PowerPoint Slide Highlights that are not in study guide
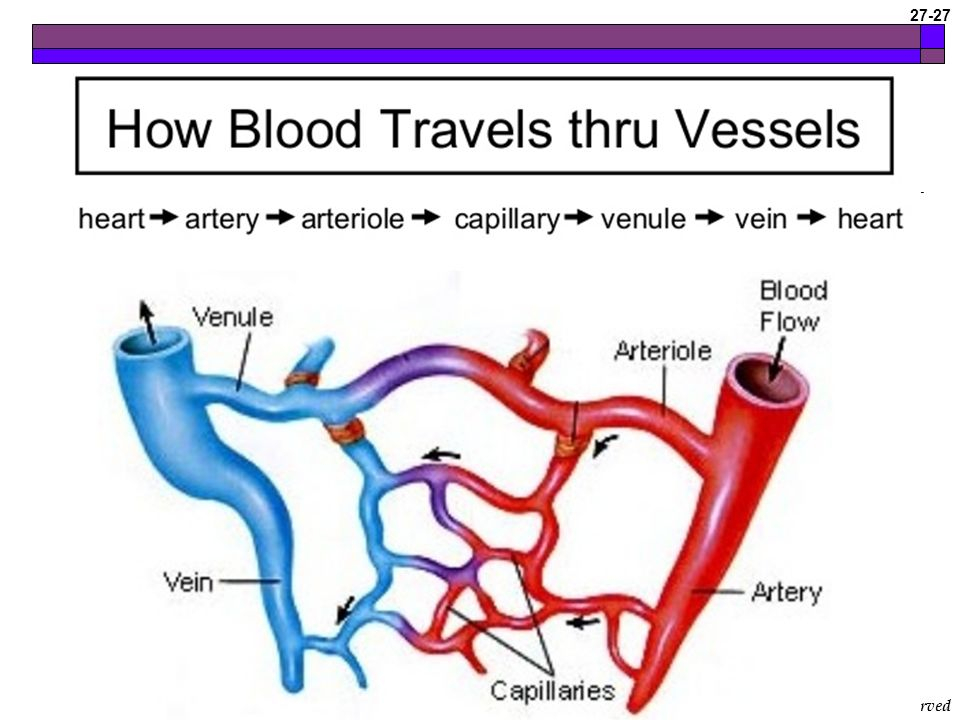
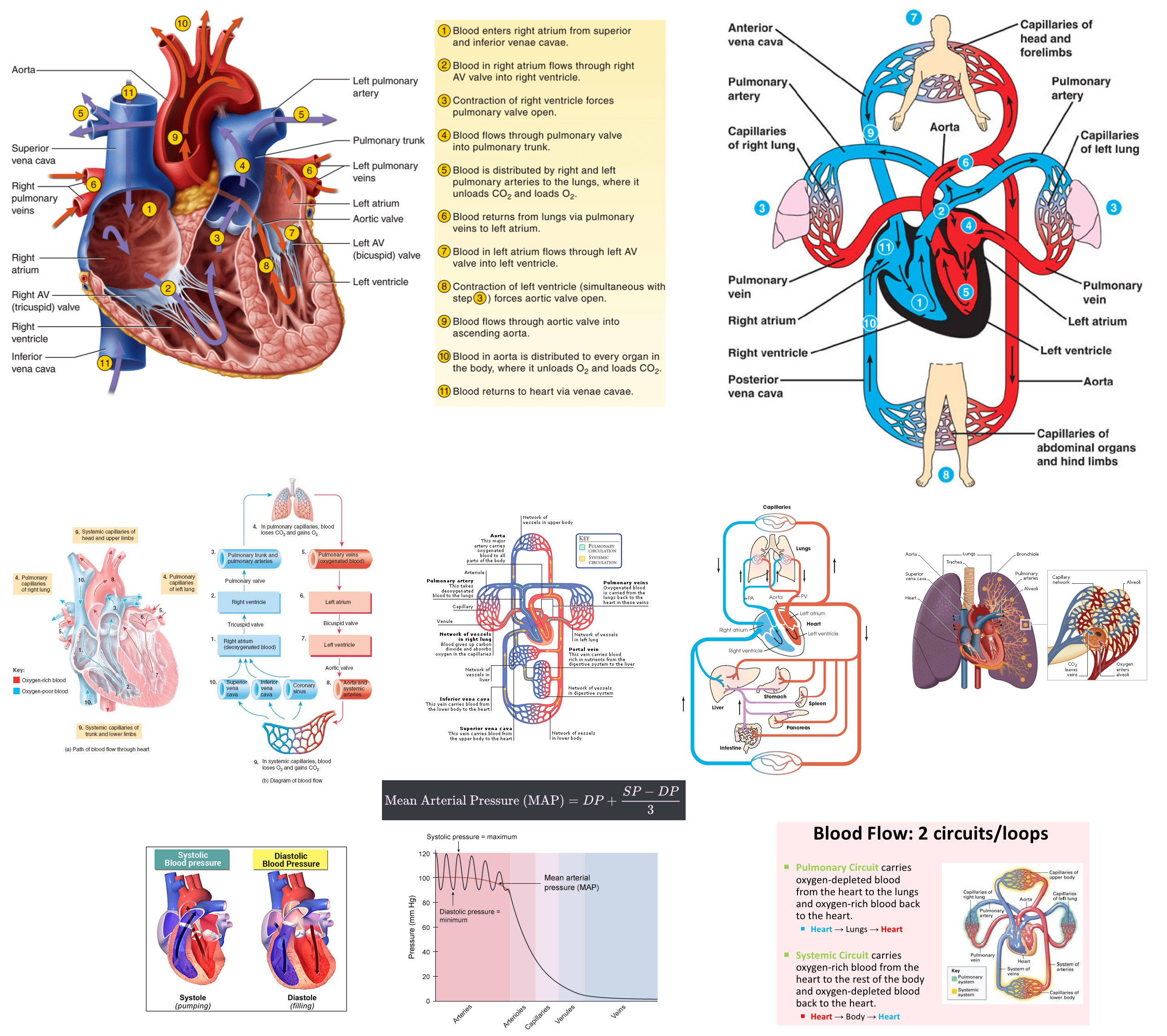
Describe the movement of blood through the heart and around the body
Describe how pressure resistance and flow are related to each other in the body
Flow occurs only when there is a difference in pressure.
Blood moves from areas of higher pressure ( arteries ) to areas of lower pressure ( veins )
If resistance is constant , increasing
Resistance is primarily influenced by the vessel diameter ( radius ) , vessel length , and blood viscosity
Poiseuille’s law quantifies this relationship :
Resistance increases dramatically with small decreases in vessel radius , as
Calculate Mean Arterial Pressure ( MAP )
Calculate Cardiac Output ( CO )
Calculate Total Peripheral Resistance ( TPR )
CVP : Central Venous Pressure ( usually negligible , approximated as 0 in many cases )
where ΔP is the pressure gradient across the systemic circulation :
and CVP is still approximately
so
Example
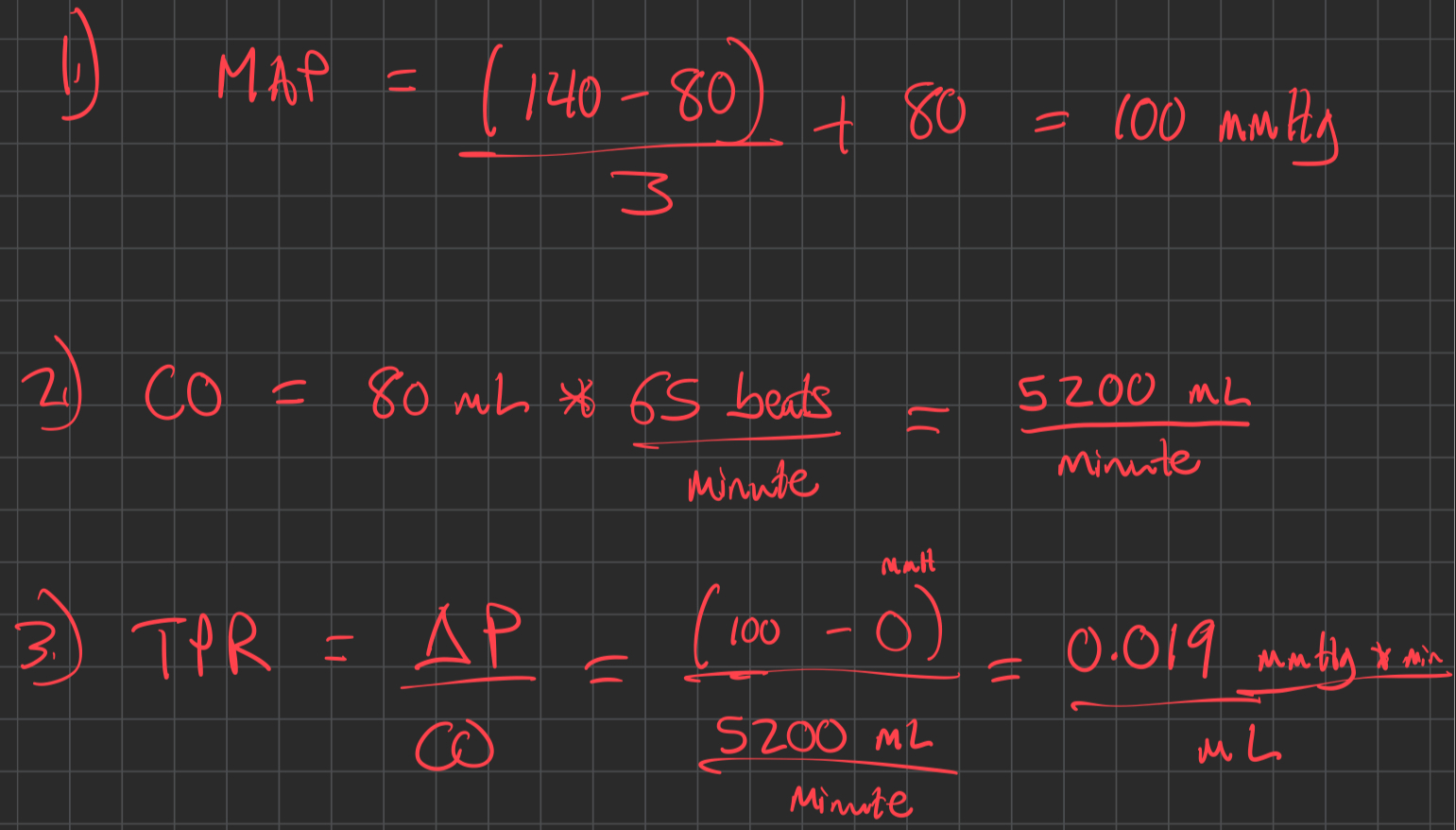
Describe the forces at play expressed by the Starling equation. What happens when they are out of balance?
The Starling equation describes the net movement of fluid (
The reflection coefficient (
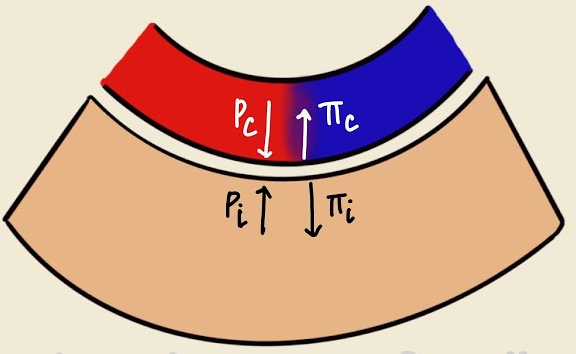
Starling Equation : Balances forces moving fluid in/out of pulmonary capillaries :
Hydrostatic pressure pushes fluid out of the capillary
Oncotic pressure pulls fluid into the capillary
Imbalance can cause pulmonary edema, impairing gas exchange.
such as increased
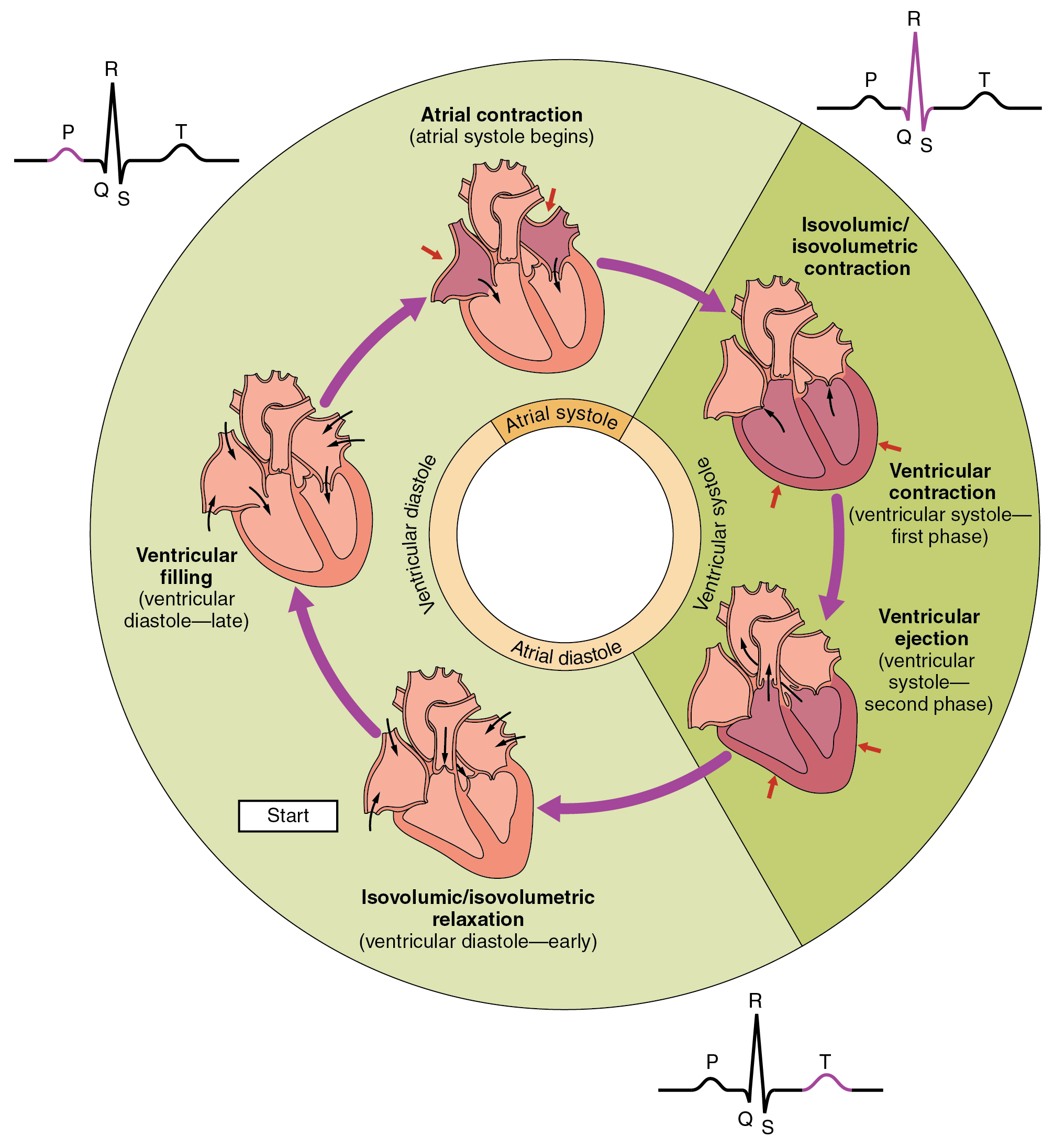
Describe the phases of the cardiac cycle
The cardiac cycle consists of systole ( contraction ) and diastole ( relaxation ) , which are divided into several phases :
Atrial Systole : The atria contract , increasing atrial pressure (
Isovolumetric Contraction : Ventricles contract, increasing ventricular pressure (
Ventricular Ejection : When
Isovolumetric Relaxation : Ventricles relax , and
Rapid Ventricular Filling : When
Reduced Ventricular Filling : Ventricular filling slows as
The cycle is coordinated by electrical signals , with pressure gradients and valve dynamics ensuring unidirectional blood flow. Imbalances in these phases can lead to conditions like heart failure or arrhythmias.
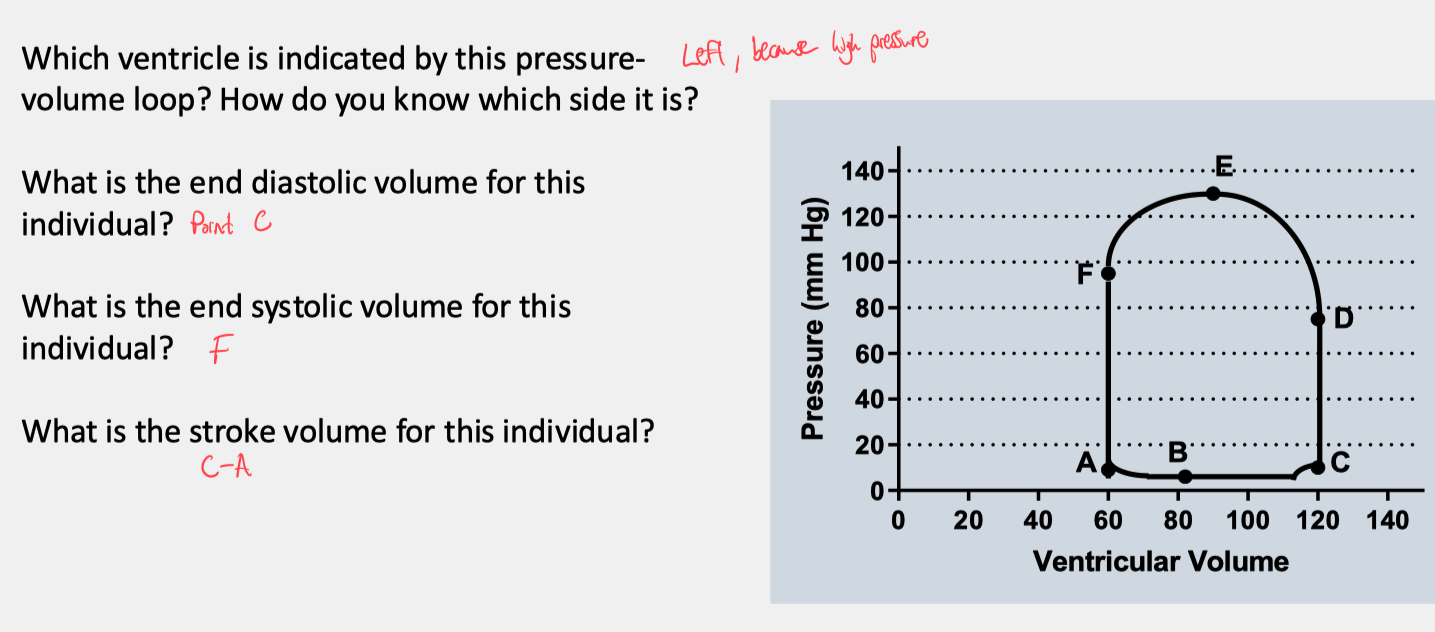
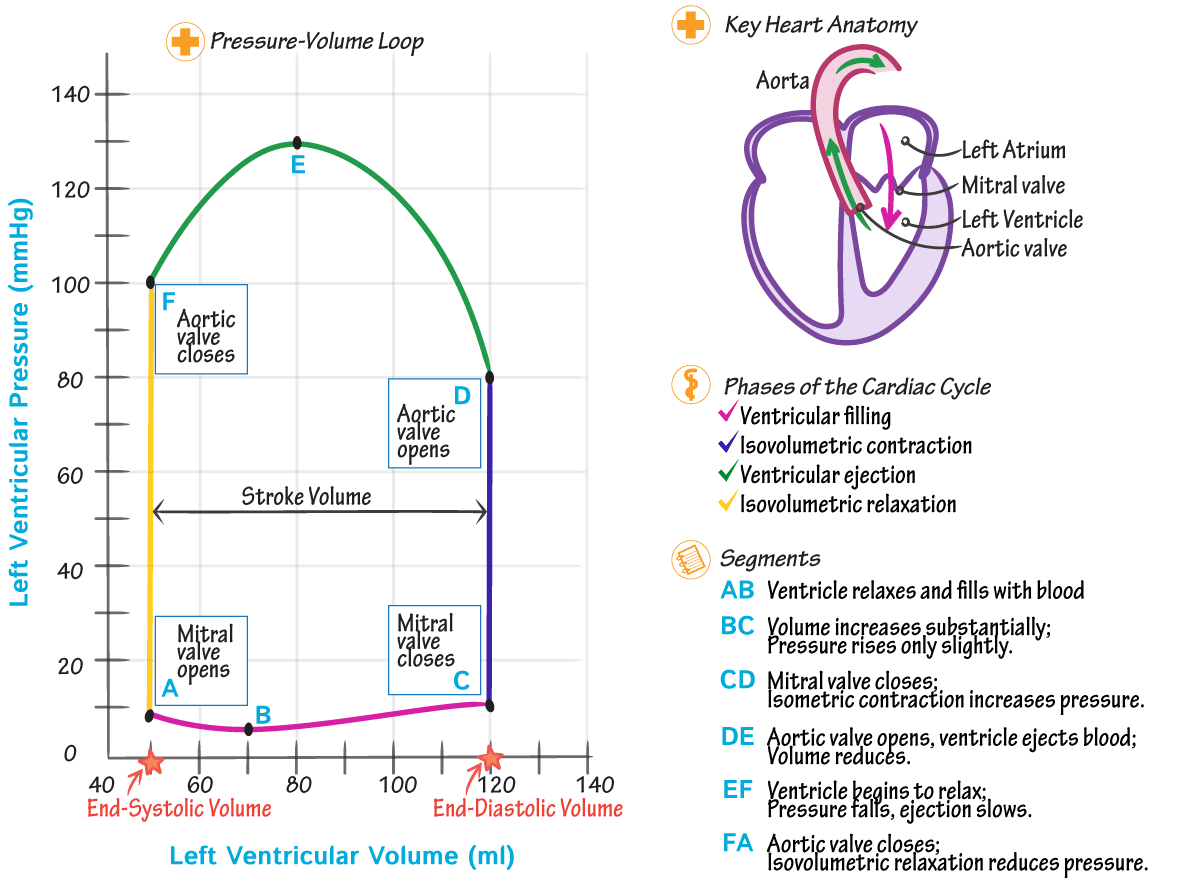
Describe and label a pressure-volume graph
Ventricular Filling ( A → B ) :
Start ( Point A ) : Mitral valve opens; Tricuspid valve opens
End ( Point B ) : Mitral valve closes; Tricuspid valve closes
Description:
Blood flows from the left atrium into the left ventricle, increasing ventricular volume significantly
Blood also flows from the right atrium into the right ventricle
Pressure rises slightly due to ventricular compliance and blood inflow
This phase represents diastole ( ventricular relaxation )
Isovolumetric Contraction ( B → C ) :
Start ( Point B ) : Mitral valve closes; Tricuspid valve closes.
End ( Point C ) : Aortic valve opens; Pulmonary valve opens.
Description:
The ventricle contracts, causing a sharp rise in pressure while the volume remains constant
Both the mitral and tricuspid valves are closed, as are the aortic and pulmonary valves until they open
Ventricular Ejection ( C → D ) :
Start ( Point C ) : Aortic valve opens; Pulmonary valve opens
End ( Point D ) : Aortic valve closes; Pulmonary valve closes
Description:
Blood is ejected from the left ventricle into the aorta, causing a decrease in ventricular volume
Blood is also ejected from the right ventricle into the pulmonary artery
Pressure initially rises as the ventricles contract but later falls as blood is ejected
The horizontal distance between Points A and C ( width of the loop ) represents the stroke volume ( SV )
Isovolumetric Relaxation ( D → F ) :
Start ( Point D ) : Aortic valve closes; Pulmonary valve closes.
End ( Point F ) : Mitral valve opens; Tricuspid valve opens.
Description:
The ventricle relaxes after ejection, causing a sharp drop in pressure with no change in volume.
Both the aortic and pulmonary valves are closed, as are the mitral and tricuspid valves until they open.
Ventricular Filling Resumes ( F → A ) :
Start ( Point F ) : Mitral valve opens; Tricuspid valve opens.
End ( Point A ) : End-systolic volume is reached.
Description:
Blood begins to flow back into the ventricle, restarting the cardiac cycle.
Pressure is low and increases gradually as the ventricle fills.
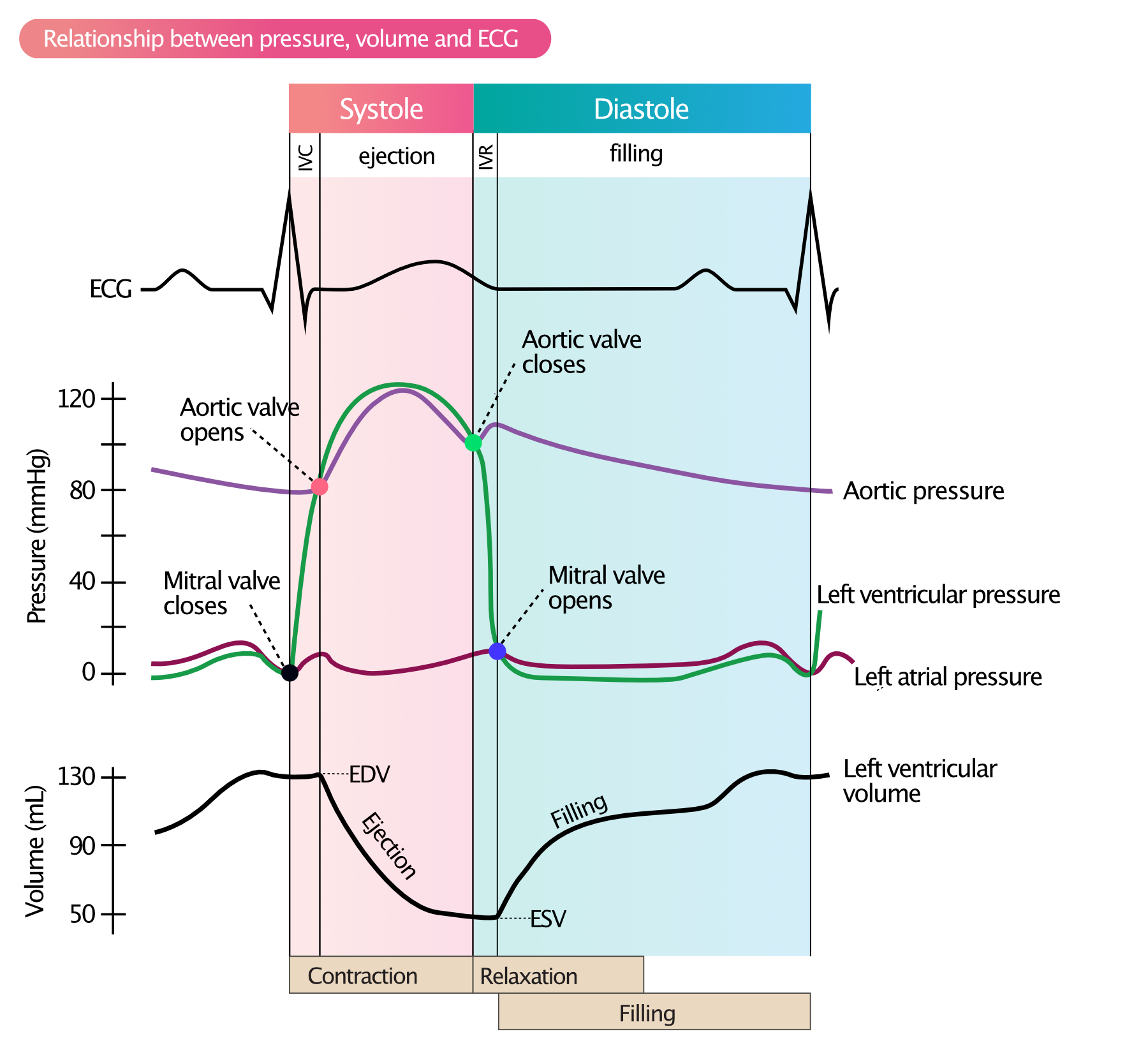
Describe the cardiac action potential in the myocardium. Which ion channels are involved?
The cardiac action potential in myocardial cells is divided into five phases ( 0 to 4 ) and involves the coordinated activity of several ion channels :
Phase 0 : Depolarization
Rapid influx of sodium ( Na⁺ ) through voltage-gated fast sodium channels ( Naᵥ )
This causes a sharp increase in membrane potential to approximately +20 mV
Phase 1 : Initial Repolarization
Closure of Na⁺ channels.
Outflow of potassium ( K⁺ ) through transient outward potassium channels ( Kᵥ )
Phase 2 : Plateau
Balance between calcium ( Ca²⁺ ) influx and potassium ( K⁺ ) efflux
Voltage-gated L-type calcium channels ( Caᵥ ) open, allowing Ca²⁺ to enter, which sustains contraction
Delayed rectifier potassium channels ( Kᵥ ) limit excessive depolarization
Phase 3 : Repolarization
Closure of Ca²⁺ channels.
Continued efflux of K⁺ through delayed rectifier potassium channels ( Kᵥ ) restores the resting membrane potential.
Phase 4 : Resting Potential
Resting membrane potential is maintained at approximately -90 mV by inward rectifier potassium channels ( Kᵢ )
Na⁺/K⁺ ATPase pumps and Na⁺/Ca²⁺ exchangers maintain ion gradients
Key Ion Channels:
Na⁺ channels ( Naᵥ ) : Phase 0 depolarization.
K⁺ channels ( Kᵥ , Kᵢ ) : Repolarization and resting potential.
Ca²⁺ channels ( Caᵥ ) : Plateau phase ( Phase 2 ).
Na⁺/K⁺ ATPase : Restores ionic balance after each action potential.
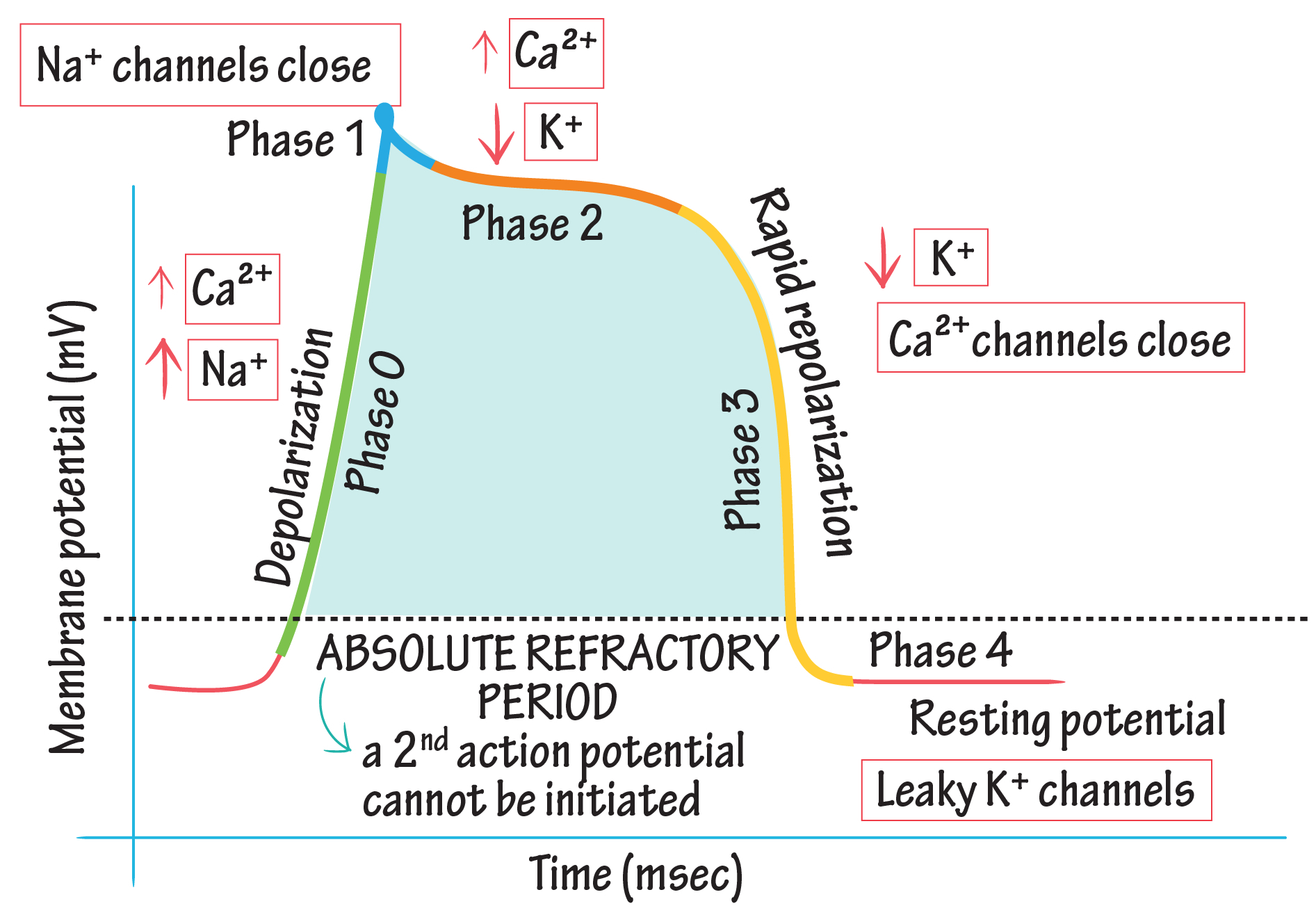
Describe the Baroreflex
The baroreflex is a negative feedback mechanism that maintains blood pressure ( BP ) homeostasis by modulating heart rate , vascular tone , and cardiac output. It involves the following steps:
Detection: Baroreceptors, stretch-sensitive mechanoreceptors located in the carotid sinus and aortic arch, detect changes in arterial pressure. Increased BP stretches the vessel walls, activating these receptors, while decreased BP reduces their activity.
Signal Transmission: Afferent signals from baroreceptors are transmitted to the medulla via the glossopharyngeal nerve ( CN IX ) from the carotid sinus and the vagus nerve ( CN X ) from the aortic arch.
Integration: The medulla’s cardiovascular centers ( nucleus tractus solitarius, vasomotor center, and cardiac center ) process these signals.
Response:
High BP: Increased baroreceptor firing inhibits sympathetic output and enhances parasympathetic activity, leading to decreased heart rate ( via vagus nerve ) , reduced cardiac contractility, and vasodilation.
Low BP: Reduced baroreceptor firing increases sympathetic output, causing vasoconstriction, increased heart rate, enhanced cardiac contractility, and elevated BP.
The baroreflex provides short-term regulation of BP. Chronic hypertension can "reset" baroreceptors to tolerate higher BP levels, reducing their sensitivity.
Describe what the sympathetic nervous system controls in the cardiovascular system
The sympathetic nervous system ( SNS ) plays a critical role in regulating cardiovascular function, primarily during stress, exercise, or situations requiring increased cardiac output. Key actions include :
Heart Rate ( Chronotropy ): The SNS increases heart rate by releasing norepinephrine, which binds to β₁-adrenergic receptors in the sinoatrial ( SA ) node, accelerating depolarization.
Cardiac Contractility ( Inotropy ): Norepinephrine binding to β₁-adrenergic receptors on ventricular myocytes enhances calcium influx via L-type calcium channels, increasing contractile force.
Conduction Velocity ( Dromotropy ): SNS stimulation speeds electrical conduction through the atrioventricular ( AV ) node by modulating ion channels, reducing AV nodal delay.
Vascular Tone ( Vasoconstriction and Vasodilation ):
Vasoconstriction: The SNS stimulates α₁-adrenergic receptors in most vascular smooth muscle, causing vasoconstriction and increasing systemic vascular resistance ( SVR ) to maintain or elevate blood pressure.
Vasodilation: In certain tissues ( e.g., skeletal muscle during exercise ), β₂-adrenergic receptor activation promotes vasodilation to enhance blood flow.
Blood Pressure Regulation: The SNS increases mean arterial pressure ( MAP ) by enhancing cardiac output ( CO ) and systemic vascular resistance ( SVR ).
These effects allow the SNS to support increased metabolic demands during stress or activity, ensuring proper perfusion of vital organs.
Give examples of endothelial-derived vasoconstrictors and dilators
Vasoconstrictors :
Endothelin-1 ( ET-1 ): A potent vasoconstrictor that binds to ETA and ETB receptors on smooth muscle, increasing calcium levels and promoting contraction.
Thromboxane A2 ( TXA2 ): Derived from arachidonic acid, it stimulates smooth muscle contraction and platelet aggregation.
Prostaglandin H2 ( PGH2 ): Acts as a vasoconstrictor precursor and amplifies vasoconstrictor signaling.
Angiotensin II ( locally produced ): Stimulates vasoconstriction via AT1 receptors on vascular smooth muscle.
Vasodilators :
Nitric Oxide ( NO ): Synthesized from L-arginine by endothelial nitric oxide synthase ( eNOS ), NO activates guanylyl cyclase in smooth muscle, leading to cGMP-mediated relaxation.
Prostacyclin ( PGI2 ): Inhibits smooth muscle contraction and platelet aggregation by increasing cAMP levels.
Endothelium-Derived Hyperpolarizing Factor ( EDHF ): Causes smooth muscle hyperpolarization via potassium channel activation, leading to relaxation.
Bradykinin: Stimulates endothelial NO and prostacyclin release, enhancing vasodilation.
The balance between these factors maintains vascular homeostasis, and imbalances can lead to conditions such as hypertension or atherosclerosis.
Cardiovascular Worksheet
How is hypoxia proposed to cause contraction in pulmonary smooth muscle ? Are there any controversies?
Yes still unclear
Low O2 , decreased mitochondrial activity , affects Kv channels
Block mitochondria = same hypoxic current
Cytochrome C and Hydrogen peroxide = activate Kv channels
byproducts of ETC
Respiratory
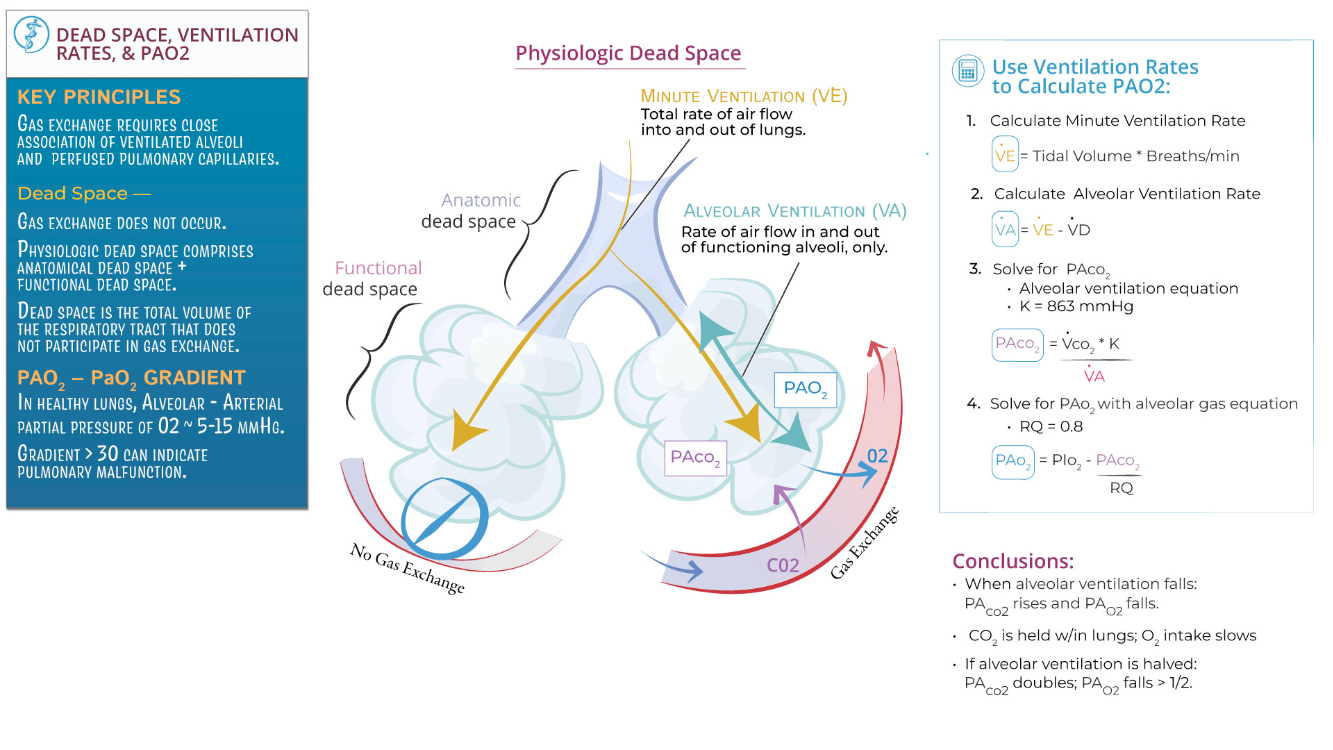
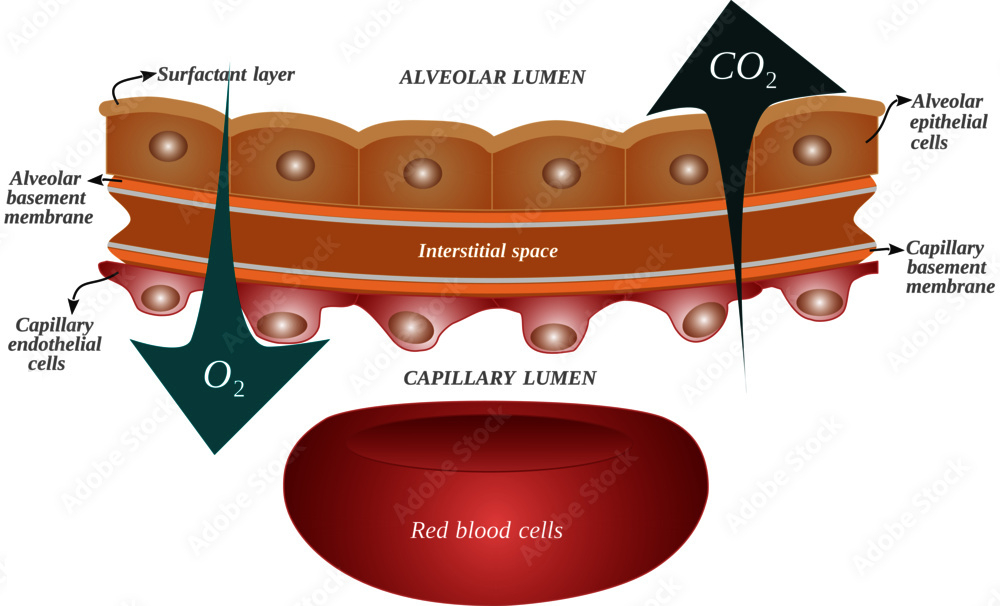
What are the requirements for diffusion of a gas across the alveolar/blood barrier?
High surface area area , low tension , strong pressure gradient , warm and moist air
Partial Pressure Gradient : Gases move down their partial pressure gradient ( e.g., oxygen from alveoli to blood, carbon dioxide from blood to alveoli ).
Surface Area : A large alveolar surface area enhances diffusion; reduced surface area ( e.g., emphysema ) impairs it.
Thin Barrier : The alveolar-capillary membrane must be thin ( ~0.5 µm ); thickening ( e.g., fibrosis, edema ) reduces diffusion efficiency.
Gas Solubility : Highly soluble gases ( e.g., carbon dioxide ) diffuse more readily than less soluble gases ( e.g., oxygen ).
Ventilation-Perfusion Matching ( V/Q Matching ) : Adequate ventilation and perfusion alignment optimize gas exchange; mismatches impair diffusion.
Hemoglobin Availability : Adequate hemoglobin is required to bind and transport oxygen effectively.
Which of the above requirements are met by the upper airways?
Partial Pressure Gradient : The upper airways contribute by humidifying and warming inspired air, ensuring a stable gradient when air reaches the alveoli.
Ventilation-Perfusion Matching ( V/Q Matching ) : The upper airways facilitate ventilation by maintaining open pathways for airflow but do not directly influence perfusion.
Gas Solubility : The upper airways condition the air by humidifying it , indirectly affecting gas solubility by preventing desiccation of the alveolar surface
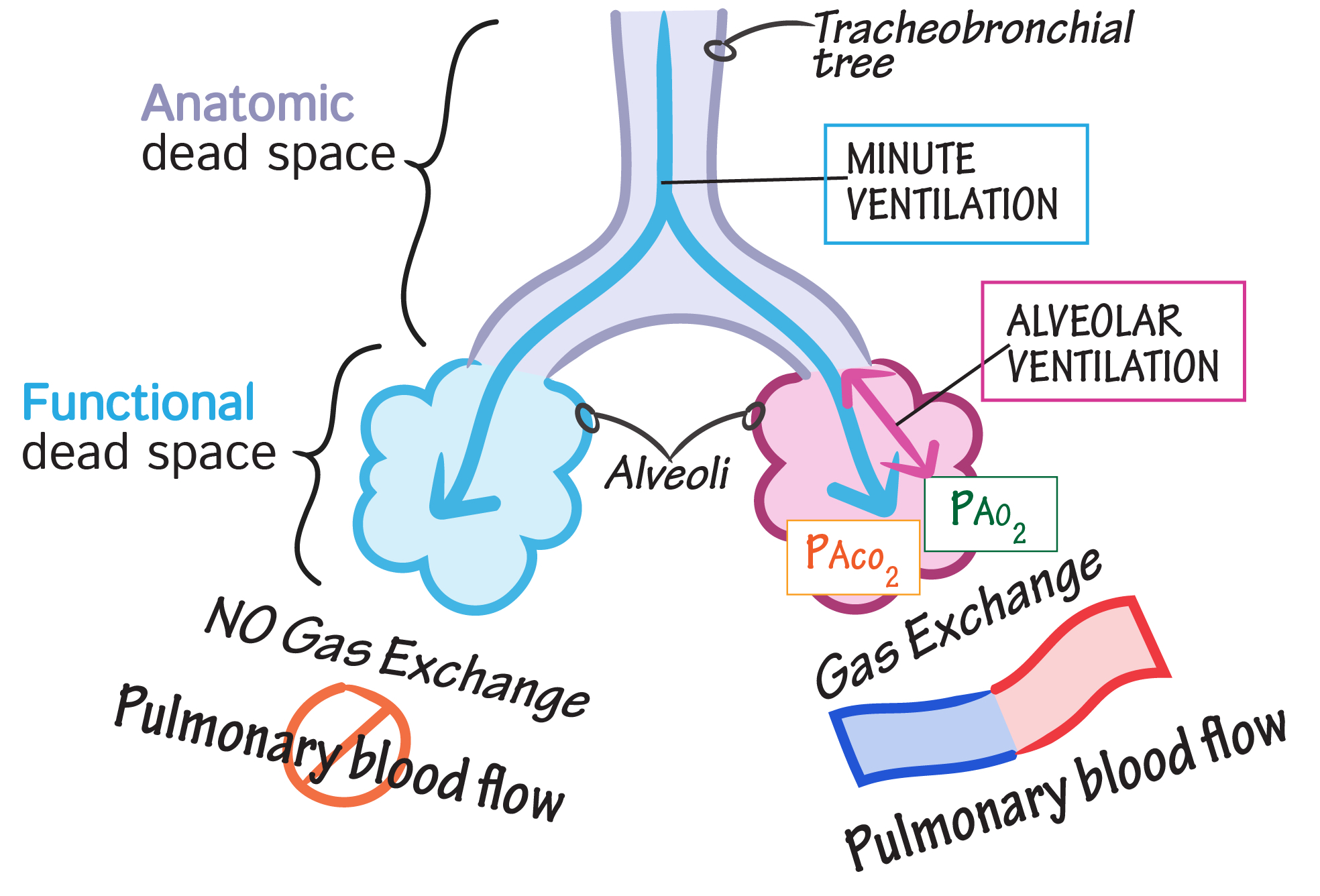
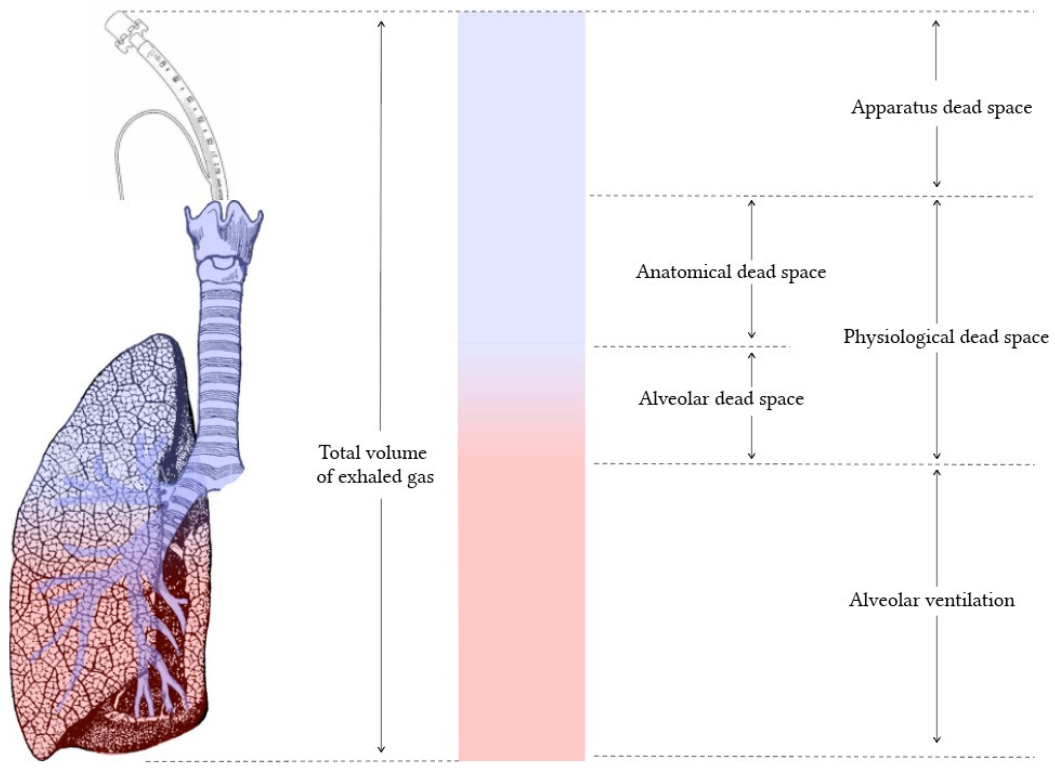
Distinguish dead space from the respiratory zone of the airways.
Dead Space: Includes airways where no gas exchange occurs ( e.g., trachea, bronchi, bronchioles ). These structures only conduct air to the respiratory zone. Anatomical dead space is typically ~150 mL in an adult, while physiological dead space includes areas where ventilation occurs without effective perfusion ( e.g., alveoli in poorly perfused regions ).
Respiratory Zone: Comprises structures involved in gas exchange ( e.g., respiratory bronchioles, alveolar ducts, alveoli ). These areas have thin walls and extensive capillary networks to facilitate diffusion of oxygen and carbon dioxide. The respiratory zone begins where alveoli first appear in the airway.
Key Distinction : Dead space supports air transport and conditioning ( humidification , filtration , and warming ) , while the respiratory zone is where actual gas exchange occurs.
Describe how a negative pressure pump is used to ventilate the airways
A negative pressure pump ventilates the airways by mimicking the natural mechanics of breathing, creating a pressure gradient that draws air into the lungs:
Mechanism: The pump generates a negative pressure around the thoracic cavity, reducing intrapleural pressure. This decreases alveolar pressure below atmospheric pressure, causing air to flow into the lungs ( inspiration ). During relaxation, the negative pressure is removed, allowing elastic recoil of the lungs and chest wall to expel air ( expiration ).
Applications: Negative pressure ventilators, such as the "iron lung," were historically used to assist patients with respiratory muscle paralysis ( e.g., polio ). These devices surround the thorax and abdomen, using external pressure changes to facilitate ventilation.
Key Difference from Positive Pressure Ventilation: Negative pressure ventilation relies on external pressure changes to create a natural airflow gradient, whereas positive pressure ventilation forces air into the lungs through an endotracheal tube or mask.
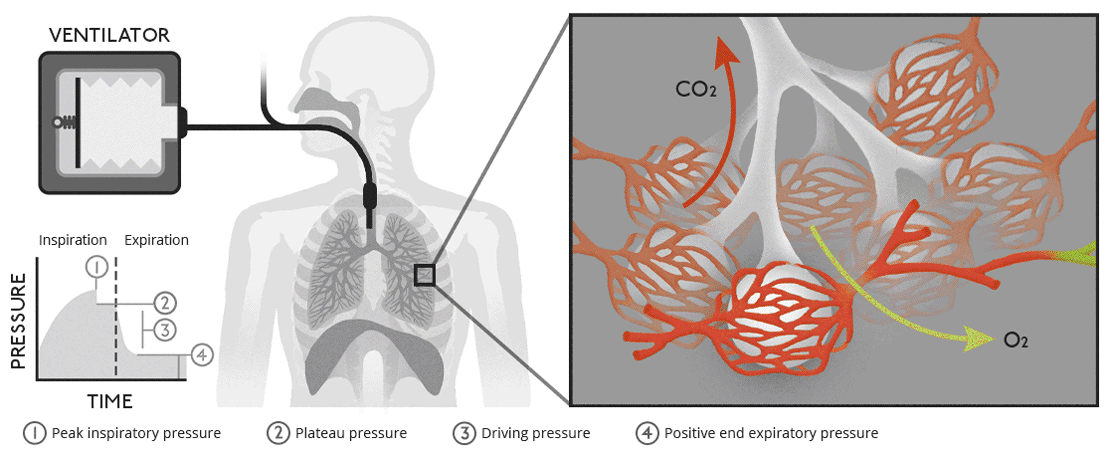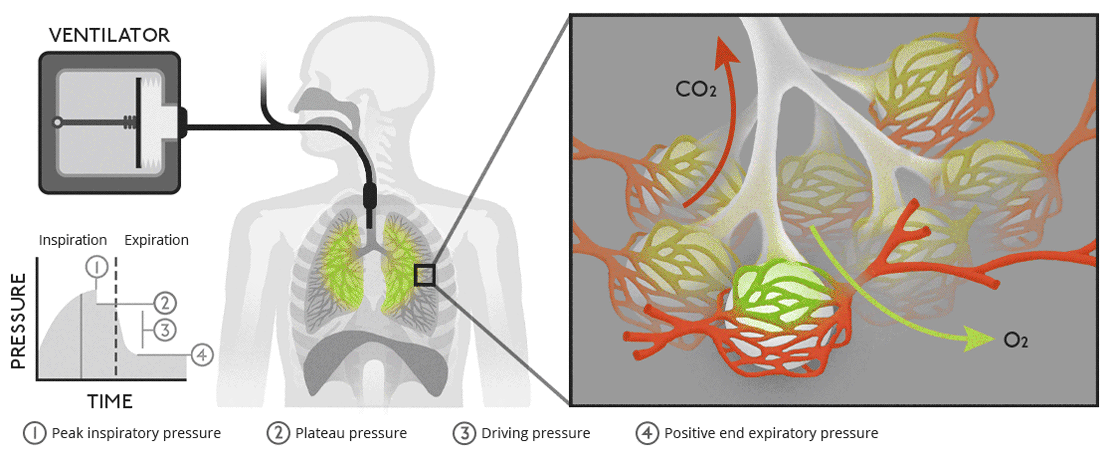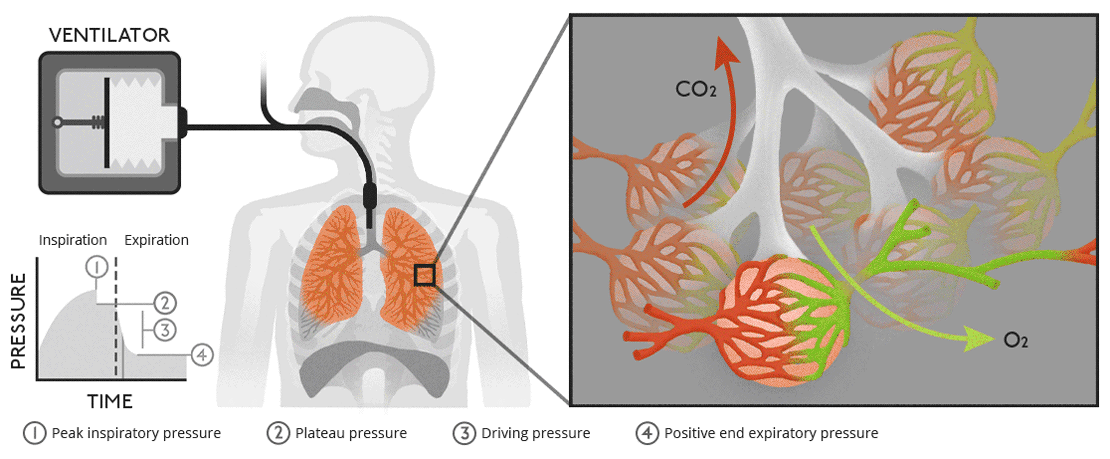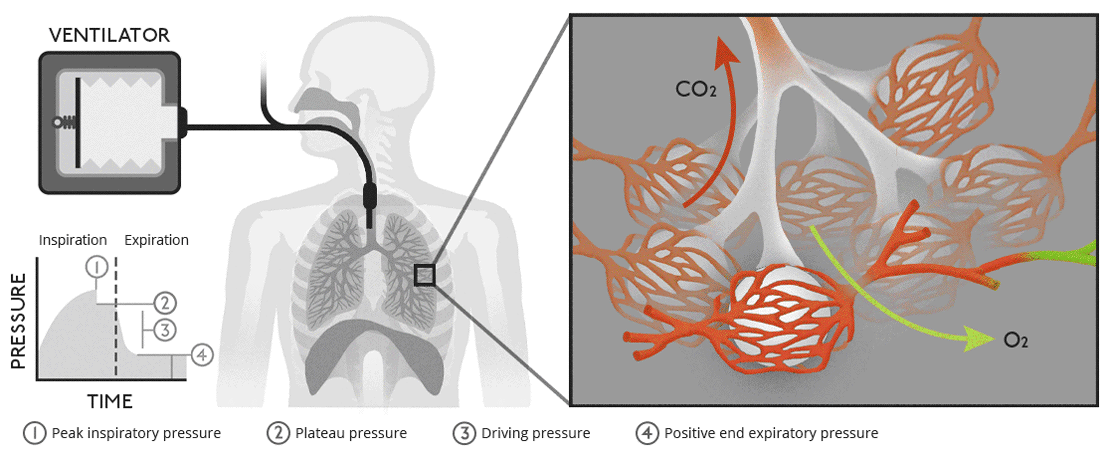
There are multiple cell types in the airways. What are these cell types and how do they differ functionally?
Ciliated Epithelial Cells: Line most of the conducting airways; their coordinated ciliary motion moves mucus and trapped particles upward toward the pharynx ( mucociliary clearance ).
Goblet Cells: Secrete mucus to trap particles and pathogens, forming part of the airway defense system. Found in the conducting zone.
Club ( Clara ) Cells: Found in terminal bronchioles; secrete surfactant-like substances to reduce surface tension and detoxify inhaled substances via cytochrome P450 enzymes.
Basal Cells: Act as stem cells for airway epithelium, capable of differentiating into other cell types ( e.g., ciliated or goblet cells ) during repair.
Type I Alveolar Cells: Thin, squamous cells in the respiratory zone; cover ~95% of the alveolar surface and facilitate gas exchange.
Type II Alveolar Cells: Cuboidal cells in the respiratory zone; secrete surfactant to reduce alveolar surface tension and serve as progenitors for Type I cells.
Macrophages: Found in alveoli and conducting airways; phagocytose pathogens, particles, and dead cells to maintain airway cleanliness.
Neuroendocrine Cells: Rare cells in the conducting airways; release signaling molecules ( e.g., serotonin ) to regulate airway tone and immune responses.
What keeps alveoli from collapsing?
Surfactant: Secreted by Type II alveolar cells, surfactant reduces alveolar surface tension by disrupting cohesive forces between water molecules. This prevents alveolar collapse, especially during expiration, and stabilizes alveoli of different sizes ( Laplace’s Law ).
Residual Volume: A baseline amount of air remains in the lungs even after maximal expiration, keeping alveoli partially inflated.
Alveolar Interdependence: Adjacent alveoli are structurally connected, so when one alveolus collapses, the surrounding alveoli exert outward traction to reopen it.
Negative Intrapleural Pressure: The pressure in the pleural space is lower than atmospheric pressure, creating a vacuum that opposes alveolar collapse.
During maximal expiration the radius of a certain alveolus is reduced by ½ what will happen to the pressure within that alveolus? What can be done to keep P constant?
When the radius of an alveolus is reduced by half during maximal expiration, the pressure within the alveolus increases due to Laplace's Law:
Where:
What Happens:
If the radius (
How to Keep
Reduce Surface Tension (
Surfactant , secreted by Type II alveolar cells , decreases surface tension.
This compensates for the reduction in radius , keeping pressure relatively constant across alveoli of varying sizes
Stabilize Alveoli ( Interdependence ) :
Alveoli are mechanically tethered , and their structural interdependence helps prevent collapse by redistributing forces
By reducing surface tension with surfactant , the alveoli maintain stable pressure , even as the radius decreases during maximal expiration
If you need to increase VE is it more effective to increase VT or frequency? Why?
Rapid , shallow breathing :
Not effective because a large proportion of each breath is wasted in dead space ( no gas exchange occurs there ).
This results in less effective alveolar ventilation.
Slower , deeper breathing :
More effective because it increases tidal volume (
allowing more air to reach the alveoli where gas exchange occurs.
The dead space has less impact on overall ventilation
To optimize gas exchange , slower , deeper breaths are more efficient than rapid , shallow ones
Describe V/Q ratio. What accounts for regional differences in V/Q ratio?
The formula for alveolar ventilation is :
Perfusion represents pulmonary blood flow, and its formula is derived from the Fick Principle :
The
Regional Differences:
At the lung apex ,
At the lung base ,
Ventilation is greater at the base due to better alveolar compliance, but the perfusion gradient is even steeper.
These differences ensure efficient gas exchange overall but can lead to mismatches in diseases affecting ventilation or perfusion.
Ventilation-Perfusion ( V/Q ) Relationships :
Matching: Optimal gas exchange occurs when ventilation matches perfusion
V/Q Mismatch :
High V/Q ( dead space ) : Ventilation without perfusion ( e.g., pulmonary embolism )
Low V/Q ( shunt ) : Perfusion without ventilation ( e.g., airway obstruction )

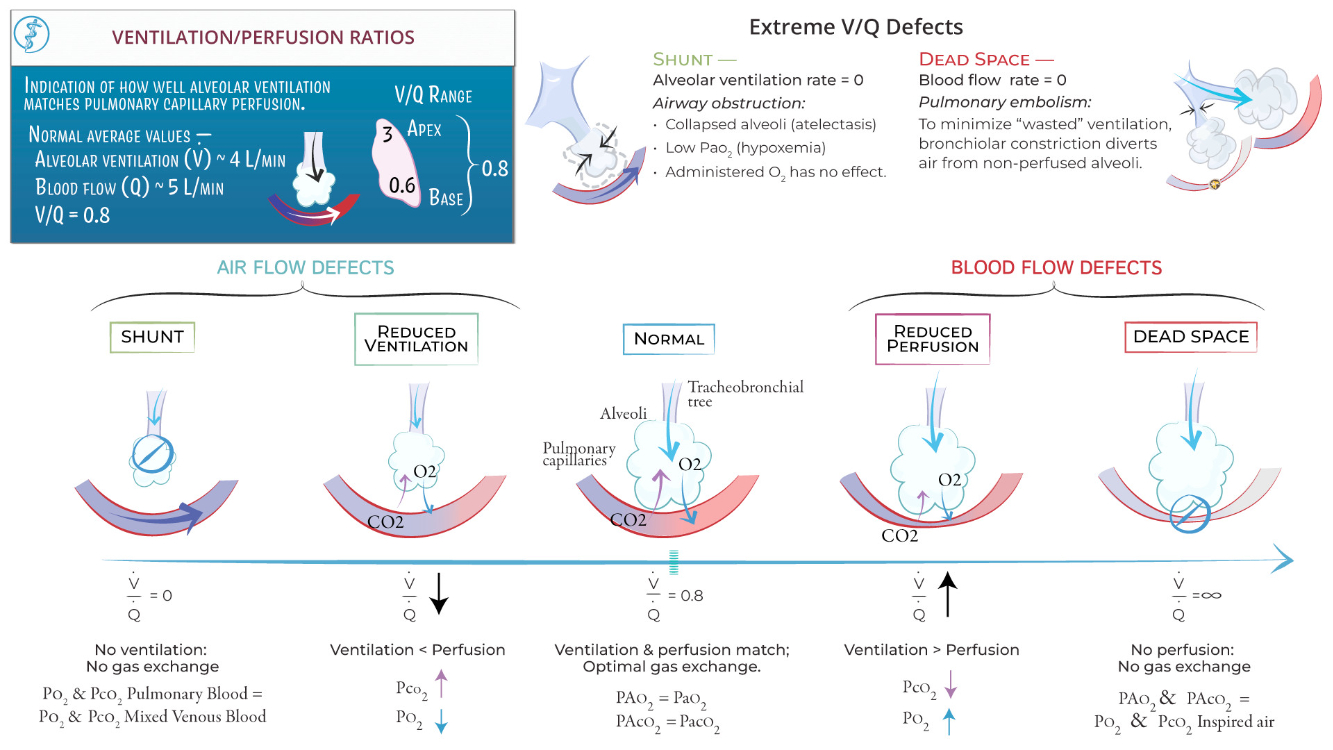
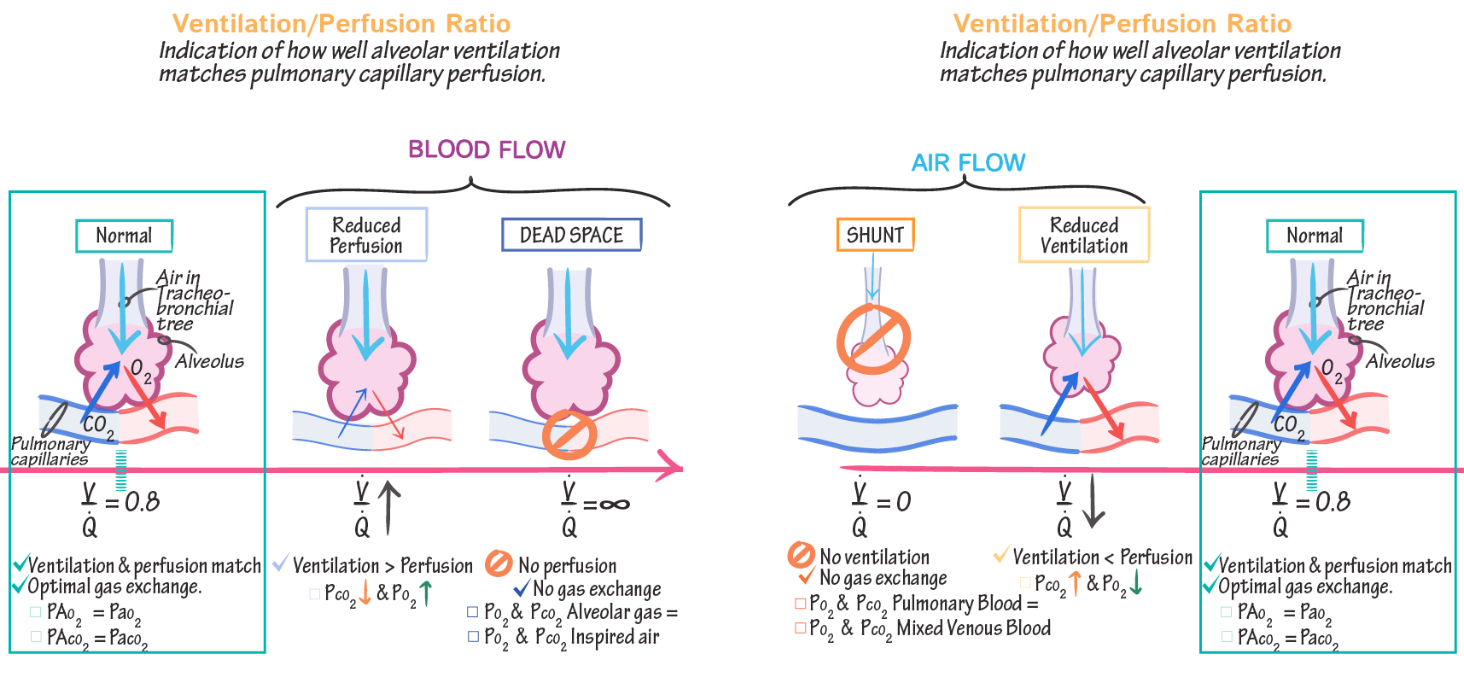
Gases move down their partial pressure gradient. In the oxygen cascade where is gas movement by diffusion? Where is gas movement by convection?
Gas Movement by Convection :
Occurs in the bulk transport of oxygen via airflow through the trachea and bronchi into the alveoli.
Continues in the blood as oxygen is carried from the lungs to tissues by the cardiovascular system.
Gas Movement by Diffusion :
Occurs at the alveolar-capillary interface, where oxygen diffuses from alveoli into the blood and carbon dioxide diffuses out.
Happens again at the tissue level, where oxygen diffuses from capillaries into cells and carbon dioxide diffuses into the blood.
Convection dominates long-distance transport, while diffusion handles short-distance gas exchange across membranes.
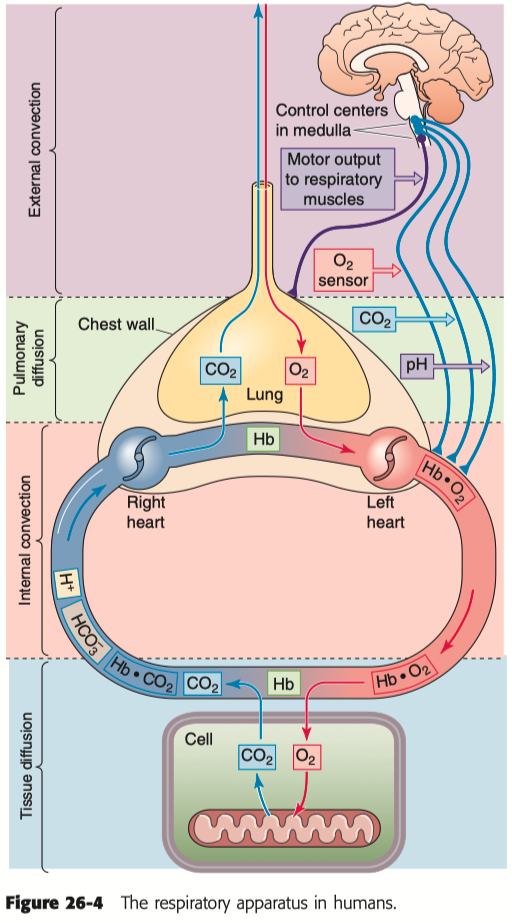
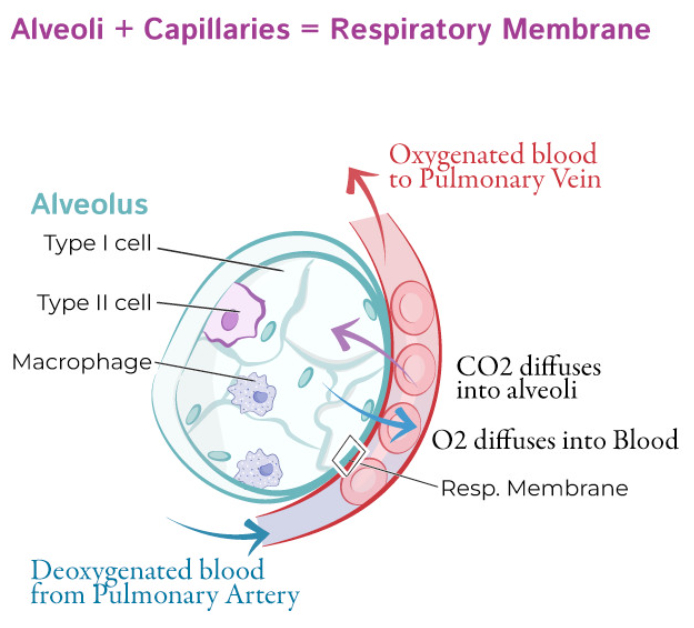
Why is hemoglobin critical in oxygen delivery to tissues.
Hemoglobin is critical because it binds oxygen in the lungs and releases it to tissues, greatly increasing blood's oxygen-carrying capacity.
Oxygen is poorly soluble in plasma; hemoglobin allows ~98% of oxygen to be transported bound to it, rather than dissolved.
Hemoglobin's cooperative binding ensures efficient oxygen uptake in the lungs ( high partial pressure ) and release in tissues ( low partial pressure ).
Without hemoglobin, the oxygen content of blood would be insufficient to meet tissue metabolic demands.
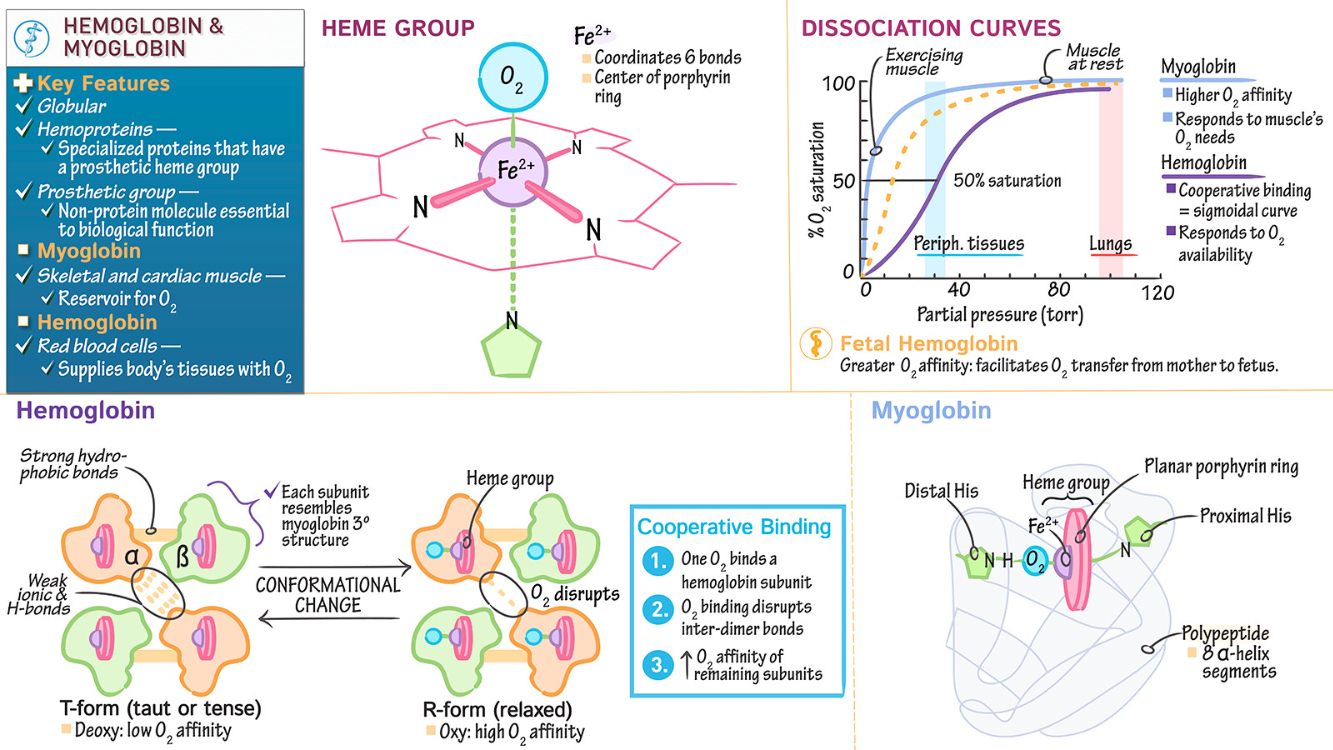
Most conditions of anoxia are readily evident by a blue hue to unpigmented or lightly pigmented skin surfaces. Name one condition of anoxia where hemoglobin saturation is complete ( and the skin is pink )
Carbon Monoxide ( CO ) Poisoning : In this condition, hemoglobin binds carbon monoxide with high affinity, forming carboxyhemoglobin, which prevents oxygen delivery to tissues. Despite anoxia, hemoglobin remains saturated, giving the skin a pink or cherry-red hue instead of cyanosis.
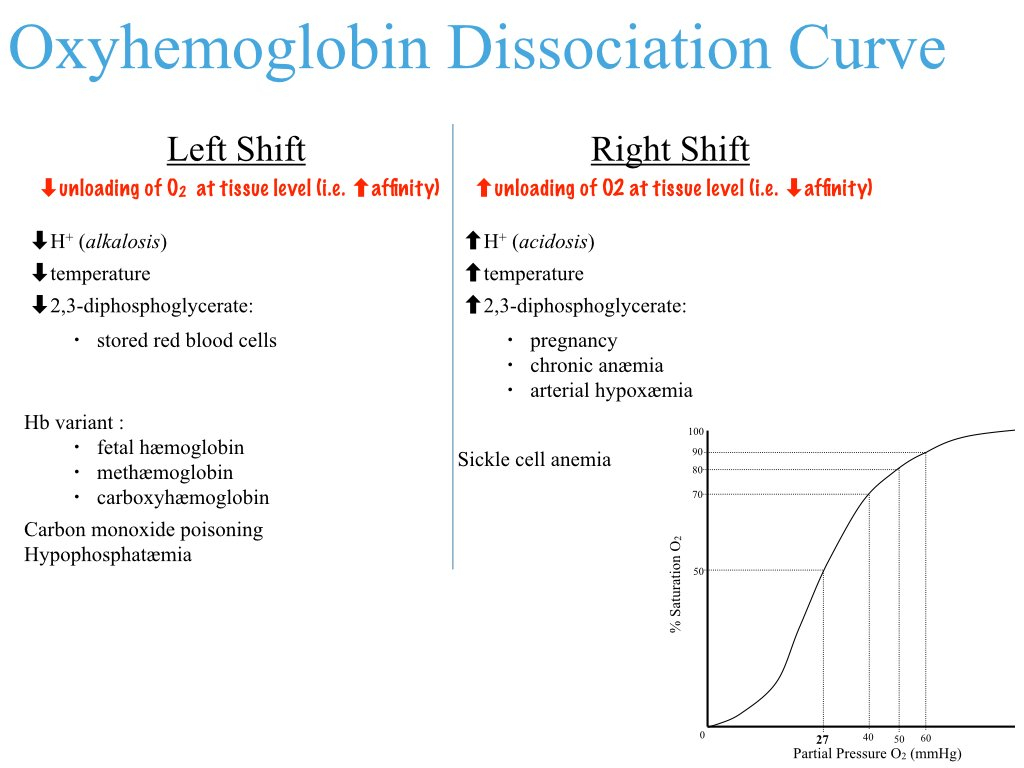
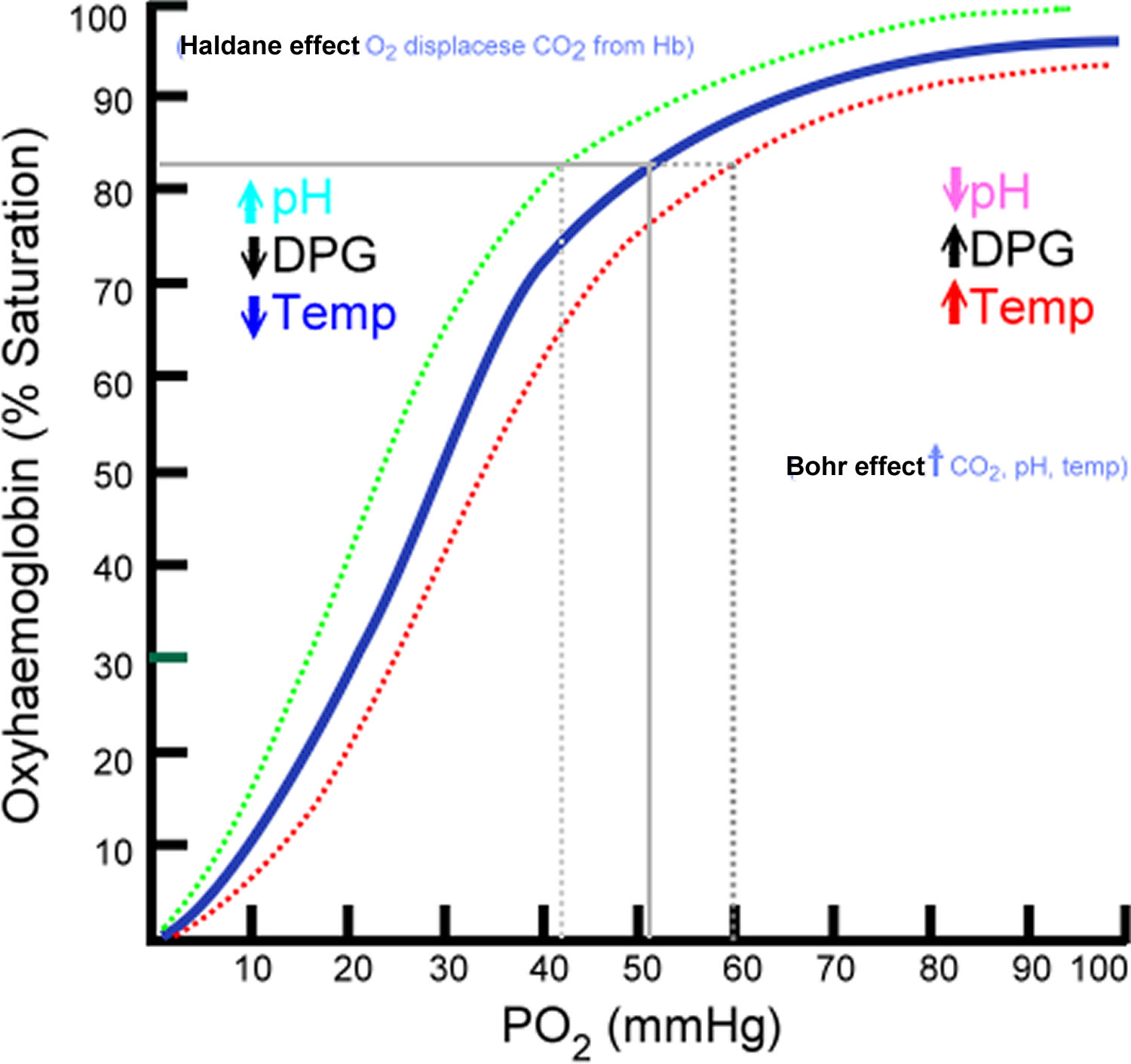
What factors right-shift the oxyhemoglobin dissociation curve? What effect on oxygen uptake/delivery does rightshifting the curve have? Under what circumstances would the curve be right-shifted?
Factors that Right-Shift the Curve: Increased
Effect on Oxygen Uptake/Delivery : A right shift reduces hemoglobin's affinity for oxygen , making it easier to release oxygen to tissues but harder to bind oxygen in the lungs.
Circumstances for Right Shift : Occurs during exercise, hypoxia, fever, or acidosis, where tissues require more oxygen to meet metabolic demands.
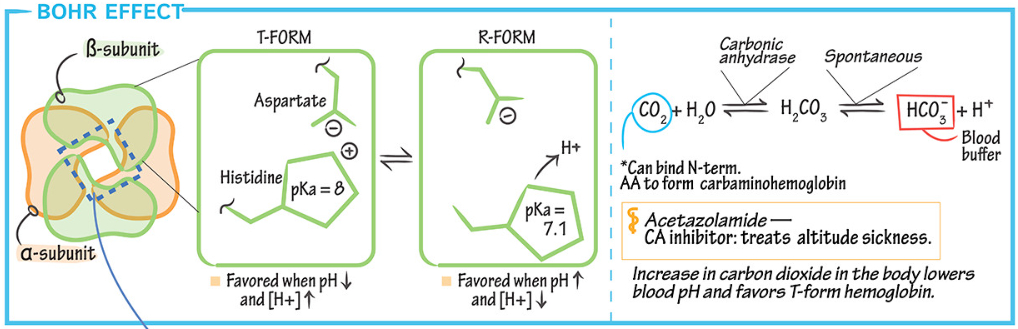
What is the Bohr effect?
The Bohr effect describes how increased carbon dioxide (
This facilitates oxygen unloading in metabolically active tissues where
It shifts the oxyhemoglobin dissociation curve to the right, enhancing oxygen delivery while reducing oxygen binding in these regions.
This mechanism ensures tissues receive more oxygen when they need it most.
Why does fetal hemoglobin have a left-shifted curve?
Fetal hemoglobin ( HbF ) has a left-shifted oxyhemoglobin dissociation curve because it binds oxygen with higher affinity than adult hemoglobin ( HbA ).
This is due to reduced binding of 2,3-BPG, which stabilizes the low-affinity state in HbA but has less effect on HbF.
The left shift allows HbF to effectively extract oxygen from maternal blood in the placenta, ensuring adequate oxygen transfer to the fetus.
What are the 3 ways CO2 is transported in the blood?
Dissolved in Plasma : About 7-10% of
Bound to Hemoglobin : Around 20-30% of
As Bicarbonate Ions (
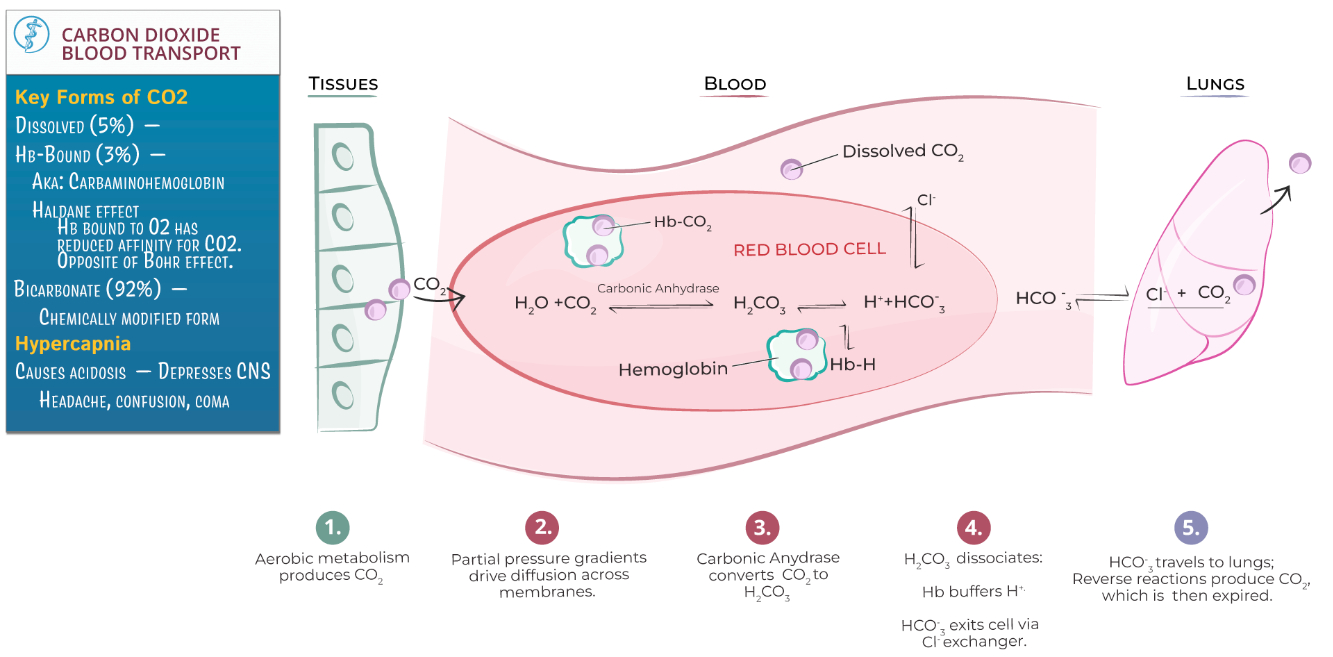
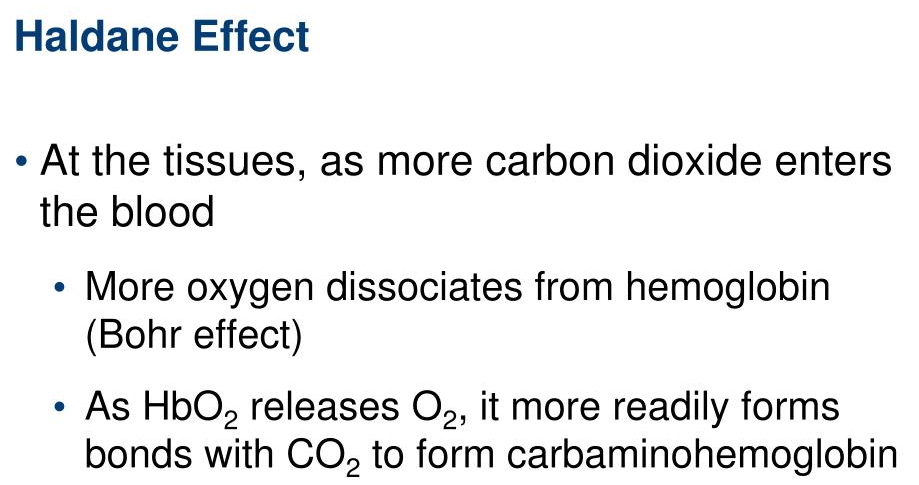
What is the Haldane effect?
The Haldane effect describes how deoxygenated hemoglobin binds carbon dioxide (
This enhances
In the lungs , oxygenation of hemoglobin reduces its ability to carry
The Haldane effect complements the Bohr effect in optimizing gas exchange during respiration.
H - emoglobins
A - affinity
LDane - Carbon Dioxide
B -
O - xygen
H - hydrogen
R - eleased in tissue
Distinguish restrictive and obstructive pulmonary diseases.
Restrictive Pulmonary Diseases: Characterized by reduced lung compliance and difficulty expanding the lungs. Examples include pulmonary fibrosis and interstitial lung disease. Spirometry shows decreased total lung capacity ( TLC ), vital capacity ( VC ), and forced vital capacity ( FVC ), but the FEV1/FVC ratio is normal or increased.
Obstructive Pulmonary Diseases: Characterized by airflow limitation due to narrowed or obstructed airways. Examples include asthma, chronic obstructive pulmonary disease ( COPD ), and bronchitis. Spirometry shows reduced forced expiratory volume in 1 second ( FEV1 ) and FEV1/FVC ratio, with normal or increased TLC due to air trapping.
Key Difference: Restrictive diseases impair lung expansion, while obstructive diseases hinder airflow.
| Feature | Restrictive | Obstructive |
|---|---|---|
| Primary Problem | Limited lung expansion (compliance issue) | Airflow limitation (resistance issue) |
| V/Q Mismatch | Mild-to-moderate low V/Q | Severe low and high V/Q, shunting, dead space |
| Hypoxemia | Diffusion impairment | V/Q mismatch, shunting |
| Hypercapnia | Rare (unless severe disease) | Common in advanced disease |
During an acute dehydration event, an individual’s intravascular colloid osmotic pressure increases ( proteins are concentrated due to water loss ). If no other parameters change to compensate for this increase, what will happen to fluid flux within a given capillary network?
Increased Colloid Osmotic Pressure: Elevated intravascular colloid osmotic pressure pulls more fluid into the capillaries from the interstitial space.
Fluid Flux Effect: Net fluid movement shifts toward absorption rather than filtration, reducing interstitial fluid volume and increasing plasma volume.
Key Outcome: This mechanism partially compensates for water loss by drawing fluid back into the intravascular compartment, but excessive dehydration can still lead to reduced tissue perfusion.
Respiratory System - Misc
Mechanics of Breathing :
Negative Pressure Pump : Diaphragm contraction increases thoracic volume , creating sub-atmospheric pressure in the lungs to draw air in
Lung Compliance : Elasticity of lung tissue and surfactant reduce work of breathing; chest wall counteracts collapse
Gas Exchange Principles :
Fick’s Law : Gas exchange depends on surface area, pressure gradient, and barrier thickness ( alveoli optimized for all three )
Pathway : Oxygen diffuses through alveolar epithelium , interstitial space , and capillary endothelium into blood
Pulmonary Circulation :
Low Pressure , High Efficiency : Thin capillaries with large surface area ensure rapid diffusion.
Hypoxic Pulmonary Vasoconstriction ( HPV ) : Redirects blood flow from poorly ventilated to well-ventilated areas, but in chronic hypoxia ( e.g., emphysema ) , can cause pulmonary hypertension and right heart failure
Control of Breathing :
Central Chemoreceptors : Monitor CO2 and H+ in cerebrospinal fluid ( CSF ); major driver of respiration
Peripheral Chemoreceptors : Detect low oxygen in the carotid and aortic bodies
Nervous Control :
Sympathetic : Dilates airways , reduces mucus secretion
Parasympathetic : Constricts airways , increases mucus production
Clinical Implications :
Obstructive Diseases : Increased airway resistance and residual volume ( e.g., asthma, COPD )
Restrictive Diseases : Reduced lung expansion and volumes ( e.g., pulmonary fibrosis )
Surfactant Deficiency : Leads to alveolar collapse ( atelectasis ) , common in premature infants ( neonatal respiratory distress syndrome )
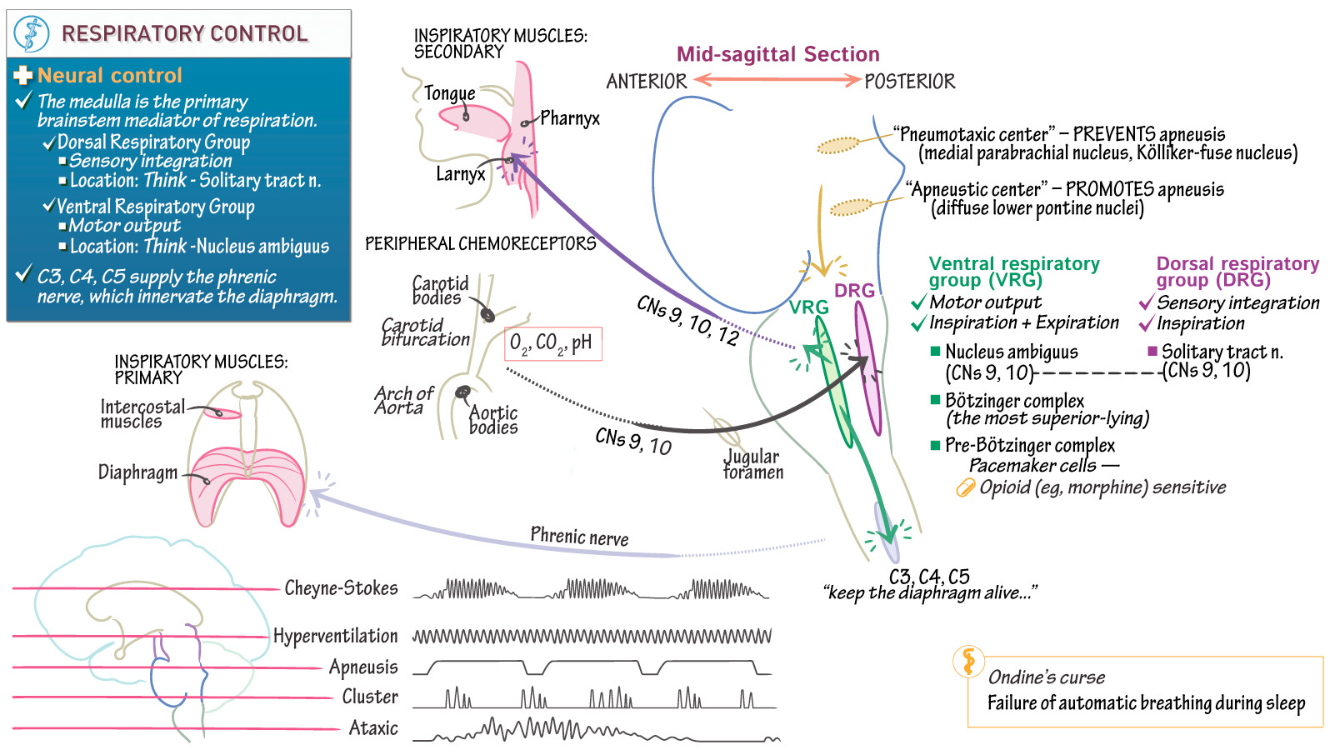
Control of Breathing
Breathing Overview :
Voluntary and Involuntary Control : Voluntary control bypasses the brainstem, originating in the motor cortex, while involuntary control is driven by the pontine-medullary respiratory network.
Phases of Breathing : Resting motor pattern includes three phases: inspiration, post-inspiration, and late expiration.
Coordination : Central integration ensures synchronization with other functions like swallowing, sniffing, and speaking.
Rhythm Generation : Rhythms originate in the pre-Bötzinger complex ( inspiration ) and the Bötzinger complex ( expiration )
Voluntary Control of Breathing :
Voluntary control bypasses respiratory centers, involving corticospinal tracts and regions like the motor cortex , premotor area , and prefrontal cortex.
fMRI and PET studies reveal activity in these areas during voluntary breath control, though the exact mechanisms remain partially understood.
Automatic Control of Breathing :
Chemoreceptor Regulation : Arterial CO2 is the primary driver via central chemoreceptors, while peripheral chemoreceptors (carotid and aortic bodies) respond faster and are stimulated by hypoxia , hypercapnia , and acidosis.
Mechanisms : In carotid bodies , hypoxia inhibits potassium channels in Type I cells, leading to depolarization, calcium entry, and neurotransmitter release. Central chemoreceptors respond to changes in extracellular pH and CO2 in cerebrospinal fluid ( CSF )
Central Chemoreception :
Neuronal Locations : Chemosensitive neurons are present in the retrotrapezoidal nucleus, medullary raphé, and ventrolateral medulla.
CO2 Sensitivity : Blood CO2 diffuses into CSF, altering pH via the bicarbonate buffer system, and modulates respiratory neuron firing through changes in extracellular and intracellular environments.
Reflex Control of Breathing :
Hering-Breuer Reflex : Activated by airway stretch receptors when tidal volume exceeds 1L, terminating inspiration.
Diving Reflex : Cold stimulation of facial receptors induces apnea, bradycardia, and peripheral vasoconstriction to conserve oxygen.
Other Reflexes : Includes the sneeze reflex triggered by nasal receptor stimulation.
Physiological Adaptations to Hypoxia :
High Altitude Acclimatization : Initial respiratory increase ( 1.65x ) is peripheral chemoreceptor-driven. Long-term acclimatization involves hyperventilation, increasing O2 uptake and reducing CO2 for acid-base stability.
Everest Adaptations : Reinhold Messner’s ascent demonstrated the extreme limits of human respiratory capacity under hypoxia.
Comparative Physiology :
Bar-Headed Goose : Adaptations such as efficient hemoglobin, high tidal volume, and insensitivity to low CO2 enable survival at altitudes where humans cannot function.
Abnormalities in Breathing Control :
Obstructive Sleep Apnea ( OSA ) : Upper airway collapse leads to hypoxia and hypercarbia, sensitizing carotid bodies and causing hypertension and heart failure.
Central Sleep Apnea ( CSA ) : Reduced ventilatory drive results in milder symptoms than OSA.
Sudden Infant Death Syndrome ( SIDS ) : Linked to ventilatory control abnormalities, potentially involving desensitized nicotinic receptors in carotid bodies.
Respiratory Pattern Generation :
Inspiratory and expiratory patterns arise from the pontine-medullary respiratory network. The pre-Bötzinger and Bötzinger complexes coordinate rhythms through excitatory and inhibitory signals.
Clinical Implications :
Reflex dysregulation in lung diseases can impair respiratory control.
Abnormal chemosensitivity, as seen in COPD and SIDS, poses challenges for regulation.
Failures in adaptive mechanisms under chronic hypoxia can result in life-threatening outcomes.
Reproduction
3 Examples of Positive Feedback
LH Surge
Oxytocin in Lactation
Oxytocin in Contractions
Describe the Cell Signalling MOLECULAR mechanisms that regulate testosterone and estrogen production
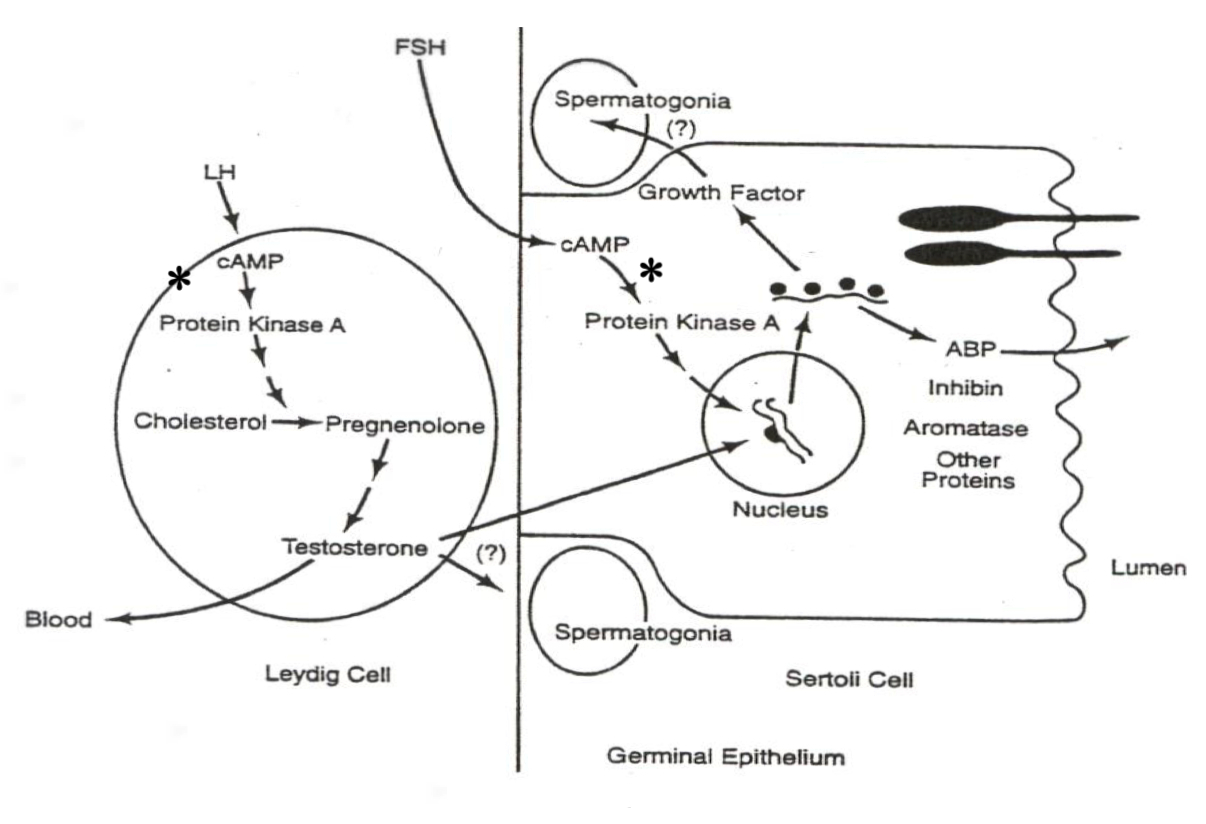
Male System ( Spermatogenesis ) :
LH in Leydig Cells :
xxxxxxxxxxLH ➡️ cAMP ➡️ PKA ➡️ Cholesterol ➡️ Pregnenolone ➡️ TestosteroneExplanation : LH activates cAMP and PKA , driving gene transcription of enzymes that convert cholesterol to testosterone in Leydig cells.
FSH in Sertoli Cells :
xxxxxxxxxxFSH ➡️ cAMP ➡️ PKA ➡️ Gene Transcription ➡️ Androgen-Binding Protein ( ABP )Explanation : FSH activates gene transcription for ABP , aiding sperm maturation and transport.
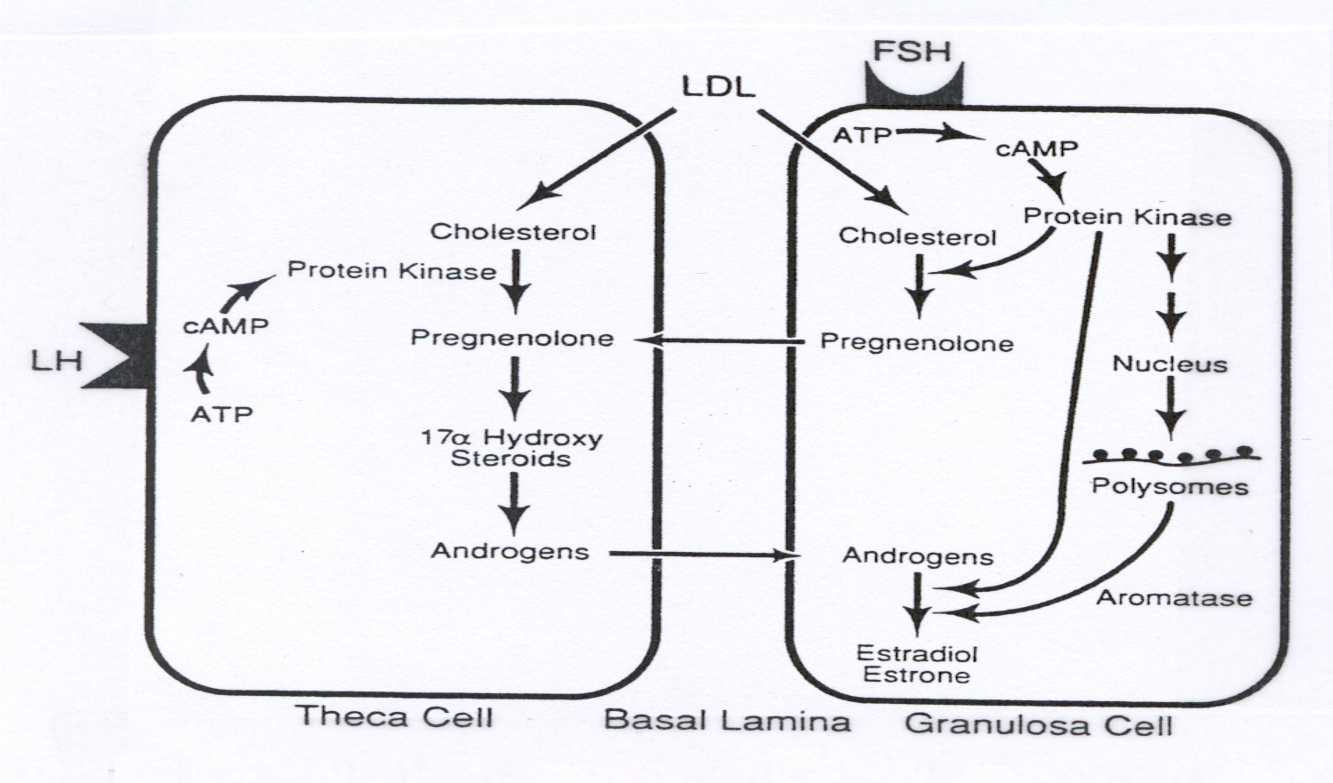
Female System ( Oogenesis and Follicle Development ) :
LH in Theca Cells :
xxxxxxxxxxLH ➡️ cAMP ➡️ PKA ➡️ Cholesterol ➡️ Pregnenolone ➡️ AndrogensExplanation : LH activates gene transcription for enzymes that convert cholesterol to androgens in theca cells.
FSH in Granulosa Cells :
xxxxxxxxxxFSH ➡️ cAMP ➡️ PKA ➡️ Gene Transcription ➡️ Aromatase ➡️ Androgens ➡️ EstrogensExplanation : FSH in granulosa cells transcribes aromatase , converting androgens ( from theca cells ) to estrogens , completing estrogen production.
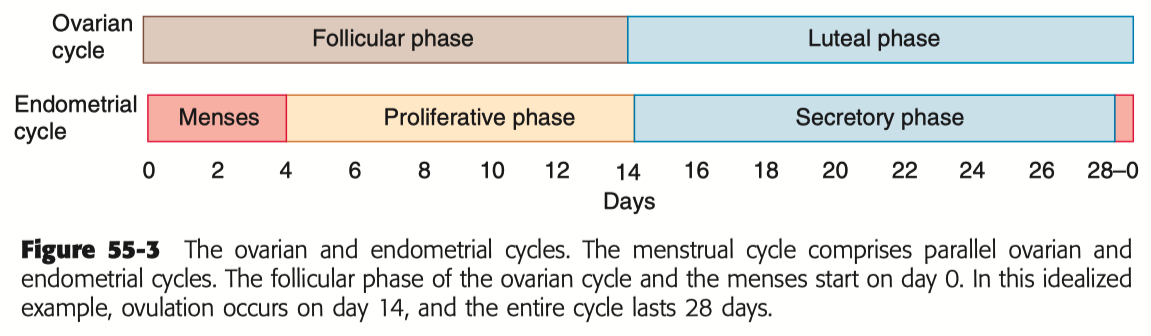
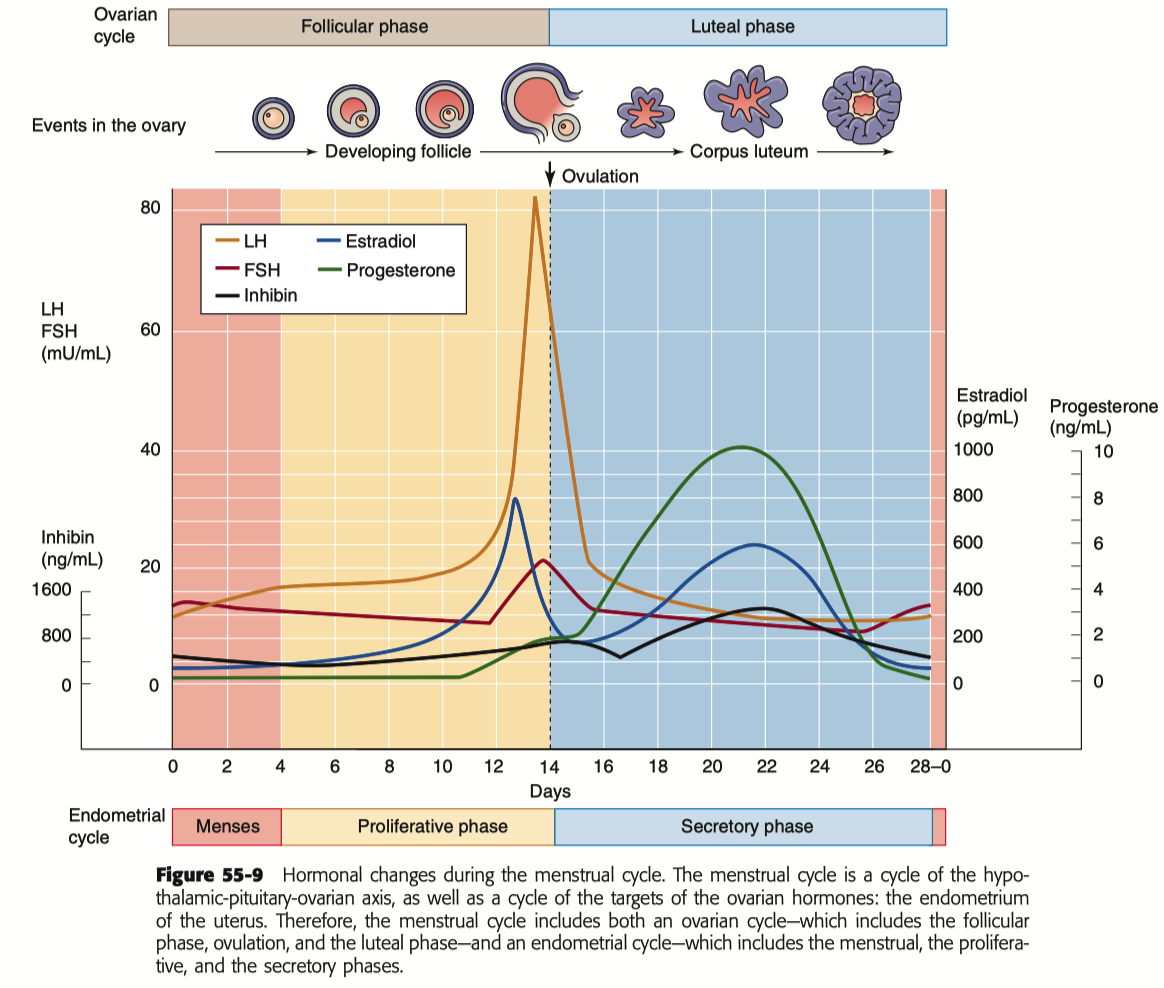
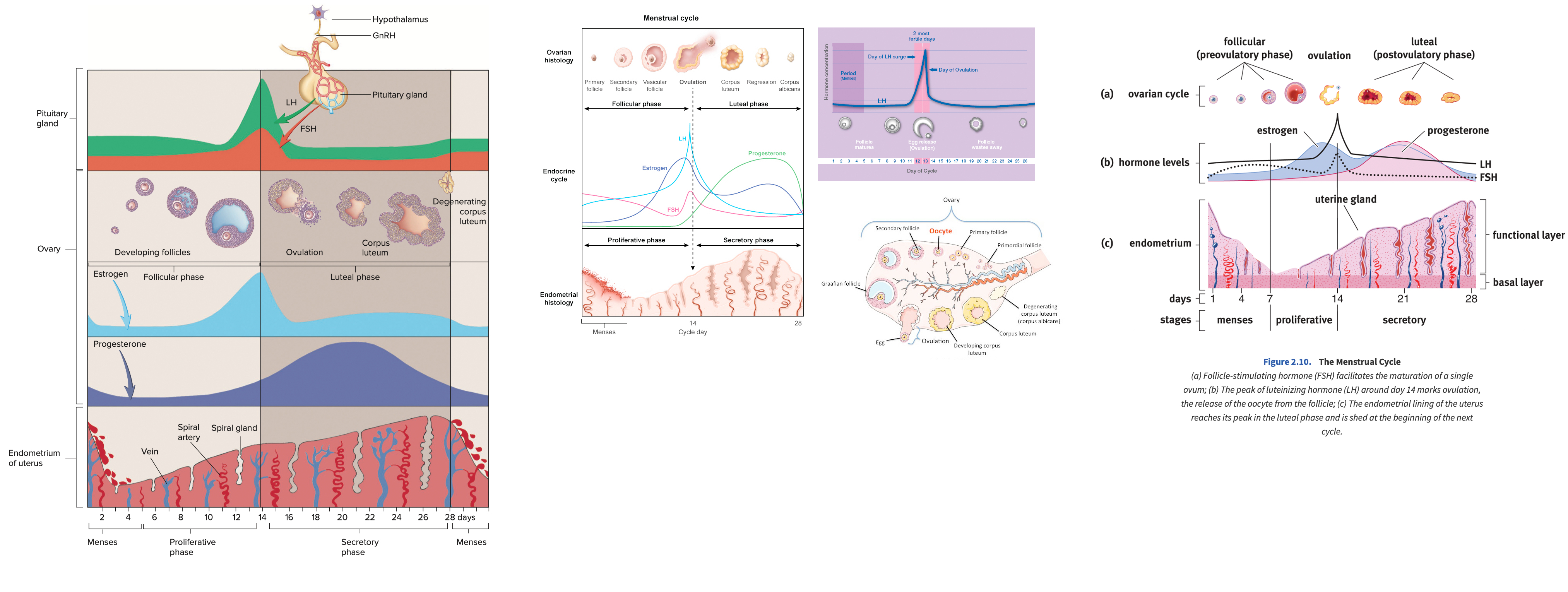
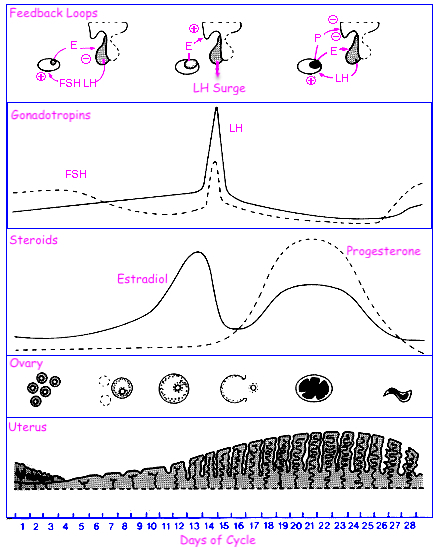
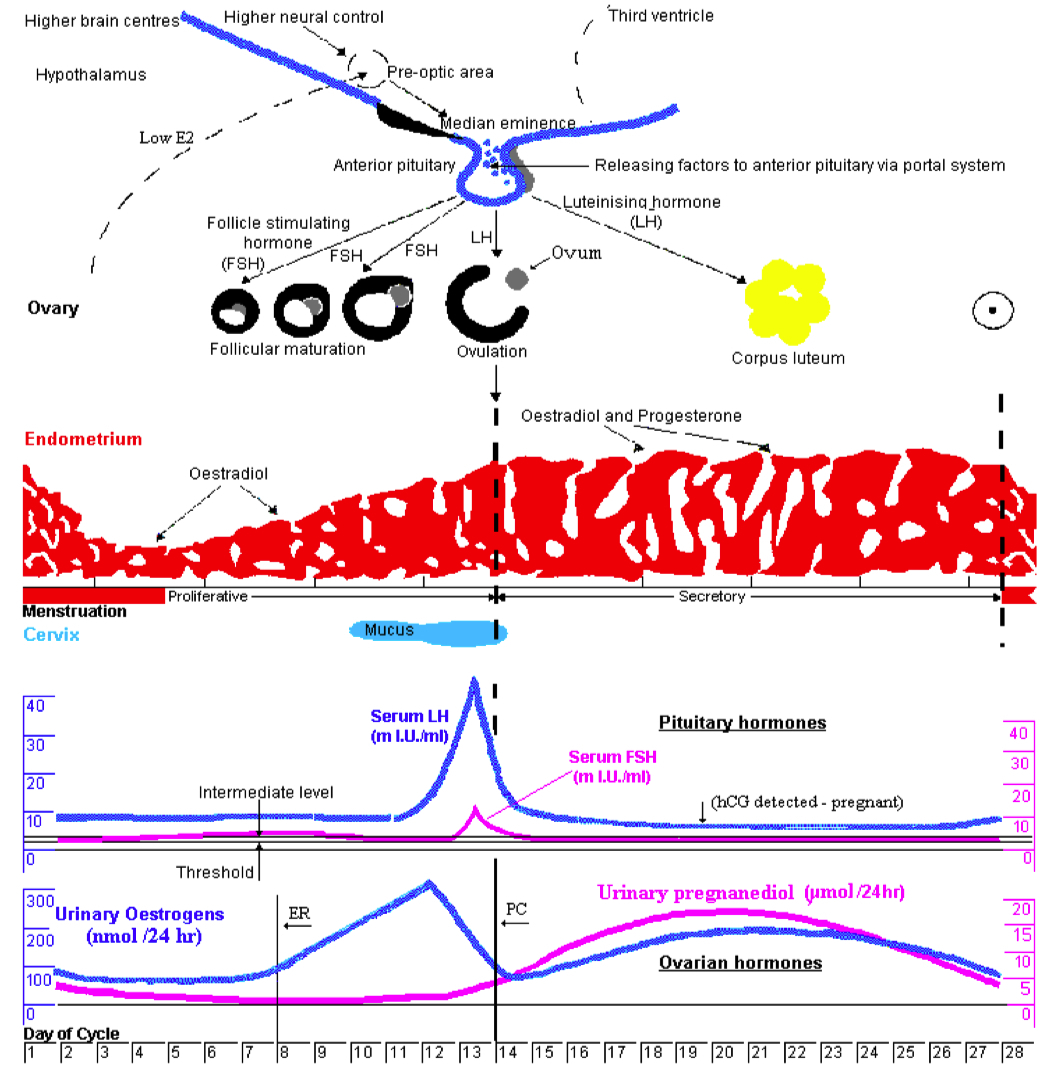
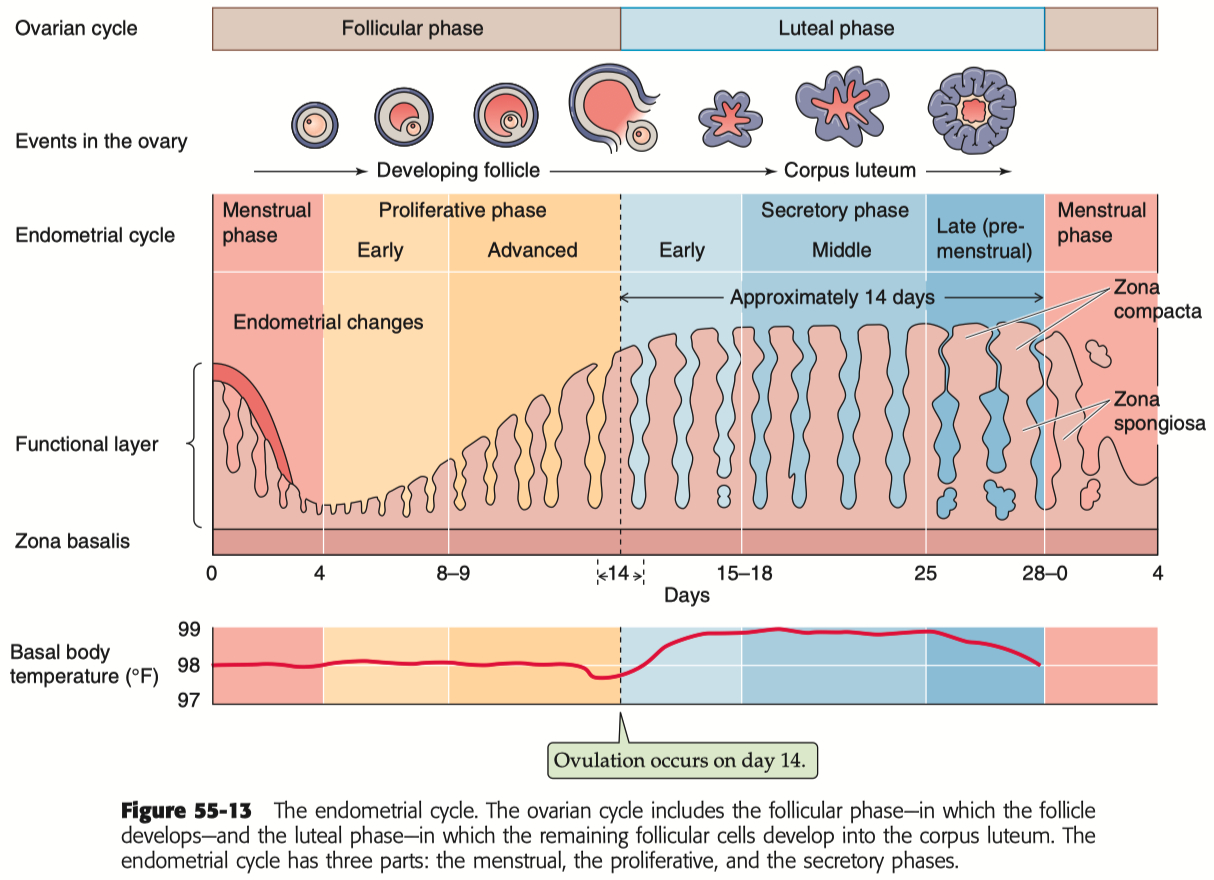
Describe the menstrual cycle in detail in relation to the ovary and uterus
Hormones :
FSH ( Follicle-Stimulating Hormone ) : Stimulates follicle development in the ovaries.
LH ( Luteinizing Hormone ) : Triggers ovulation through the LH surge.
Estrogen : Rises during the follicular phase, supporting uterine lining growth; drops after ovulation.
Progesterone : Rises post-ovulation during the luteal phase to support the endometrium for potential implantation.
Sequence of Hormone Events :
Follicular Phase ( Days 1-14 ) : FSH and estrogen levels gradually increase , supporting follicle development.
Ovulation ( Day 14 ) : Estrogen hits a threshold, causing a positive feedback loop that triggers the LH surge. This surge leads to ovulation.
Luteal Phase ( Days 14-28 ) : Progesterone rises as the corpus luteum forms , maintaining the uterine lining. If no fertilization occurs, progesterone drops , leading to menstruation.
Embryology
Describe what constitutes an embryo - what is the makeup
Cellular Composition :
Zygote : The single-cell stage resulting from the fusion of a sperm and an egg, marking the beginning of the embryo.
Blastomeres : As the zygote undergoes mitotic divisions ( cleavage ) , it forms smaller, undifferentiated cells called blastomeres.
Embryonic Stem Cells : These are pluripotent cells that arise during the early stages ( e.g., inner cell mass of the blastocyst ) and have the potential to differentiate into various cell types.
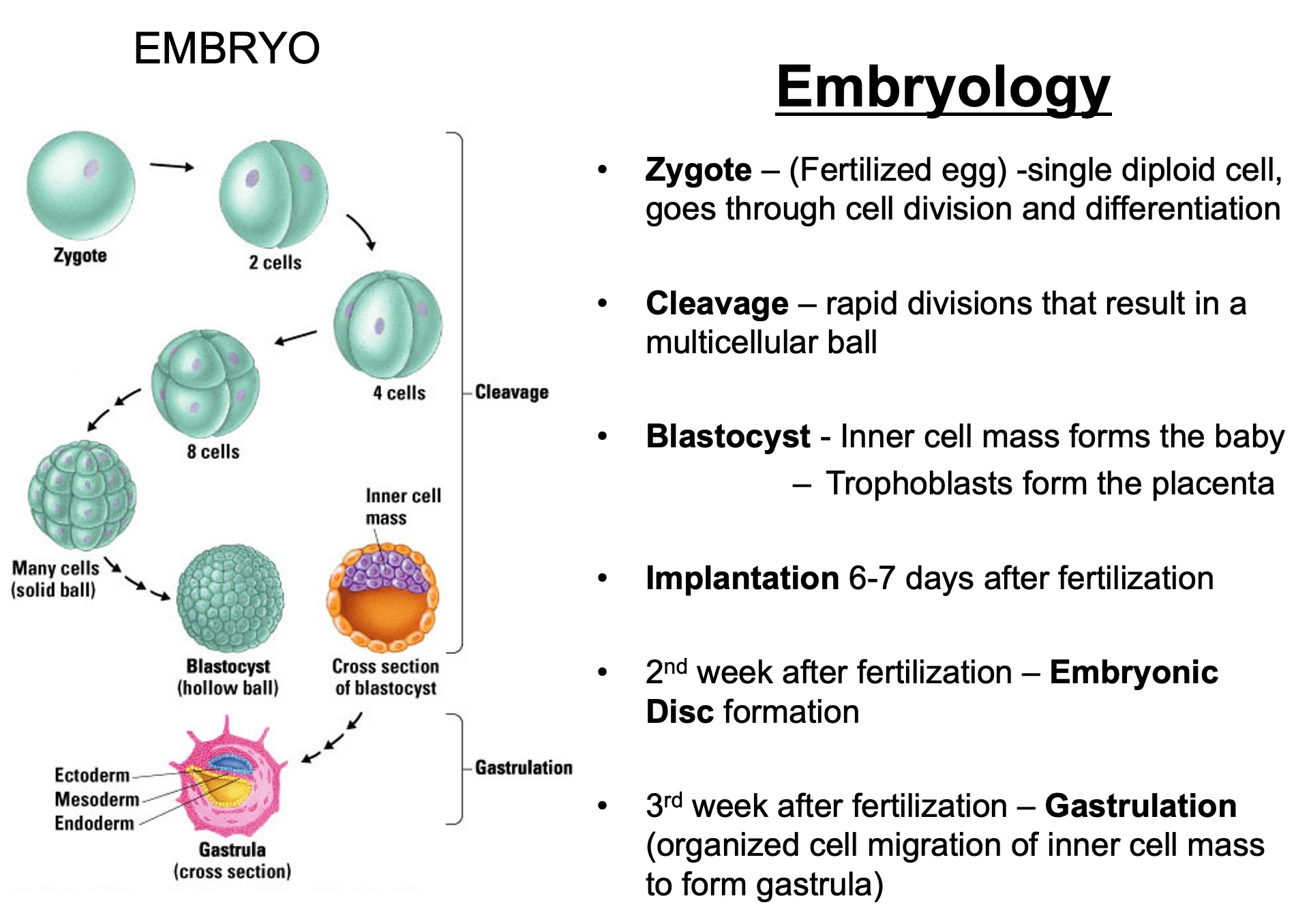
Zygote : Day 0 ( fertilization )
Blastomeres : Day 1-3 ( cleavage )
Morula : Day 3-4 ( solid ball of cells )
Blastocyst : Day 5-6 ( hollow structure , first differentiation )
Bilaminar Disc : Week 2 ( epiblast and hypoblast form )
Trilaminar Disc : Week 3 ( gastrulation creates germ layers )
Organogenesis : Weeks 4-8 ( development of organs and systems )
Fetal Stage : Week 9 onward ( growth and maturation )
By Week 9 , it is no longer referred to as an embryo but as a fetus
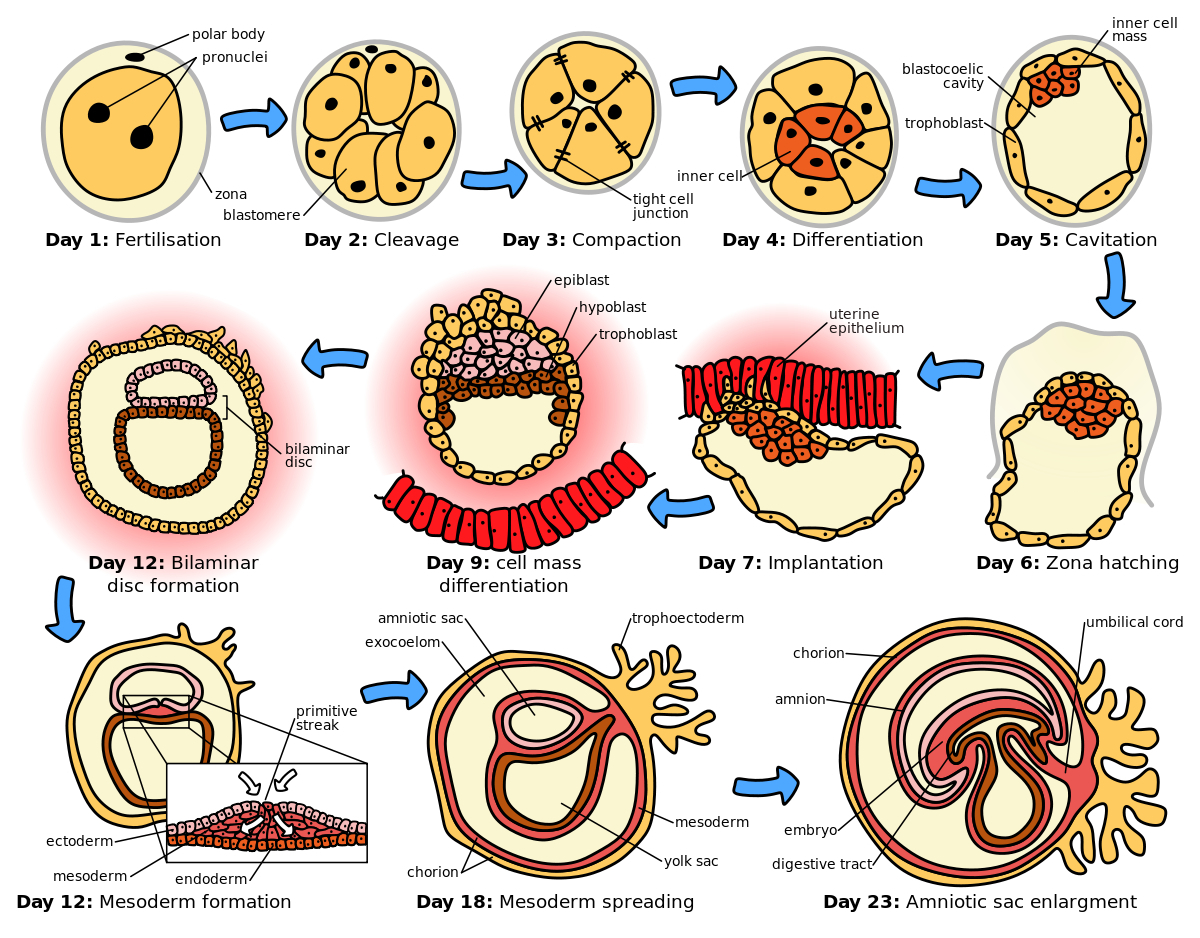
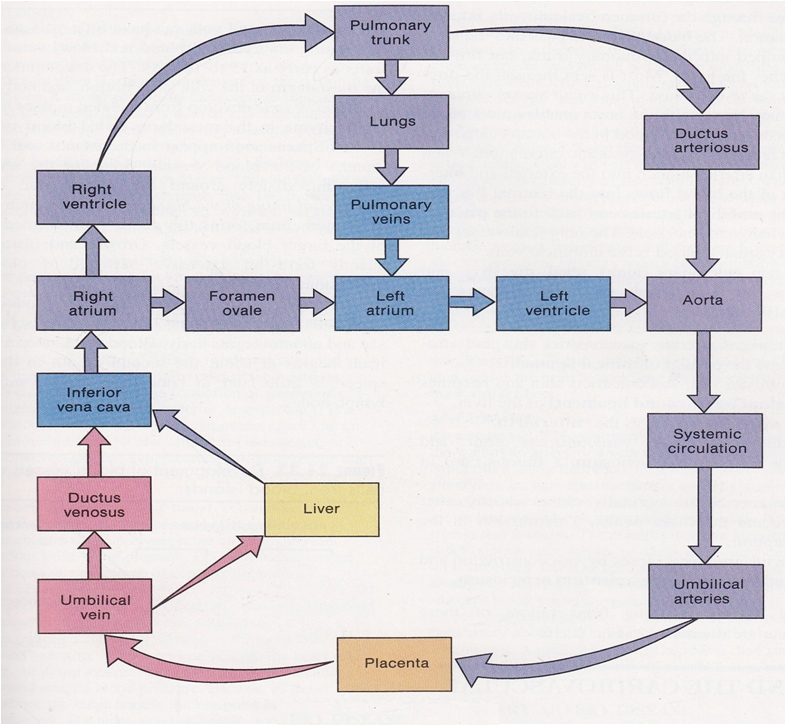
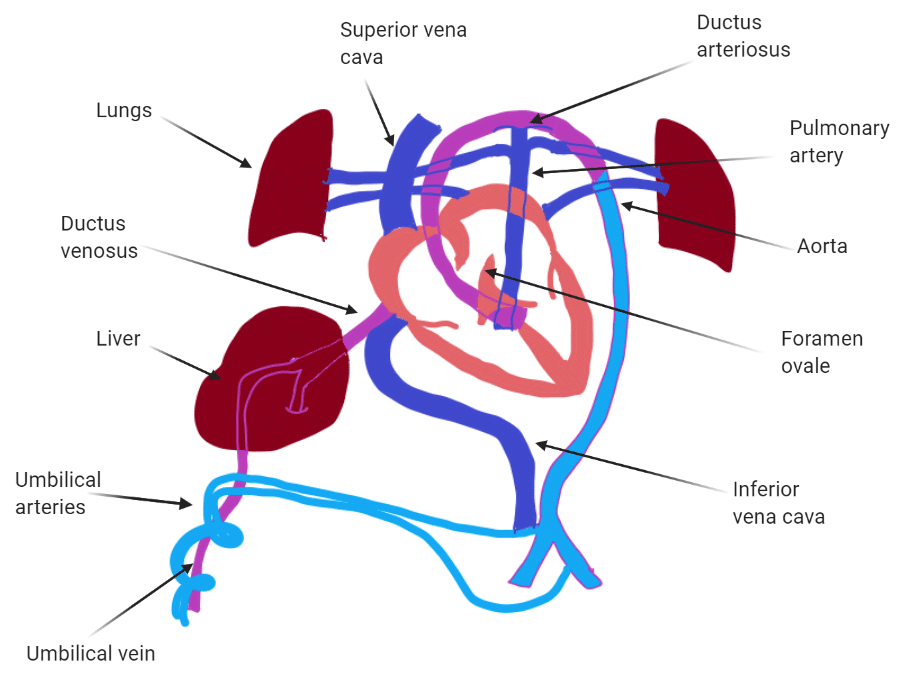
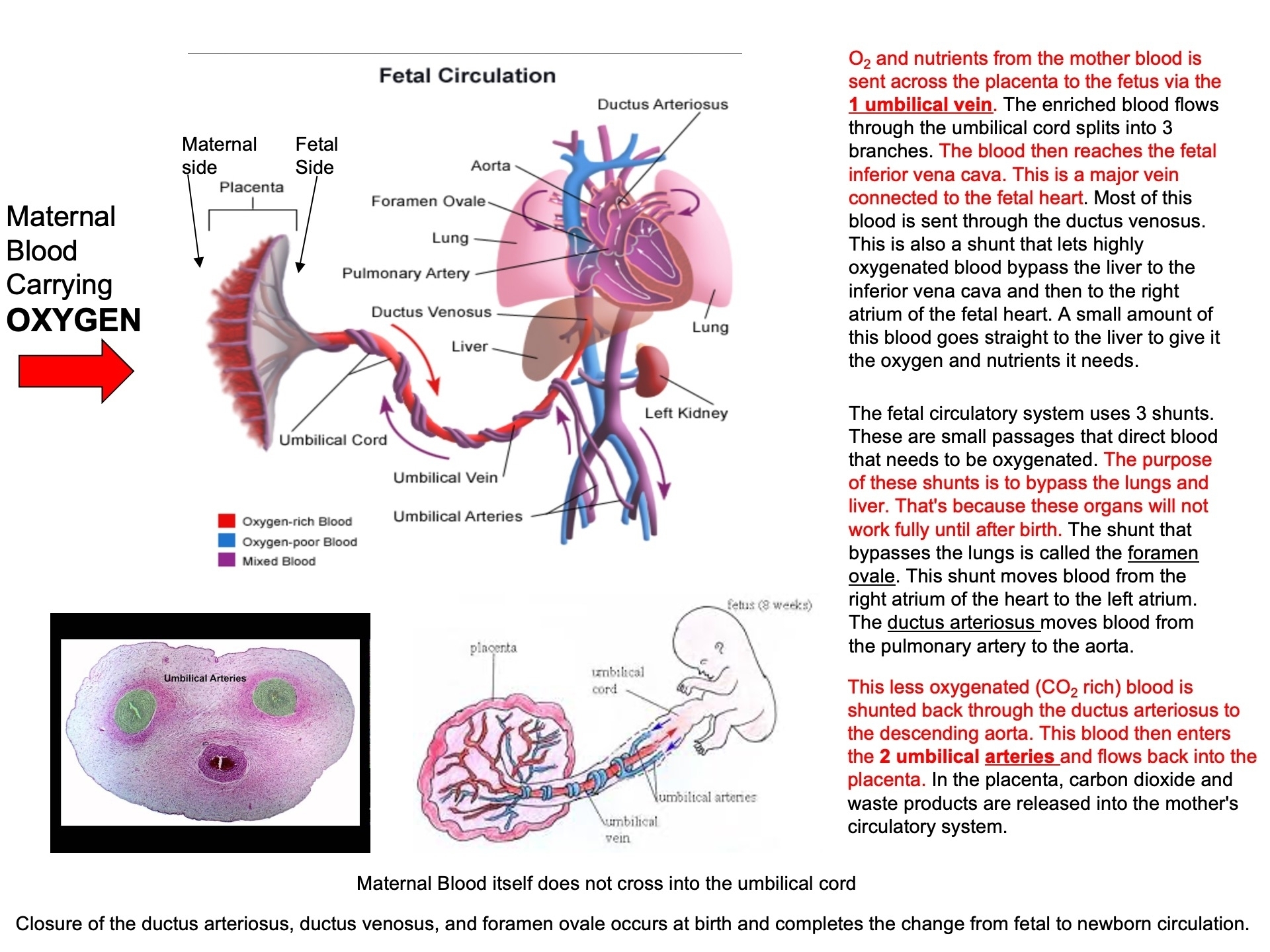
Describe why the umbilical cord is important and how it works
Acts as the conduit connecting the embryo/fetus to the placenta.
Directly connects to the fetal liver and subsequently to the fetal heart via the umbilical vein.
Carries oxygenated blood from the placenta to the fetus.
Carries deoxygenated blood and waste from the fetus to the placenta via the umbilical arteries.
Umbilical Cord Stem Cells :
Multipotent
you bank them at birth and then don't have to worry about donor rejection problems
expensive over time
Typing Memorization Game :
Route of Maternal Blood Oxygen to Baby
Maternal Lungs → Pulmonary veins → Left atrium → Left ventricle → Aorta → Uterine arteries → Placenta ( intervillous space )
Placenta → Oxygen diffuses into fetal capillaries → Umbilical vein :
Liver Branch : A portion of oxygenated blood enters the fetal liver via smaller branches of the umbilical vein
Bypass Liver ( Ductus Venosus ) : The rest bypasses the liver through the ductus venosus , merging with the inferior vena cava ( IVC )
IVC → Right atrium → Foramen ovale → Left atrium → Left ventricle → Aorta → Fetal tissues
Route of CO₂ from Baby Back to Mother
Fetal Tissues ( CO₂ produced ) → Fetal veins → Right atrium → Right ventricle → Pulmonary artery :
Bypass Lungs ( Ductus Arteriosus ) : Most blood bypasses the lungs via the ductus arteriosus , joining the descending aorta
Descending Aorta → Umbilical arteries → Placenta
Placenta ( CO₂ diffuses into maternal blood ) → Uterine veins → Inferior vena cava → Right atrium → Right ventricle → Pulmonary arteries → Maternal lungs ( CO₂ exhaled )