as the electrophysiological evidence indicates (Fig. 7; Ref. 9), then the cell treatment with cytochalasin B should also modulate the effect of the mitochondrial inhibitors on IKv characteristics."
Abstract
Mitochondria are proposed to be a major oxygen sensor in hypoxic pulmonary vasoconstriction (HPV), a unique response of the pulmonary circulation to low oxygen tension. Mitochondrial factors including reactive oxygen species, cytochrome c, ATP, and magnesium are potent modulators of voltage-gated K+ (Kv) channels in the plasmalemmal membrane of pulmonary arterial (PA) smooth muscle cells (PASMCs). Mitochondria have also been found close to the plasmalemmal membrane in rabbit main PA smooth muscle sections. Therefore, we hypothesized that differences in mitochondria localization in rat PASMCs and systemic mesenteric arterial smooth muscle cells (MASMCs) may contribute to the divergent oxygen sensitivity in the two different circulations. Cellular localization of mitochondria was compared with immunofluorescent labeling, and differences in functional coupling between mitochondria and Kv channels was evaluated with the patch-clamp technique and specific mitochondrial inhibitors antimycin A (acting at complex III of the mitochondrial electron transport chain) and oligomycin A (which inhibits the ATP synthase). It was found that mitochondria were located significantly closer to the plasmalemmal membrane in PASMCs compared with MASMCs. Consistent with these findings, the effects of the mitochondrial inhibitors on Kv current (IKv) were significantly more potent in PASMCs than in MASMCs. The cytoskeletal disruptor cytochalasin B (10 μM) also altered mitochondrial distribution in PASMCs and significantly attenuated the effect of antimycin A on the voltage-dependent parameters of IKv. These findings suggest a greater structural and functional coupling between mitochondria and Kv channels specifically in PASMCs, which could contribute to the regulation of PA excitability in HPV.
the pulmonary circulation represents a vascular bed with unique properties. Under physiological conditions this vasculature is a highly oxygenated, low-pressure system. When oxygen tension drops during, for example, chronic obstructive pulmonary disease, a response known as hypoxic pulmonary vasoconstriction (HPV) occurs, diverting the blood flow to the higher oxygenated areas to match ventilation to perfusion. The mechanisms involved in this response are complex and currently incompletely understood (14, 48, 52). Such responses do not occur in the systemic circulation, which dilates when oxygen tension is reduced to maximize blood supply to all parts of the body.
Smooth muscle cell contraction is critical to vasoconstriction; however, to date no definitive mechanism can account for how these cells sense changes in oxygen tension or how increased contractility occurs. The response is considered to be a complex, multifactorial phenomenon ultimately contributing to elevated intracellular Ca2+ concentration ([Ca2+]i), essential for formation of the calcium-calmodulin complex and activation of the myosin light chain kinase. Hypoxic inhibition of voltage-gated K+ (Kv) channels, which results in membrane depolarization and voltage-dependent Ca2+ influx in pulmonary arterial (PA) smooth muscle cells (PASMCs), was originally suggested as one of the important factors leading to increased [Ca2+]i during HPV (3, 36, 47, 51). Currently, mitochondria, the main consumers of cellular oxygen during respiration, are considered to be one of the putative oxygen sensors in pulmonary smooth muscle cells (18, 22, 50, 53), and it has been postulated that Kv channels may be regulated by an effector of mitochondrial origin (3, 22). This idea is supported by the observations that 1) inhibitors of the mitochondrial electron transport chain (mETC) such as rotenone and antimycin A, acting at complexes I and III, respectively, and the mitochondrial uncoupler FCCP mimic the effect of hypoxia on Kv current (IKv) in PASMCs (3, 57) and ductus arteriosus (23) and 2) mice lacking the active gp91phox subunit of the membrane-bound NADPH oxidase preserve the effect of hypoxia on IKv in PASMCs (5). Numerous products of mitochondrial metabolism have also been shown to activate Kv channels, including cytochrome c (34) and hydrogen peroxide in pulmonary and coronary arterial smooth muscle cells (39, 40), while reducing agents inhibit Kv channels (27, 28, 38). In addition, we recently reported (9) that that the voltage-dependent characteristics of IKv in PASMCs are potently modulated via a mitochondrion-mediated Mg2+-dependent mechanism.
Although products of mitochondrial metabolism are currently established to have effects on Kv channels, the exact reasons why these mediators and channels existing in all circulatory beds should behave differently in the pulmonary circulation in response to hypoxia remain unknown. One possibility could be functional differences of the mitochondria present in different vascular beds (22, 37). Another possibility could be that the intracellular locality of mitochondria in relation to other organelles and the cellular membrane may vary in the different vascular beds. Using electron microscopy imaging, Vallières et al. (46) demonstrated the presence of mitochondria close to the plasmalemmal membrane in rabbit main PA smooth muscle sections. Such close proximity between mitochondria and the plasmalemmal membrane, if it specifically exists in PASMCs, could be responsible for the opportunistic detection of a mitochondrion-dependent mediator by Kv channels.
No systematic comparison of mitochondrial distribution in smooth muscle cells (SMCs) isolated from resistance PAs and systemic arteries has yet been performed. Therefore, the main aim of this study was to investigate the differences in cellular localization of mitochondria in PASMCs and mesenteric arterial SMCs (MASMCs, as an example of systemic circulation) with fluorescence confocal imaging. The functional differences between the inhibition of mitochondria and IKv characteristics in both cell types were investigated with the patch-clamp technique using the inhibitor of complex III of the mETC antimycin A and the inhibitor of the mitochondrial F0/FI ATP synthase oligomycin. These inhibitors were chosen because they have a most pronounced effect on IKv in rat PASMCs
Materials and Methods
Materials.
All standard chemicals were purchased from BDH Merck or Fisher. Collagenase type XI and type P were obtained from Sigma and Roche, respectively. Papain, dithiothreitol, mETC inhibitor antimycin A (a mixture of antimycins from Streptomyces species), and ATP synthase inhibitor oligomycin were all obtained from Sigma; cytochalasin B was purchased from Calbiochem. Brefeldin A BODIPY 558/568, MitoTracker Green FM, di-8-ANEPPS, and BAPTA-AM were purchased from Invitrogen. Cell isolation.
Male Wistar rats (225–300 g) were killed by cervical dislocation as approved by the local United Kingdom Home Office inspector, in accordance with Schedule 1 as prescribed and approved by the Home Office and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Pub. No. 85-23, revised 1996). Small intrapulmonary arteries (3rd–5th order) were microdissected. Isolation of PASMCs (using 1 mg/ml type XI collagenase, 0.5 mg/ml papain, and 1 mM dithiothreitol and 20 min incubation at 37°C) were performed as previously described (43). SMCs from small mesenteric arteries (MA) (3rd–4th order) (MASMCs) were isolated in a similar manner except that 2 mg/ml collagenase and 1 mg/ml papain were used and the tissue incubation in the enzyme solution was increased to 30 min. Confocal microscopy.
Small aliquots of the suspension of freshly isolated PASMCs or MASMCs were transferred to the experimental chambers and diluted with a saline buffer composed of (mM) 120 NaCl, 6 KCl, 0.5 CaCl2, 1.2 MgCl2, 12 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH. The sarcoplasmic reticulum (SR) in the SMCs was stained by 30-min incubation of the myocytes with 2 μM brefeldin A BODIPY 558/568 [absorbance (Abs)/emission (Em) = 559 nm/568 nm], which was previously demonstrated to stain the SR in rat gastric myocytes (54). The validity of this staining was further confirmed in myocytes in which intracellular calcium stores were visualized with the low-affinity [kd(Ca) = 42 μM] fluorescent Ca2+ indicator fluo-3FF (12). This approach was demonstrated previously to reveal a well-developed subplasmalemmal SR network in both vascular (11) and visceral (12) SMCs.
Mitochondria were stained by 30-min incubation of SMCs with 1 μM MitoTracker Green FM (Abs/Em = 490 nm/516 nm) (24). The plasmalemmal membrane was labeled by incubating the cells for 3 min with 10 μM di-8-ANEPPS (Abs/Em = 488 nm/605 nm) (41). An experimental chamber containing cells was placed on the stage of an Axiovert 100 M inverted microscope attached to a LSM 510 laser-scanning unit (Zeiss, Oberkochen, Germany). Confocal imaging was performed with a Zeiss plan-Apochromat 40× 1.3 numerical aperture oil-immersion objective. The SCSi interface of the confocal microscope was hosted by a Pentium PC (32-bit Windows NT 4.0 operating system) running LSM510 software (Zeiss). To visualize the three-dimensional distribution of the SR and mitochondria in the cell, z-sectioning (series of 40 x-y images taken from a confocal optical section <0.6 μm and with a z-step of 0.3 μm) was performed. Brefeldin A BODIPY fluorescence was excited by the 488-nm line of a 200-mw argon ion laser (Laser-Fertigung, Hamburg, Germany), and the emitted fluorescence was detected with 505- to 550-nm band-pass emission filter. MitoTracker Green fluorescence was excited by the 543-nm line of a 5-mw HeNe ion laser (Laser-Fertigung), and the emitted fluorescence signal was captured at wavelengths >560 nm. Di-8-ANEPPS fluorescence was excited by the 458-nm line of a 200-mw argon ion laser, and the emitted fluorescence was captured at wavelengths >585 nm. For all desired laser lines the illumination intensity was set with an acoustooptical tunable filter. Averaging of two frames was used to reduce noise. The gains were such that the red and green fluorescent signals were approximately of the same intensity but below the saturation of the photomultiplier. To avoid any bleed-through, imaging was performed with the multitrack mode of an LSM 510 (sequential capturing of images with 2 separate photomultipliers). A threshold was set to remove subsignal noise from the images. The adequacy of the imaging protocol applied to the double-labeled myocytes was confirmed by control experiments on the single-labeled cells. It is worth noting that, although the z-sectioning was performed through the entire cell volume, the distribution of the SR and mitochondria was analyzed only in x-y images taken from the half of each SMC located closer to the objective lens. This shortens the length of the optical path and thus minimizes erroneous measurements caused by waveguide dispersion of light, which occurs when the wave propagates through any inhomogeneous structure such as a cell.
Electrophysiology.
Cells were placed in a chamber with a volume of 100–200 μl and continually superfused (∼1 ml/min) with a physiological saline solution (PSS) or a test solution via a five-barrel pipette at room temperature. PSS contained (mM) 140 NaCl, 5 KCl, 1.5 CaCl2, 1.2 MgCl2, 10 HEPES, and 10 glucose, pH = 7.2. Control pipette solution contained (mM) 140 KCl, 0.5 MgCl2, 10 HEPES, 10 EGTA, and 0.5 CaCl2, pH = 7.2, and was used for recording unless otherwise stated. Cells were dialyzed with pipette solution for 5 min before currents were recorded. The effects of inhibitors were studied a minimum of 5 min after addition. Paxilline (1 μM) and glibenclamide (10 μM) to block Ca2+-activated and ATP-sensitive K+ currents, respectively, were present in all extracellular recording solutions, isolating the voltage-dependent K+ channels from other K+ conductances as previously described (43). To evaluate cell size, whole cell capacitance transient currents were measured with a 10-mV hyperpolarizing step after stable whole cell access was achieved in each cell. Cell membrane capacitance (Cm) was then calculated as an area under a capacitance transient and expressed in picofarads.
IKv amplitude was measured with a 200-ms voltage step applied from the holding potential of −80 mV to membrane potentials between −100 and +50 mV in 10-mV increments with a frequency of 0.1 Hz. After membrane depolarization cells were repolarized to −20 (PASMCs) or 0 (MASMCs) mV for 160 ms to measure tail currents, which were used to assess changes in the steady-state activation of IKv. A more positive step potential in MASMCs was required to increase the size of tail currents because of the significantly smaller current amplitude in these cells. Current-voltage (I-V) curves were constructed from the tail current measures 2–3 ms after repolarization and fitted with the following equation: where Va and ka are the half-activation potential and the e-fold steepness of the activation dependence (the slope factor of activation), respectively, and Vm is membrane potential. IKv block was calculated as a percentage of the current at +50 mV in control conditions from the same cell.
IKv inactivation was measured with a two-pulse protocol consisting of a 10-s conditioning pulse stepping in 10-mV increments from −100 to +40 mV, followed by a test potential to +60 mV for 200 ms. Current at the end of the test pulse was measured, normalized to the maximal current recorded, and plotted versus conditioning potential. The resulting I-V curve was fitted to the following equation: where Vh and kh are the half inactivation potential and the e-fold steepness of the inactivation dependence (the slope factor of inactivation), respectively, and A denotes the noninactivating component of the current.
Data analysis and statistics.
Data were analyzed and presented with pCLAMP 8 (Axon Instruments), Microsoft Excel, and Microcal Origin 6.0 software. Data are expressed as means ± SE. Statistical comparisons were performed with a paired or unpaired two-tailed t-test, with P < 0.05 deemed significant unless stated otherwise. When normality test failed (Shapiro-Wilk test) the Wilcoxon signed rank test and the Mann-Whitney test were used for comparison of paired and unpaired data, respectively. Paired statistical tests were used to compare the difference in parameters obtained in the control condition and in the presence of mitochondrial inhibitor in the same cell, whereas unpaired tests were used to compare differences between two groups of cells (e.g., PASMCs vs. MASMCs).
Results
Differences in spatial distribution of mitochondria in PASMCs and MASMCs.
The distribution of mitochondria [stained with MitoTracker Green FM (24)] in relation to the SR [stained with brefeldin A BODIPY 558/568 (54)] was compared in SMCs isolated from rat small PAs and MAs. The association between mitochondria and SR elements was assessed with the confocal z-sectioning protocol described in materials and methods. This approach revealed that in PASMCs, on movement from the cell bottom toward the middle of the myocyte depth (Fig. 1A), the fluorescent signal from MitoTracker Green appeared in the x-y confocal optical slices (Fig. 1C) before that of brefeldin A BODIPY (Fig. 1B). In PASMCs, in individual x-z and y-z optical cross sections of the myocyte MitoTracker Green fluorescence (mitochondria) was detected distinctly closer to the cell periphery than that of brefeldin A BODIPY (SR) (Fig. 1, D and E). In MASMCs, however, brefeldin A BODIPY fluorescence staining the SR appeared before or simultaneously with MitoTracker Green fluorescence (Fig. 2B). Such opposing juxtaposition between the SR and mitochondria in MASMCs compared with PASMCs becomes more evident from the comparison of individual x-z and y-z optical cross sections of the myocyte MitoTracker Green (mitochondria) and brefeldin A BODIPY (SR) fluorescent signals (Fig. 2, C and D).
To quantify these differences, the total area occupied by pixels showing MitoTracker Green and brefeldin A BODIPY fluorescence was analyzed in four PASMC and MASMCs and plotted against the optical slice number in Fig. 3. This analysis clearly shows the existence of an area close to the surface of PASMCs that is enriched with mitochondria (proportional to the area with MitoTracker Green fluorescence), while in MASMCs a similar area is predominantly occupied with the SR (proportional to the area with brefeldin A BODIPY fluorescence). Figure 3 also shows diagrammatic representations of these areas, comparing PASMCs with MASMCs.
The existence of the subplasmalemmal pool of mitochondria in PASMCs, but not in MASMCs, was further studied by double staining of both types of cells with MitoTracker Green and di-8-ANEPPS, selective fluorescent probes for mitochondria and the plasmalemmal membrane, respectively (Fig. 4). Figure 4A shows x-y confocal images taken though the middle of a representative PASMC. As can be seen clearly in Fig. 4A, MitoTracker Green fluorescence often appeared to be “fused” (within ∼200 nm, the limit of lateral resolution of our confocal microscope) with di-8-ANEPPS fluorescence shown in red (plasmalemma). Conversely, in MASMCs, a clear gap between the two fluorescent signals was observed (Fig. 4B). To quantitatively compare relative distances between mitochondria and the plasma membrane in the two cell types, the spatial profiles of fluorescent signals recorded for di-8-ANEPPS (red) and MitoTracker Green (green) were constructed across the shortest distance between the center of each mitochondrion and the plasma membrane (shown by yellow lines in Fig. 4, C and D, for PASMC and MASMC, respectively). Histograms of distribution of mitochondria according to their distance from the plasma membrane measured in six PASMCs and six MASMCs are compared in Fig. 4E. The histogram bin size was set to 0.5 μm, which is 2.5-fold greater than the lateral optical resolution of our imaging system determined with 0.2-μm fluorescent beads. This analysis clearly shows that in PASMCs the majority of peripheral mitochondria are localized between 0.5 and 1.5 μm from the plasma membrane, compared with ∼1.5–3.5 μm in MASMCs. On average, the mean distance between peripheral mitochondria and the plasma membrane calculated as described above was twofold greater in MASMCs (2.4 ± 0.05 μm, n = 405) than in PASMCs (1.2 ± 0.05 μm, n = 235) (P < 0.0001; Fig. 4F).
Comparison of IKv characteristics in PASMCs and MASMCs.
In view of the locality of the mitochondria to the plasma membrane demonstrated above, it could be postulated that the mitochondrion-mediated effects on Kv channels that occur in PASMCs may exist in MASMCs but would be less effective. Consequently, IKv was isolated pharmacologically (as described in materials and methods), and its electrophysiological properties were compared in PASMCs and MASMCs under identical experimental conditions.
With the IKv activation protocol featured in Fig. 5A, whole cell IKv was recorded in both PASMCs and MASMCs. The amplitude of the current recorded from a representative MASMC (Fig. 5C) was considerably smaller than in a PASMC (Fig. 5B). On average, the differences in IKv amplitude were significantly different between −40 and +50 mV. For example, at 0 and +50 mV, the mean amplitude of IKv was equal to 69 ± 5 and 270 ± 17 pA (n = 60), respectively, in MASMCs and 446 ± 20 and 1,369 ± 59 pA (n = 184), respectively, in PASMCs (P < 0.0001). On the other hand, the size of MASMCs, calculated from Cm, was significantly larger than that of PASMCs [16.3 ± 0.6 pF (n = 60) vs. 11.1 ± 0.3 pF (n = 184); P < 0.0001]. The smaller IKv and the larger cell size in MASMCs result in significant differences in the whole cell current density in the two cell types (Fig. 5D), which was 10- to 18-fold greater between −30 and 0 mV and 8- to 9-fold greater between 0 and +50 mV in PASMCs compared with MASMCs.
Comparison of the steady-state activation of IKv measured from the normalized I-V curves (which were derived from the IKv tail current measurements) from the representative PASMC and MASMC shown in Fig. 5, B and C, indicates that IKv activation in MASMC was shifted by ∼6 mV to more positive membrane voltages and was steeper than in PASMC (Fig. 5E). These differences in the activation parameters were significant (Table 1).
Comparison of IKv inactivation measured with the voltage protocol shown in Fig. 6A from the representative PASMC (Fig. 6B) and MASMC (Fig. 6C) demonstrates that IKv inactivation in PASMCs is relatively slow and incomplete compared with that in MASMCs. The normalized IKv recorded during the test pulse was plotted against the prepulse potential, and resulting dependencies were fitted to the equation described in materials and methods, yielding the parameters of IKv inactivation, the half-inactivation potential Vh, the slope factor kh, and the noninactivating component A (Fig. 6D). Statistical comparison of these parameters showed that IKv in MASMCs is inactivated at more negative membrane potentials, its dependence on the conditional prepulse potential is steeper, and it has less noninactivated current than IKv in PASMCs; these differences in inactivation characteristics were significant (Table 1).
Therefore, direct comparison of the steady-state activation and inactivation of IKv measured under identical experimental conditions in both cell types clearly demonstrates that PASMCs have significantly greater current amplitude and more available and less inactivated Kv channels in the negative range of membrane potentials compared with MASMCs.
Mitochondrial inhibition regulates IKv more potently in PASMCs.
Inhibition of the mETC was previously shown to inhibit IKv in PASMCs (3, 23, 57). We recently showed (9) that steady-state activation and IKv block were significantly affected by mitochondrial inhibition in PASMCs, with the most pronounced effects observed for antimycin A (inhibiting the mETC at complex III) and oligomycin (blocking the ATP synthase). These two mETC inhibitors therefore were chosen to verify whether functional coupling between mitochondria and Kv channels in PASMCs is greater than in MASMCs, as would be predicted based on the difference in the distribution of mitochondria in the two cell types as demonstrated in Figs. 1–4.
The different sites of action of antimycin A and oligomycin in mitochondria are schematically depicted in Fig. 7A. The differences in the mean parameters for IKv activation and inactivation due to mitochondrial inhibition with antimycin A and oligomycin in PASMCs and MASMCs are comprehensively analyzed in Table 2, while Fig. 7,B–D, compares the relative changes in IKv activation, inactivation, and block in PASMCs to those in MASMCs. Although both inhibitors caused significant negative shifts in IKv inactivation (Fig. 7C) and significant decreases in IKv amplitude at +50 mV (Fig. 7D) in both cell types, all these effects were significantly smaller in MASMCs than in PASMCs (Fig. 7, B–D). Similarly, antimycin A had a significantly greater effect in PASMCs than in MASMCs (Fig. 7B). Oligomycin, on the other hand, caused significant differences in IKv activation only in PASMCs and not in MASMCs (Fig. 7B and Table 2). Furthermore, inhibitor-induced changes in other parameters, such as ka, kh, and A, were less pronounced and overall not significantly different in MASMCs from those in PASMCs (Table 2). Thus differences in potency of the effects of the two different mitochondrial inhibitors in two cell types correlate with the difference in mitochondrial distribution described above.
Disruption of cytoskeleton with cytochalasin B alters both peripheral mitochondria distribution and antimycin A-dependent modulation of IKv.
A link between mitochondria and the cytoskeleton has been demonstrated previously in cardiomyocytes (2), neurons (25), human coronary arterioles (19), and pulmonary endothelial cells (1) but not in PASMCs. If the cytoskeleton is involved in juxtaposition of mitochondria and the plasma membrane in PASMCs, then disruption of the cytoskeleton should alter the association between mitochondria and the plasma membrane and at the same time attenuate the effect of the mitochondrial inhibitors on IKv. The effect of treatment of PASMCs with 10 μM cytochalasin B, a disrupter of F-actin microfilaments (1, 17, 19), on distribution of mitochondria was visualized with MitoTracker Green and confocal imaging. Figure 8, A and B, compare distribution of mitochondria (shown in green) in relation to the plasma membrane (stained with di-8-ANEPPS and shown in red) in a single PASMC before and 10 min after incubation with cytochalasin B, respectively. To reduce possible errors resulting from changes in the cell shape due to a disruption of the cytoskeleton, partially contracted PASMCs were used in these experiments. As can be seen in Fig. 8, cytochalasin B did not significantly change the cell shape; however, the pattern of green fluorescence across the whole cell was altered, suggesting that the mitochondria are associated with F-actin filaments in PASMCs. Importantly, the distribution of mitochondria at the submembrane regions was altered, as can be seen at an increased magnification in Fig. 8, A and B, bottom. Similar effects were observed in the other eight PASMCs studied.
The above results suggest that if Kv channels in the plasma membrane and peripheral mitochondria are functionally coupled in PASMCs as the electrophysiological evidence indicates (Fig. 7; Ref. 9), then the cell treatment with cytochalasin B should also modulate the effect of the mitochondrial inhibitors on IKv characteristics. To test this prediction, the effect of 10 μM cytochalasin B on antimycin A-induced changes in IKv activation, inactivation, and block was assessed. Cytochalasin B caused a decrease in IKv amplitude by 35.7 ± 6.1% (n = 5, P < 0.01) but itself did not have any significant effect on the steady-state activation or inactivation parameters of IKv (Table 3). Importantly, the effect of antimycin A on IKv activation was completely abolished (Fig. 8C) and antimycin A-induced changes in IKv inactivation were significantly attenuated (Fig. 8D) in the presence of cytochalasin B. Thus the ability of cytochalasin B to inhibit the antimycin A-induced changes in the voltage-dependent IKv characteristics correlates well with cytochalasin B-mediated changes in mitochondrial distribution in the vicinity of the plasma membrane, providing direct evidence for close functional coupling between Kv channels and peripheral mitochondria in PASMCs. Pretreatment of cells with the cytoskeletal disruptor did not, however, significantly change the antimycin A-mediated inhibition of IKv (Fig. 8E), suggesting that other factors are likely to contribute to the IKv block caused by the mitochondrial inhibitors.
Intracellular Mg2+ is involved in antimycin A-mediated changes in IKv inactivation.
We recently demonstrated (9) that dialysis of PASMCs with Na2ATP and EDTA, which are more potent chelators of Mg2+ than Ca2+, compared with EGTA present in the pipette solution, significantly attenuated the effects of the mitochondrial inhibitors on IKv activation and IKv block. Considering that the effect of the mitochondrial inhibitors on the steady-state inactivation of IKv has not been studied previously, it is important to demonstrate whether changes in IKv inactivation induced by the mitochondrial inhibitors are also mediated by intracellular Mg2+. To test this possibility, the effect of antimycin A and oligomycin was studied in cells dialyzed with pipette solutions containing 5 mM EDTA or 5 mM Na2ATP, respectively. Figure 9, A and B, clearly demonstrate that the relative changes in Vh caused by both inhibitors were significantly reduced under these conditions.
We previously found (9) that cell dialysis with increased Mg2+ concentration also mimicked the effects of the mitochondrial inhibitors on IKv activation and block. When the steady-state inactivation of IKv was measured in cells dialyzed with 5 mM instead of 0.5 mM MgCl2 in the pipette solution but in the absence of mitochondrial inhibitors, a significant leftward shift by ∼7 mV in the inactivation dependence was observed. The mean Vh was changed from −23.9 ± 1.1 mV for 0.5 mM MgCl2 (n = 73) to −30.7 ± 2.8 mV for 5 mM MgCl2 (n = 12, P < 0.026). The noninactivating component A of IKv was also decreased under this condition, from 0.28 ± 0.01 (0.5 mM) to 0.21 ± 0.02 (5 mM) (P < 0.03). No significant changes in kh were observed [11.2 ± 0.4 (n = 72) in 0.5 mM MgCl2 vs. 10.4 ± 0.5 mV (n = 12) in 5 mM MgCl2]. Furthermore, antimycin A-induced changes in Vh were significantly attenuated (Fig. 9C), whereas changes in kh and A remained similar (compare Tables 2 and 4) under these conditions.
Discussion
Subplasmalemmal localization of mitochondria provides structural basis for functional interaction with Kv channels in PASMCs.
Although the amount of intracellular diffusible mediators in close vicinity to the Kv channel cannot be precisely quantified, the overall local cellular changes could be substantial, providing that diffusion processes in the vicinity of the Kv channels are limited. The existence of a submembrane compartment formed by the SR and called the “superficial barrier” has been previously proposed in systemic vascular SMCs [recently reviewed by Poburko et al. (35)]. Indeed, a tight juxtaposition between the plasmalemmal membrane and the SR has been directly visualized in vascular (11) and visceral (12) SMCs. In myocytes isolated from intralobar rat PAs, we previously demonstrated (42) the presence of a restricted diffusion space by measuring the changes in the K+ gradient during the development of IKv. This phenomenon has not been reported in other vascular SMCs. Comparison of the relative distribution of mitochondria and the SR in PASMCs and MASMCs clearly demonstrates the presence of a distinctive population of mitochondria close to the plasmalemmal membrane and relatively separate from the SR in PASMCs but not in MASMCs, where mitochondria are more closely associated with the SR (Figs. 1–4). This novel and important observation demonstrates the presence of a subplasmalemmal pool of mitochondria in PASMCs that are potentially functionally associated with the Kv channels, as our electrophysiological evidence shows. Such compartmentalization, similar to that formed by the SR in other types of vascular SMCs (35), could be primarily responsible for the lack of sensitivity of IKv to hypoxia in mesenteric arteries previously reported by others (31, 56, 58). Notably, close association between the plasma membrane and mitochondrion has previously been shown by electron microscopy (46). Although the full physiological significance of the existence of the distinctive subplasmalemmal population of mitochondria in PASMCs remains to be established, it is possible that a close juxtaposition of the plasma membrane and mitochondria in PASMCs might be important in polarization of mitochondrion-mediated signals [e.g., reactive oxygen species (ROS) release] in a similar manner to the “mitochondrial belt” involvement in cytosolic Ca2+ signal polarization in pancreatic acinar cells (45).
The ability of the F-actin filament disrupter cytochalasin B to alter mitochondrial distribution in PASMCs suggests that the cytoskeleton contributes to cellular organization of mitochondria in PASMCs. Whether mitochondria are similarly associated with the cytoskeleton in MASMCs remains to be established. Interestingly, inhibition of F-actin filament polymerization with cytochalasin suppressed mitochondrial ROS production induced by stretch in bovine pulmonary endothelial cells (1) and by flow in human coronary arterioles (19). Whether the cytoskeleton is involved in the generation of ROS in PASMCs has not been yet investigated. Our data clearly suggest that in PASMCs mitochondria are spatially arranged in the distinctive layer separate from the SR, forming a compartment with restricted diffusion in the subplasmalemmal space, thus providing a structural basis for a better functional coupling between mitochondria and Kv channels in PASMCs than in systemic arterial myocytes. The correlation between the disruption of the distribution of peripheral mitochondria by cytochalasin B and significant attenuation of the effects of antimycin A on IKv activation and inactivation directly supports this conclusion. Mechanisms involved in Kv channel regulation by mitochondria in PASMCs.
The electrophysiological evidence presented in Fig. 9, demonstrating that addition of Mg2+-chelating agents into the pipette solution inhibits changes in Vh caused by antimycin A and oligomycin, clearly suggests that an increase in intracellular concentration of Mg2+ plays a key role in the modulation of IKv inactivation by mitochondrial inhibitors. The ability of the increased pipette Mg2+ concentration first to mimic and then to attenuate the effect of antimycin A (Fig. 9C) also supports this notion. We previously demonstrated (9) that similar pipette interventions significantly attenuated changes in the IKv activation and IKv block caused by antimycin A and oligomycin in PASMCs, thus suggesting that changes in mitochondrion-mediated changes in intracellular Mg2+ concentration represent one of the major mechanisms controlling the functional activity of Kv channels. On the other hand, the failure of cytochalasin B to attenuate IKv block, while inhibiting changes in IKv activation and inactivation (Fig. 8, C–E), suggests the presence of other factors (e.g., ROS) that may influence the ability of Mg2+ to interact with Kv channels. The identification of these factors and the establishment of their importance in mitochondrion-mediated and Mg2+-dependent modulation of IKv in PASMCs in normoxic and hypoxic conditions will entail further experimental work. Stronger functional coupling exists between mitochondria and Kv currents in PASMCs than in MASMCs.
To analyze the differences in functional coupling between mitochondria and Kv channels in PASMCs and MASMCs, the inhibitors of complex III (antimycin A) and of the ATP synthase (oligomycin) were used because, as we previously reported (9), their effects on IKv activation and IKv block in PASMCs are substantially stronger compared with other mETC inhibitors (rotenone, myxothiazol, and cyanide) and the mitochondrial uncoupler CCCP. Comparison of the effects of antimycin A and oligomycin on IKv activation and IKv block in the two cell types clearly demonstrated significantly lesser effects in MASMCs than in PASMCs (Fig. 7, B and D). Notably, the effect of oligomycin on IKv activation was not significant in MASMCs. In addition, we also compared the effect of the two inhibitors on IKv inactivation, which represents another important voltage-dependent characteristic of Kv channels that, together with the steady-state activation, determines the availability of Kv channels at membrane potentials negative to 0 mV (26). Similar to IKv activation and IKv block, changes in IKv activation induced by both inhibitors were also significantly smaller in MASMCs than in PASMCs (Fig. 7C), as would be predicted from the different distribution of mitochondria in PASMCs and MASMCs.
The tighter spatial and functional coupling between Kv channels and mitochondria, one of the proposed oxygen sensors (18, 49), in PASMCs can contribute to the difference in sensitivity of IKv to hypoxia in the pulmonary and systemic arterial SMC reported previously (22, 31, 56, 58). This could be further enhanced by the difference in the functional activity of mitochondria. Thus Michelakis et al. (22) demonstrated a significantly greater rate in basal production of mitochondrial ROS in PASMCs than in renal arterial SMCs, while Rathore et al. (37) found that mitochondrial ROS were selectively increased by hypoxia in PASMCs but not in MASMCs. It is currently difficult to directly measure changes in the concentration of mitochondrial ROS or other mitochondrial metabolites such as ATP, cytochrome c, or magnesium ions [all of which modulate the activity of Kv channels in PASMCs (3, 8, 9, 34, 38, 59)] in submembrane compartments in intact cells. In this instance, ion channel characteristics, which can be measured, quantified, and even calibrated with different intracellular solutions, can serve as useful markers. Indeed, ATP-sensitive K+ (KATP) channels and Ca2+-sensitive K+ (BKCa) channels have been studied to assess changes in ATP concentration and [Ca2+]i, respectively, directly beneath the plasma membrane (13, 44). Similarly, as our results demonstrate, voltage-dependent parameters of IKv, a dominant K+ current in PASMCs, can serve as useful markers of changes in the confined subplasmalemmal environment in close vicinity to the Kv channel in PASMCs.
Distinct differences in Kv channel activity between PASMCs and MASMCs.
To investigate mechanisms of HPV, which are specific to the pulmonary circulation, MAs are often used as a negative control. Although the voltage-dependent characteristics of IKv, such as steady-state activation and inactivation, have been reported previously in rat PASMCs (27, 43) and MASMCs (15, 21, 30), no direct comparison between the two cell types has been performed under identical experimental conditions to determine whether there is a significant difference in IKv properties in the two cell types. Also, in this study, IKv was isolated in both PASMCs and MASMCs by elimination of BKCa and KATP currents with paxilline and glibenclamide, respectively. In other studies a low extracellular Ca2+ concentration (15, 21) and a high concentration of ATP in the pipette (15, 21, 30), respectively, were used in MASMCs for this purpose. It is noteworthy that the amplitude and the parameters for activation and inactivation of IKv measured under our experimental conditions in MASMCs were not dissimilar to those reported by others. For example, the mean midpoints for IKv activation and inactivation of −10 and −39.4 mV (30), −11 and −40 mV (21), and −3.5 and −33.9 mV (15) are comparable with the −9.4 and −37 mV, respectively, we report in the present study (Table 1), suggesting that that our experimental conditions and pharmacological agents used for current isolation do not have a substantial effect on the voltage-dependent properties of IKv in MASMCs.
Comparison of the IKv amplitude, cell size, and steady-state activation and inactivation characteristics of the current measured in identical experimental conditions revealed significant differences in all three groups of characteristics in PASMCs and MASMCs. The most significant differences were found in the whole cell current amplitude (larger in PASMCs) and the cell size (larger for MASMCs), giving between 8- and 18-fold differences in the range of membrane potential between −30 and +50 mV of cells with PASMCs. Also, the midpoint for IKv inactivation was significantly shifted to more negative potentials by ∼13 mV in MASMCs compared with PASMCs. Although a relatively small shift to more positive membrane potentials (∼4 mV) was seen in the midpoint for IKv activation, the effect was nevertheless significant. Finally, the differences in the slope factors for both activation and inactivation dependencies were also significantly different between PASMCs and MASMCs.
Since native Kv channels are believed to be heteromultimers that are formed by more than one Kv1.x α-subunit in both PASMCs (6, 7, 33, 60) and MASMCs (10, 20, 30, 55), the differences in the voltage-dependent characteristics may be due to a different heteromultimeric assembly of the Kv channels in the two cell types. In addition, differences in levels of expression or isoform of accessory Kv β-subunits, found in both cell types (7, 10, 30, 33, 60), could also contribute to the differences in IKv parameters we observed between two cell types. Also, the presence of functional Kv channels formed by the Kv2.1 α-subunit, a member of another Kv channel family, cannot be ruled out (6, 10, 20, 29, 60). Indeed, between two and four small-amplitude single-channel Kv currents were found in rat (60), human (32), and mouse (16) PASMCs. Although heterogeneity in distribution of different Kv currents was also observed in different PASMCs (4, 43), the presence of multiple voltage-dependent Kv components in a single cell was not obvious because of the homogeneity of the activation and inactivation dependencies (Figs. 5 and 6). It is possible that Kv channel heterogeneity observed at the single-channel level may contribute to some variability in the voltage-dependent parameters for IKv between cells.
It is worth mentioning that the 2- to 3-fold difference in single-channel conductance for the delayed currents encoded by Kv1.x and Kv2.x α-subunits is unlikely to explain the at least 10-fold increase in the whole cell IKv density between −40 and 0 mV in PASMCs compared with MASMCs. Similarly, the differences in the IKv activation and inactivation parameters alone between the two cell types could only predict a 2.6- to 3-fold increase in the steady-state open probability of Kv channels available in the same voltage range (calculated with the mean values for IKv activation and inactivation parameters listed in Table 1 and the theoretical model described in Ref. 26). It is therefore likely that the number of functional Kv channels expressed per unit of the cell membrane area should also be greater in PASMCs than in MASMCs.
In conclusion, our findings demonstrate the presence of a distinctive layer of mitochondria at the cell periphery in PASMCs, forming a restricted diffusion cytosolic space between the mitochondria and plasmalemmal membrane and thus providing a structural basis for a close functional interaction between the Kv channels and mitochondria as supported by the electrophysiological evidence. The smaller density of Kv channels in MASMCs alongside the greater diffusion space between the mitochondria and the cell membrane may provide one plausible explanation for the differences in cellular responses during hypoxia.