Digestion
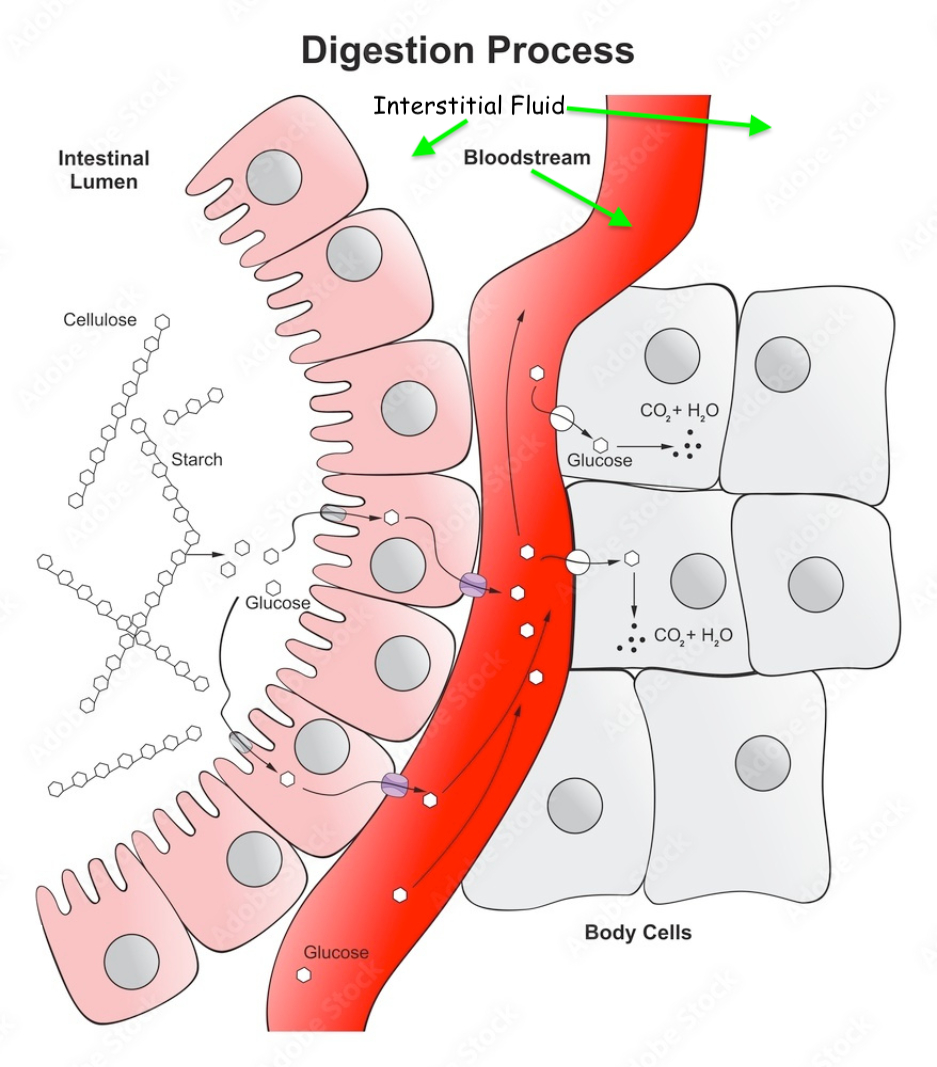
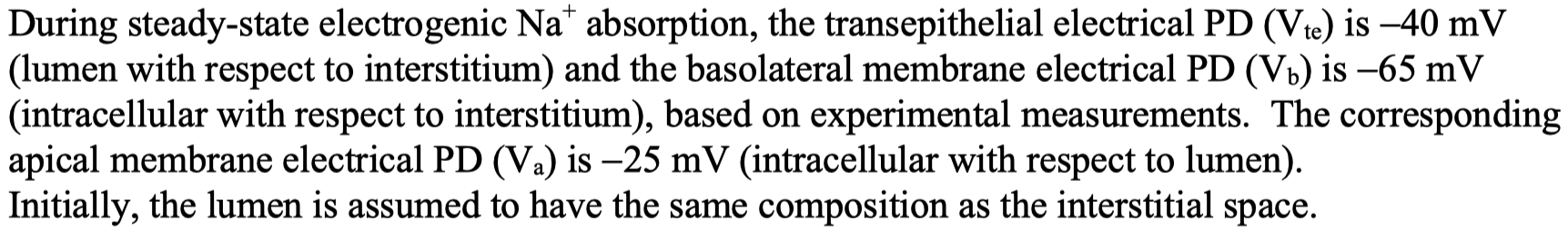
this means the lumen is more negative than the interstitial space by
this means the inside of the enterocyte ( cytosol ) is more negative than lumen by
this means the inside of the enterocyte ( cytosol ) is more negative than the interstitial fluid by
Now we have to unpack the wording of these values
And remember how an electrode / potential recording works
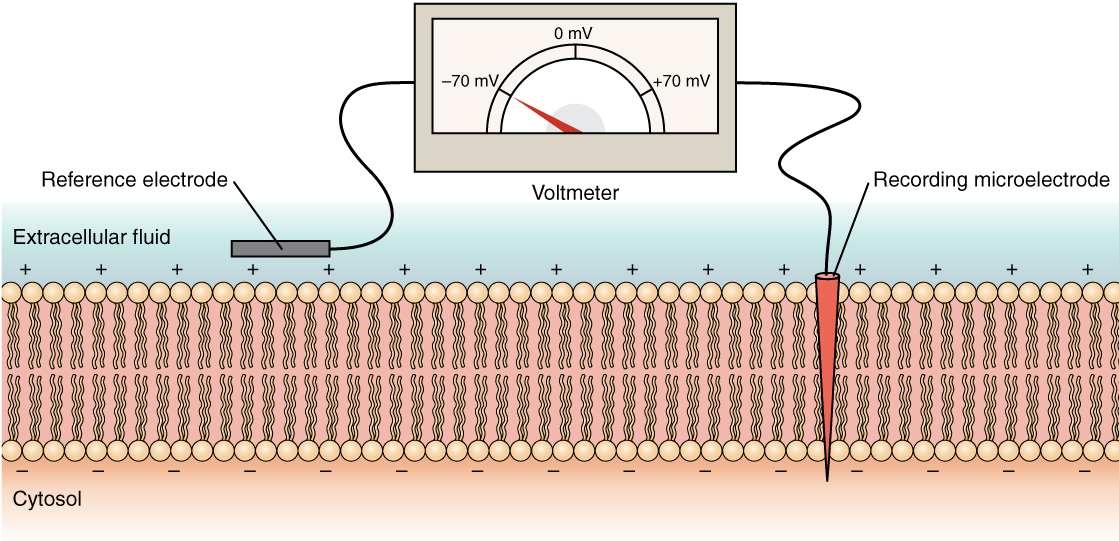
They put the ground / reference electrode into the extracellular fluid ( lumen )
They put the recording electrode into the cytosol
They device reads
You could word this then as
so this is how the electrode was placed for
For
You might be tempted to just plug in the values of
But see the trans epithelial current
Words are getting in the way here.
Just put assign values like this , on either side of the membranes
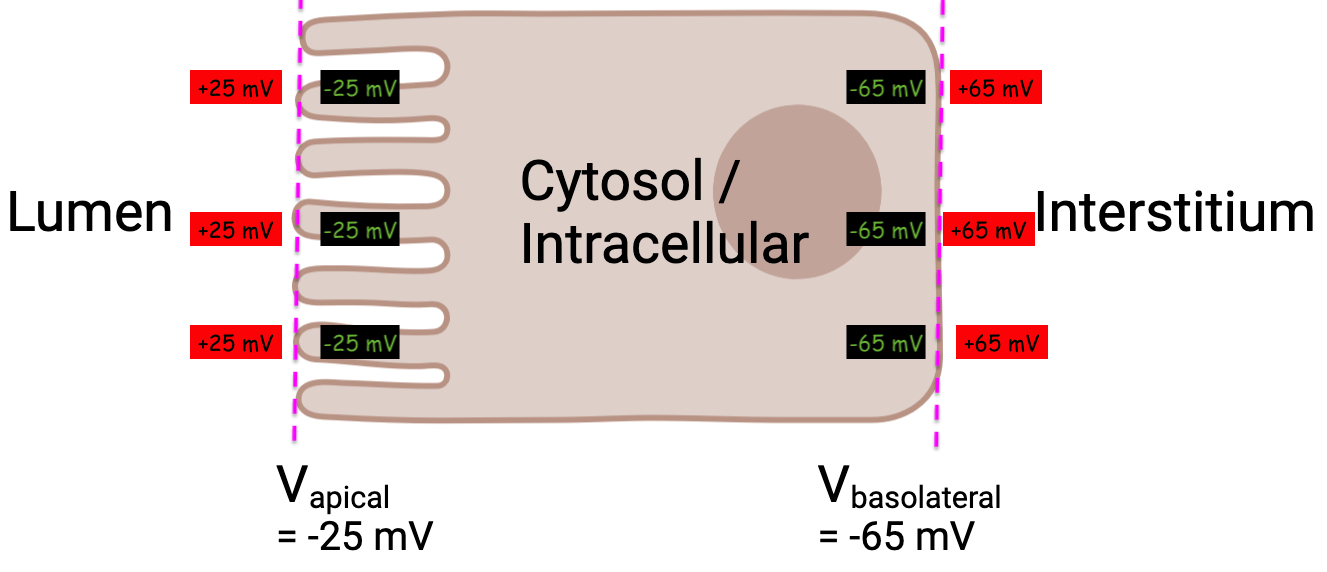
Now we can use our formula again , and we can verify the same value as in the question prompt :
This reminds me of the way the nernst equation written on the card.
I would just completely ignore the
either with a positive 60 , or with a negative 60
the negative 60 version is because you flipped the log values
I personally , always use the
if you have a negative ion , chloride for instance ,
Just write it as :
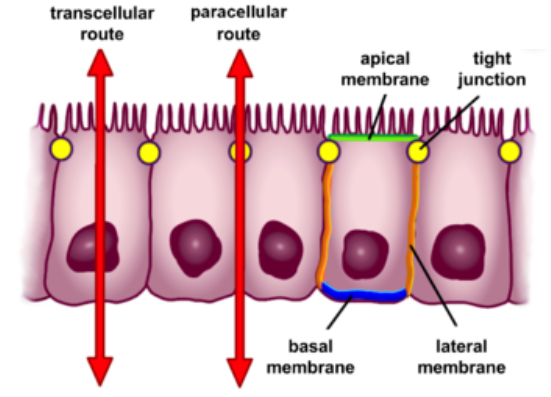
The Trans-Epithelial Potential Difference (TEPD) across epithelial cells, like those in the kidney and intestines, acts as an electrical gradient that influences the movement of ions and water.
TEPD represents the electrochemical gradient that governs the movement of ions and water across the epithelial barrier, driven by two forces:
Electrical Gradient: The difference in charge (voltage) across the epithelium.
Concentration Gradient: The difference in ion concentration across the membrane.
These forces drive ions to move from regions of higher concentration or charge to lower concentration or opposite charge, a fundamental principle of physics often described by the Nernst equation.
The movement of water is driven osmotically based on solute (primarily ion) concentrations.
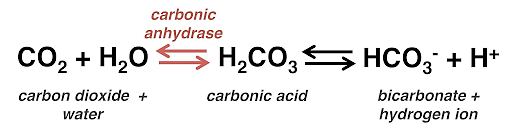
Summary: TEPD and Its Role in Ion and Water Transport
Na⁺/K⁺ ATPase creates the low intracellular Na⁺ concentration and positive interstitial charge, establishing the TEPD.
Na⁺ flows passively into epithelial cells, driven by the Na⁺ gradient and the negative TEPD.
Cl⁻ follows Na⁺ to maintain electrical neutrality, assisted by channels like CFTR and antiporters like the Cl⁻/HCO₃⁻ exchanger.
K⁺ is recycled via K⁺ channels, helping sustain the TEPD and facilitating ion transport.
Water follows the osmotic gradient set up by ion reabsorption, moving passively through aquaporins or paracellularly.
Hormonal regulation adjusts these processes, ensuring proper electrolyte and fluid balance in the body.
Kidney vs. Intestines: How TEPD is Used in Different Tissues
In the intestines, TEPD primarily facilitates nutrient (e.g., glucose) and electrolyte absorption, which indirectly supports water absorption necessary for maintaining fluid balance in the body.
In the kidney (e.g., proximal tubule, loop of Henle), TEPD drives reabsorption of electrolytes and water crucial for urine concentration and volume regulation.
Students can describe serial processing of meal components by the gastrointestinal organ system. Students can describe epithelial flow pathways supporting transepithelial absorption and secretion.
we don't do it all at once
each component specializes
makes it easier for the next section
Process of Saliva Secretion and Osmotic Forces Leading to Cell Lysis:
Primary Secretion (Isotonic Fluid Production):
Acinar cells in salivary glands produce an initial secretion.
This primary fluid is isotonic to plasma, meaning it has a similar concentration of solutes like sodium (Na⁺) and chloride (Cl⁻).
Water follows the ions into the lumen through osmosis, maintaining an equal solute concentration inside the lumen and the surrounding cells.
Ductal Modification of Saliva (Hypotonic Fluid):
The isotonic fluid moves from the acinar cells into the ducts of the salivary glands.
In the ducts, Na⁺ and Cl⁻ are reabsorbed, while K⁺ and HCO₃⁻ are secreted into the saliva.
The ducts are impermeable to water, so although ions are reabsorbed, water stays in the saliva.
This process causes the final saliva to become hypotonic (lower in solute concentration compared to plasma).
Contact with Cells (Osmotic Gradient Creation):
When this hypotonic saliva comes into contact with cells, like bacteria, an osmotic gradient is established.
The inside of the bacterial cell has a higher solute concentration compared to the hypotonic saliva outside.
Osmotic Water Influx:
Water flows into the bacterial cells by osmosis because water moves from areas of lower solute concentration (hypotonic saliva) to areas of higher solute concentration (inside the bacterial cell).
Cell Swelling and Lysis:
As water enters the bacterial cell, the cell swells.
If the bacterial cell membrane or wall cannot withstand the increased internal pressure from the water influx, the cell can lyse (burst) due to the osmotic pressure.
Gastric Secretions
Acid secretion
Parietal Cells
Intrinsic factor
helps absorb B12
Chief Cells
release pepsin
Microbiome
cellulase
Pancreas
digests everything
most important digestive organ
amylases , peptidases , lipases
dumps bi-carb everywhere to buffer stomach acid
carbonic anhydrase system
keeps saying that maybe metabolism isn't enough carbon to generate the amount of CO2 you need to run this system ?
Liver
also bicarb
and bile acids
Passive Nutrient Absorption in Brush Boarder Membrane
di-saccharides
lactose
sucrose
proteins
amino acids
fats
fluids = bulk of reabsorption happens distally
nutrient reabsorption happens proximally in intestine
Misc
peristalsis mixes and moves it along
control is neural and hormonal
cephalic phase - sensory
salivary , gastric , and pancreatic secretions
gastric phase - enteroendocrine cells
g-cells produce gastrin = stimulates H+ screation and pepsinogen release
s-cells produce secretin = stimulates pancreatic bicarbonate secretion
i-cells produce CCK = stimulates enzyme release from acinar cells
l-cells produce peptide-YY = satiety
Students can describe cellular mechanisms for solute and water absorption and secretion.
Absorption: Nutrients and ions are absorbed primarily in the small intestine through both cellular pathways (via transport proteins) and paracellular pathways (between cells). Na⁺/K⁺-ATPase creates a gradient that drives the uptake of nutrients and water.
Secretion: Cells in the GI tract also secrete electrolytes and enzymes necessary for digestion.
For example, chloride is secreted through channels like CFTR , facilitating water movement by osmosis
Solute absorption involves active transport mechanisms like the Na⁺/K⁺-ATPase , which maintains a low intracellular Na⁺ concentration, allowing Na⁺ and nutrients to enter cells via co-transporters
Water follows solutes passively through aquaporins or paracellular routes driven by osmotic gradients established by solute transport
Students can predict steady-state cellular conductive properties during absorption and secretion.
In steady-state conditions, the membrane potential and ion gradients ( such as Na⁺ and K⁺ ) are maintained by the Na⁺/K⁺ pump, ensuring that absorption ( e.g., of Na⁺ ) is balanced with secretion ( e.g., of Cl⁻ ).
The trans-epithelial potential difference ( TEPD ) remains stable due to balanced ion flows
Students can predict maximal concentration and electrical gradients attainable by cellular solute transport mechanisms.
The maximal gradient is set by the electrochemical driving force determined by ion concentrations and the TEPD. For instance, the Na⁺/K⁺-ATPase can maintain intracellular Na⁺ at low levels, allowing high rates of nutrient co-transport
Use the table
write out driving force equations for each ion involved
multiply across based on how many ions are flowing
add up the absolute value of all membrane potentials
convert to energy using faraday constant
Students can predict physiological outcomes to changes in dietary nutrients.
An increase in dietary fats triggers the release of bile acids from the liver for emulsification , while proteins and carbohydrates stimulate pancreatic enzyme release.
Changes in dietary fiber affect water retention and transit times in the colon, influencing stool consistency
Students can describe endocrine/neural control of digestion and absorption of nutrients.
The Cephalic Phase involves neural control via the vagus nerve, stimulating saliva, gastric acid, and enzyme secretion in anticipation of food.
The Gastric Phase is regulated by gastrin, which increases HCl production in response to stomach distention and proteins.
The Intestinal Phase uses hormones like secretin and cholecystokinin ( CCK ) to regulate bicarbonate and enzyme secretion , coordinating digestion
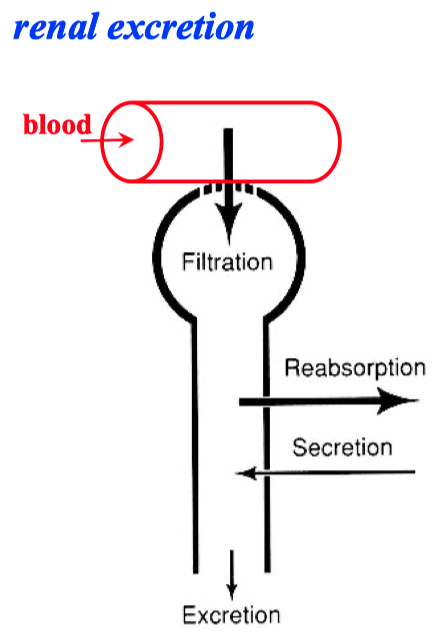
Renal System
Topic 1
Kidney = multi-stage filter
TALH = thick ascending limb of henle
Cell Swelling avoided by :
sodium-potassium-ATPase
creates initial ion gradient
potassium leak channels
maintain a negative cell potential
Both #1 and #2 items together make sodium and chloride concentrations high outside of the cell
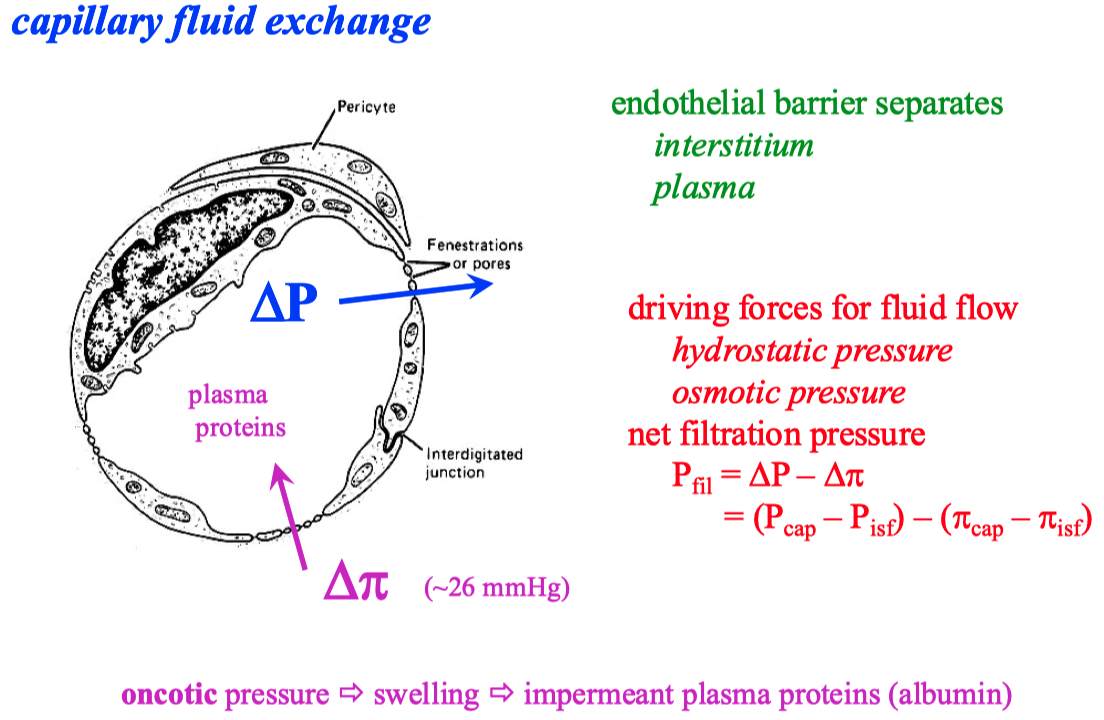
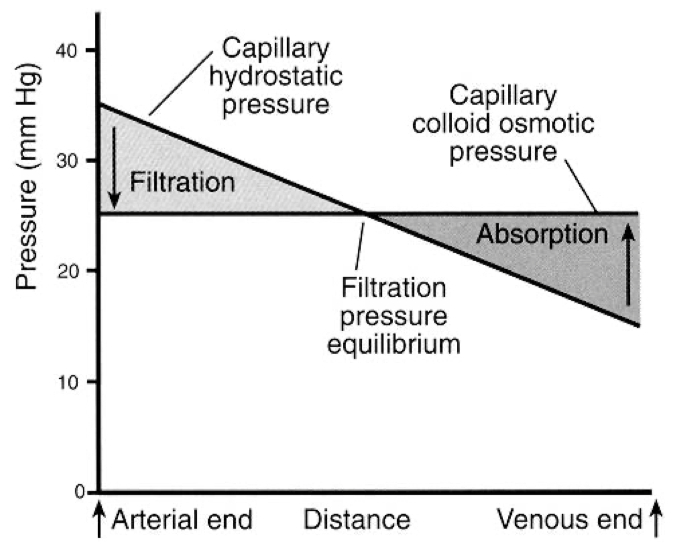
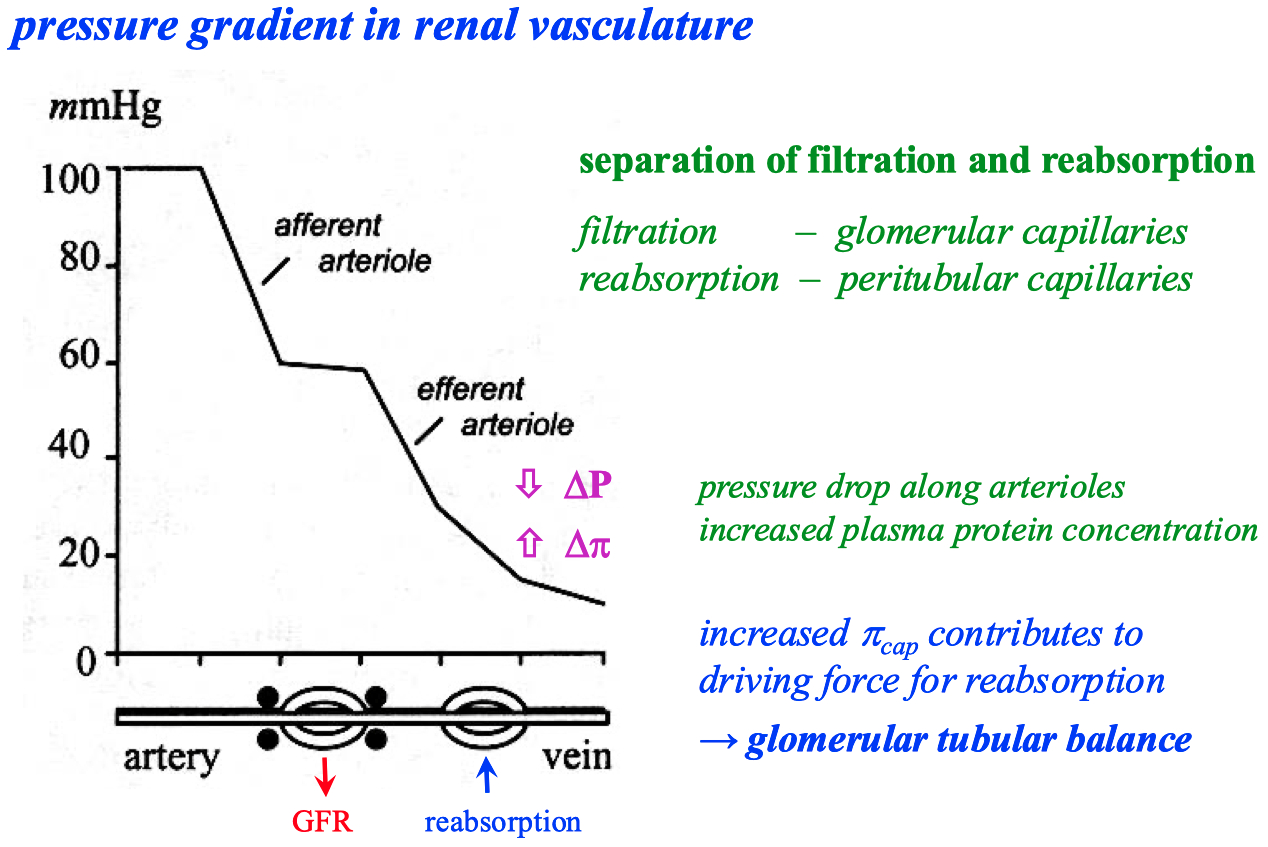
There is a pressure drop in capillaries , from arterial end to the venous end
Hydrostatic Pressure (P): This is the pressure exerted by the fluid inside the blood vessels. It tends to push water and solutes out of the capillary into the interstitial space (filtration).
Oncotic (or Colloid Osmotic) Pressure (π): This is the osmotic pressure exerted by plasma proteins (mainly albumin), which tends to pull water back into the capillary (absorption).
Pressure Differences from Arterial to Venous End:
Arterial End: The hydrostatic pressure is higher at the arterial end of the capillary (typically around 35 mmHg). This high pressure pushes fluid out of the capillary into the surrounding tissue (filtration).
Venous End: As blood moves through the capillary, hydrostatic pressure drops significantly (to about 15 mmHg). Meanwhile, oncotic pressure (around 25 mmHg) remains relatively constant throughout the capillary. By the time blood reaches the venous end, the oncotic pressure becomes greater than the hydrostatic pressure, creating a net pull of fluid back into the capillary (absorption)
From a physics perspective, this pressure drop is analogous to energy dissipation in a fluid flow system. The movement of blood through the narrow capillaries causes a loss of kinetic energy (due to resistance), which manifests as a drop in pressure. The oncotic pressure, which is relatively constant due to the steady presence of proteins, eventually overtakes the diminishing hydrostatic pressure, leading to a reversal of fluid movement back into the vessel.
Topic 2
Paracellular Pathway = Filtration
Cellular Pathway = Absorption
Nephron :
glomerulus = filter
proximal tubule = absorption of fluid
diluting segment = selective salt absorption
distal tubule = selective water ( or salt ) absorption
Topic 3
Glomerulus :
podocytes support against pressure
has fenestration pores
prevents blood cells and plasma proteins from entering nephron
afferent arteriole = in flow
efferent arteriole = exit flow
Macula Densa = boundary between proximal and distal sections
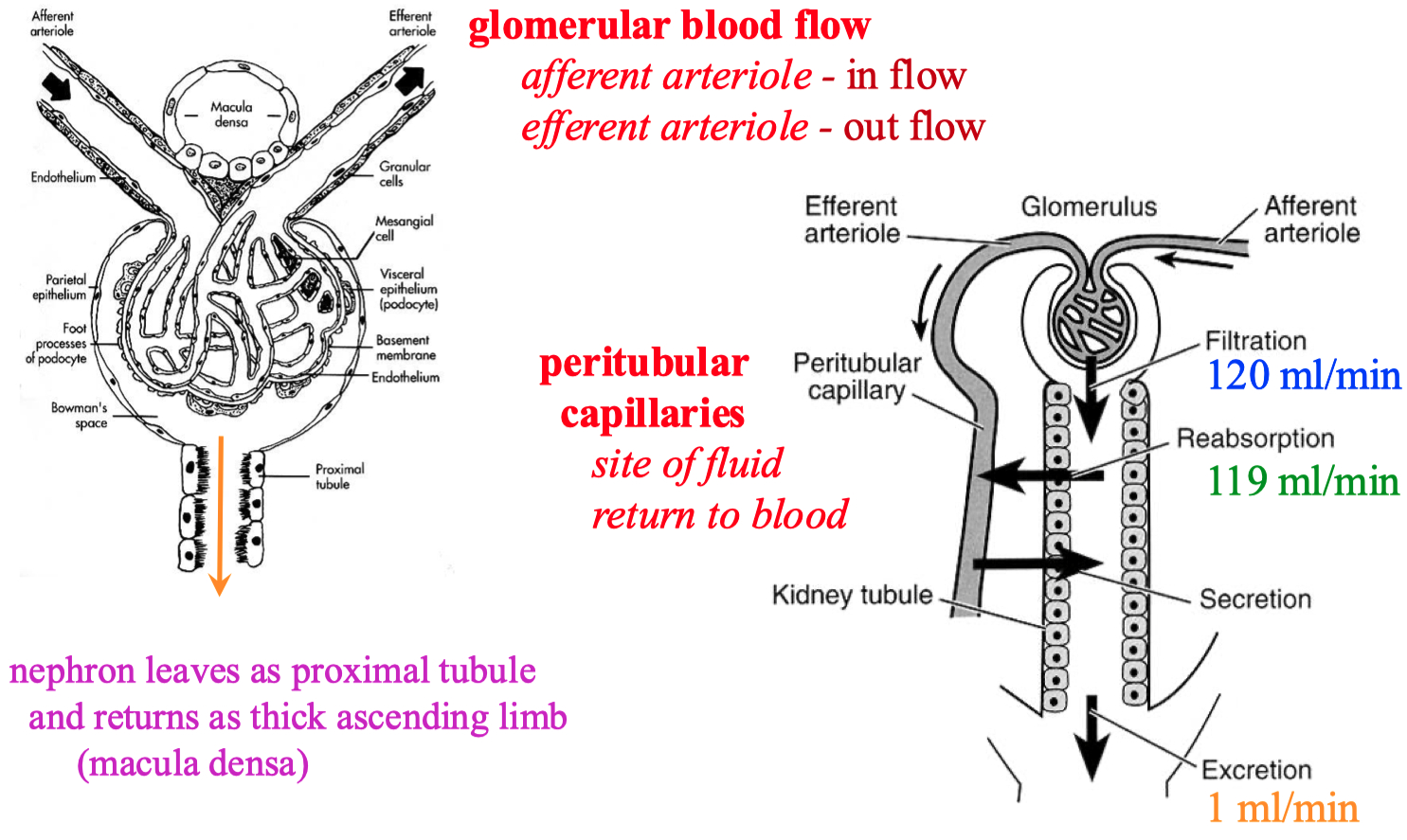
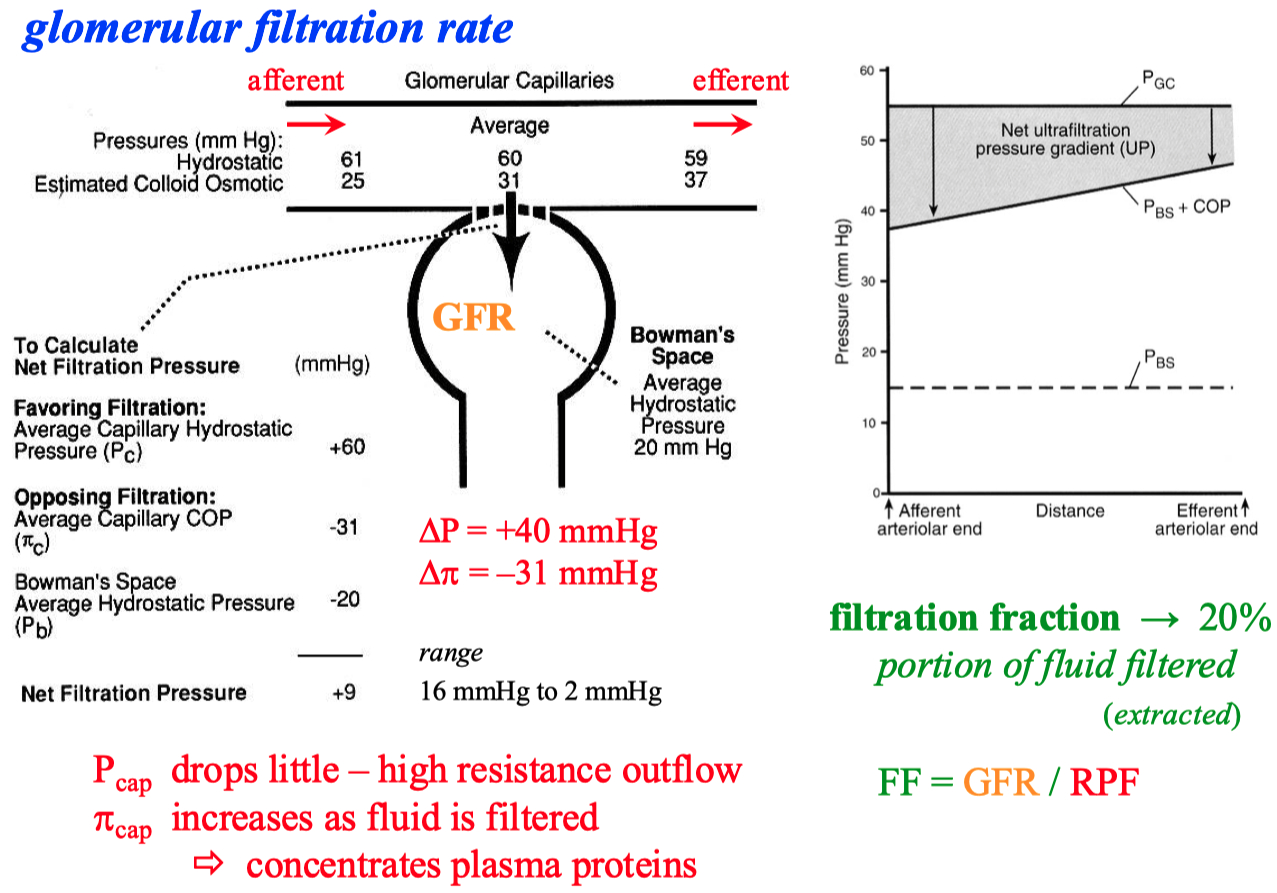
There are ways the body can autonomically control / constrict both the afferent and efferent arterioles
myogenic response ( Bayliss reflex )
maintains steady hydrostatic pressure of 60 mmHg
The glomerulus can also sense if there is high amounts of NaCl in the lumen , or just high osmolarity in general
it will decrease GFR
renin ?
Topic 4
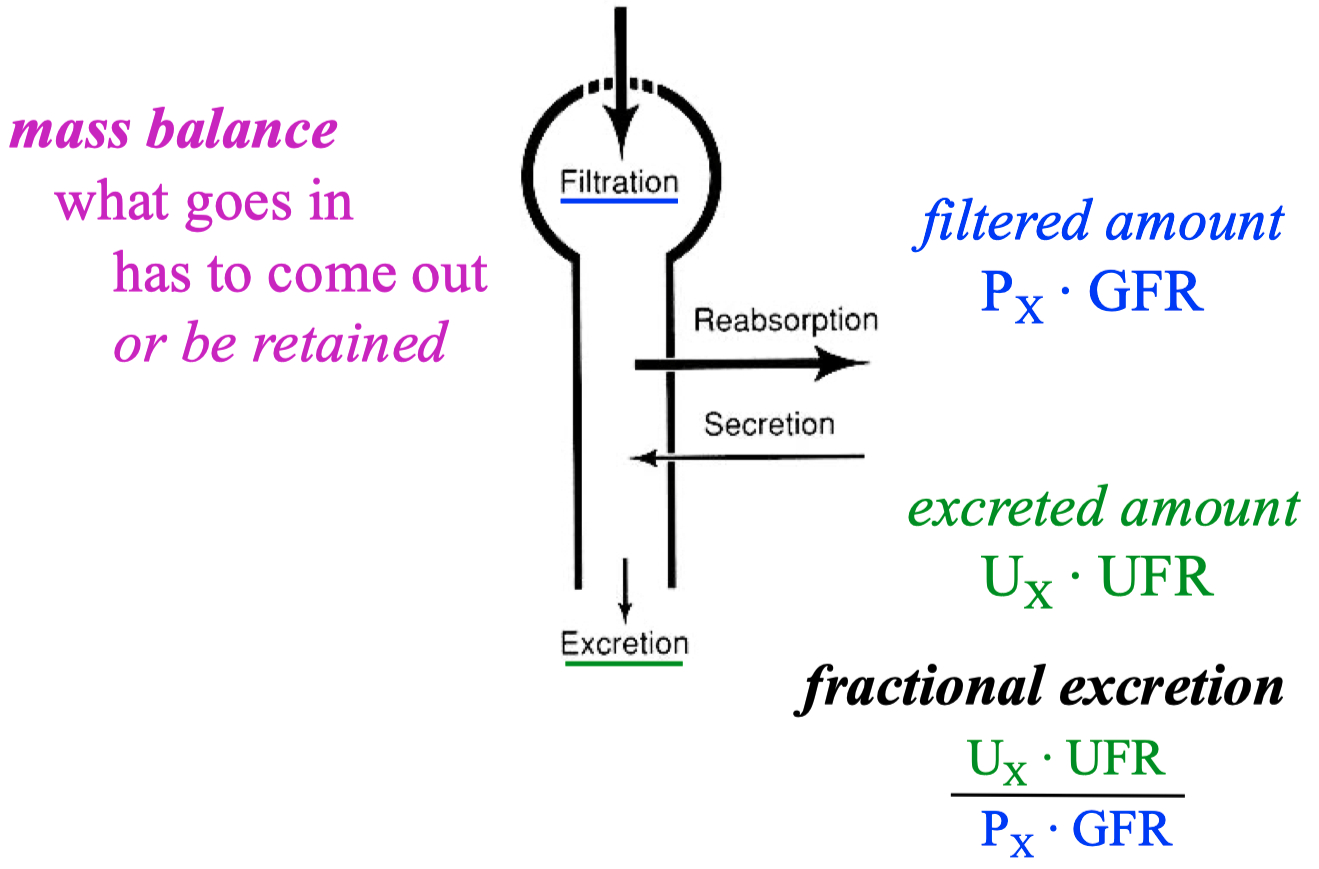
A good marker to measure GFR :
something filtered , but not re-absorbed , and not secreted
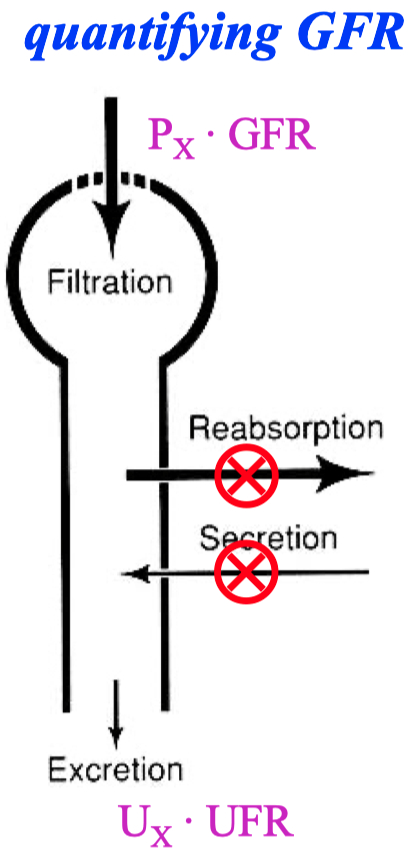
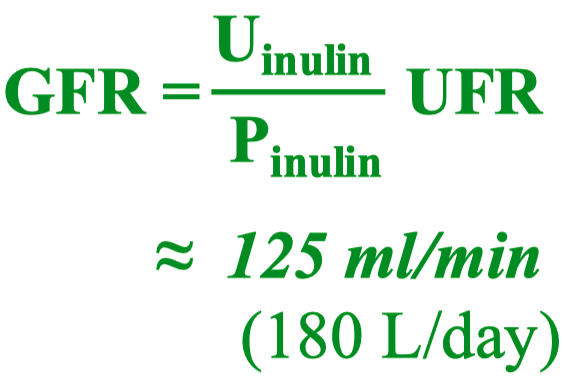
You can use inulin or creatinine
UFR = urine flow rate
this is equal to 1 for creatine , because creatinine is freely filtered and NOT reabsorbed or secreted by the kidneys
for water though :
Topic 5
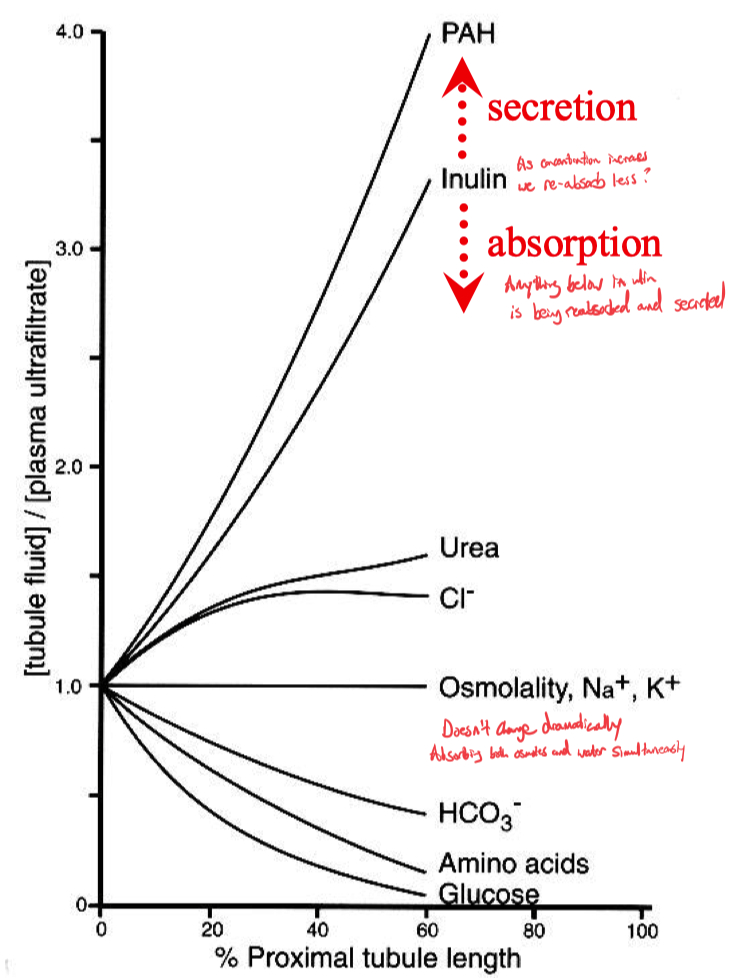
2/3 of all the filtrate is re-absorbed in the proximal tubule
basically , reabsorb all the fluid
reabsorb all the valuable things
Na , Cl , HCO3 , K , Ca , Mg , PO4 , water ( isotonic ) , glucose , amino acids
leave everything undesirable trapped in the lumen of the nephron
products of metabolism , organic cations , anions
its a convoluted tubule , help with absorption ( stirring )
On the inverse graph thing ,
inulin concentration is on the Y-axis ,
it increases in concentration as you go vertically
but simultaneously it shows the concentration of water as being the inverse
this is just saying that as we go through the nephron , we are increasing the concentration of inulin ( our perfect GFR recording molecule , because it doesn't get re-absorbed or secreted , it just gets filtered straight through to the collecting duct ).
ok and as expected , by the time we get to the collecting duct , we are left with virtually zero water left ( 1 / 1000 ) because it has all be re-absorbed
Driving Forces for Absorption :
Capillaries :
hydrostatic
osmotitc
Nephron :
osmotic
Bicarbonate Absorption ;
Apical = Sodium \ Proton Exchanger
Basolateral = Sodium \ Bi-Carb Co-Transporter
To get trans epithelial flow , you need different transporter types on apical vs basolateral sides
if they were the same , there would be no gradient
Bicarb gets concentrated as we absorb all of the water
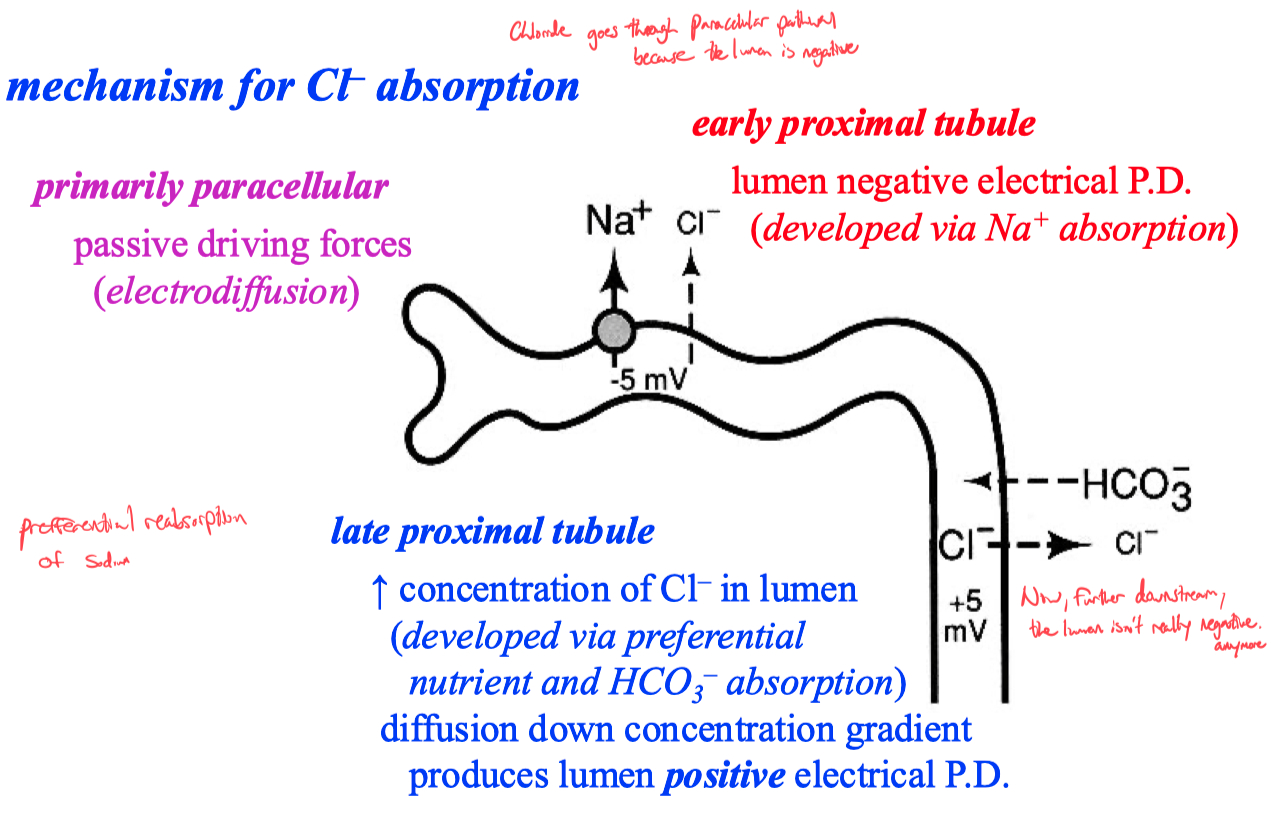
absorbed = filtered - secreted
Topic 6
Proximal Nephron = most absorption
Distal Nephron = fine adjustment
A vs B Type Distal Tubule Cells
Topic 7
How does the structure of the kidney allow us to re-absorb water against its concentration gradient ?
No aquaporins in ascending limb
Counter Current Exchange = down , and then back up ( hairpin movement )
maintains gradient
The ascending limb does have Na \ K \ 2Cl co transporters at the top
at the last possible moment , we throw away NaCl into the intermedullary space
NaCl enters outer medulla from TALH
Urea enters inner medulla from IMCD
We need capillaries to receive fluid , and the cells need oxygen.
so capillaries do counter-current exchange as well
Proximal Tubule = continual absorption
Thin Descending Limb = continual absoprtion
Late Distal Tubule = low H2O permeability
ADH = causes distal nephron / collecting ducts to insert aquaporins
allows for water reabsorption ( dehydrated )
Glomerulus has "compact" osmotic gradient , due to its shape
The Proximal Tubule has a large-scale osmotic gradient
Free Water Clearance ?
ADH / Vasopressin :
upregulates aquaporin expression in distal nephron and collecting ducts
increases urea permeability for inner medullary collecting duct
stimulates NaCl absorption in thick ascending limb
increases blood flow to juxtamedullary glomeruli
dilation = increased flow rate
Diabetes Insipidus = lack of ADH release ➡️ urinate tons of water
Topic 8
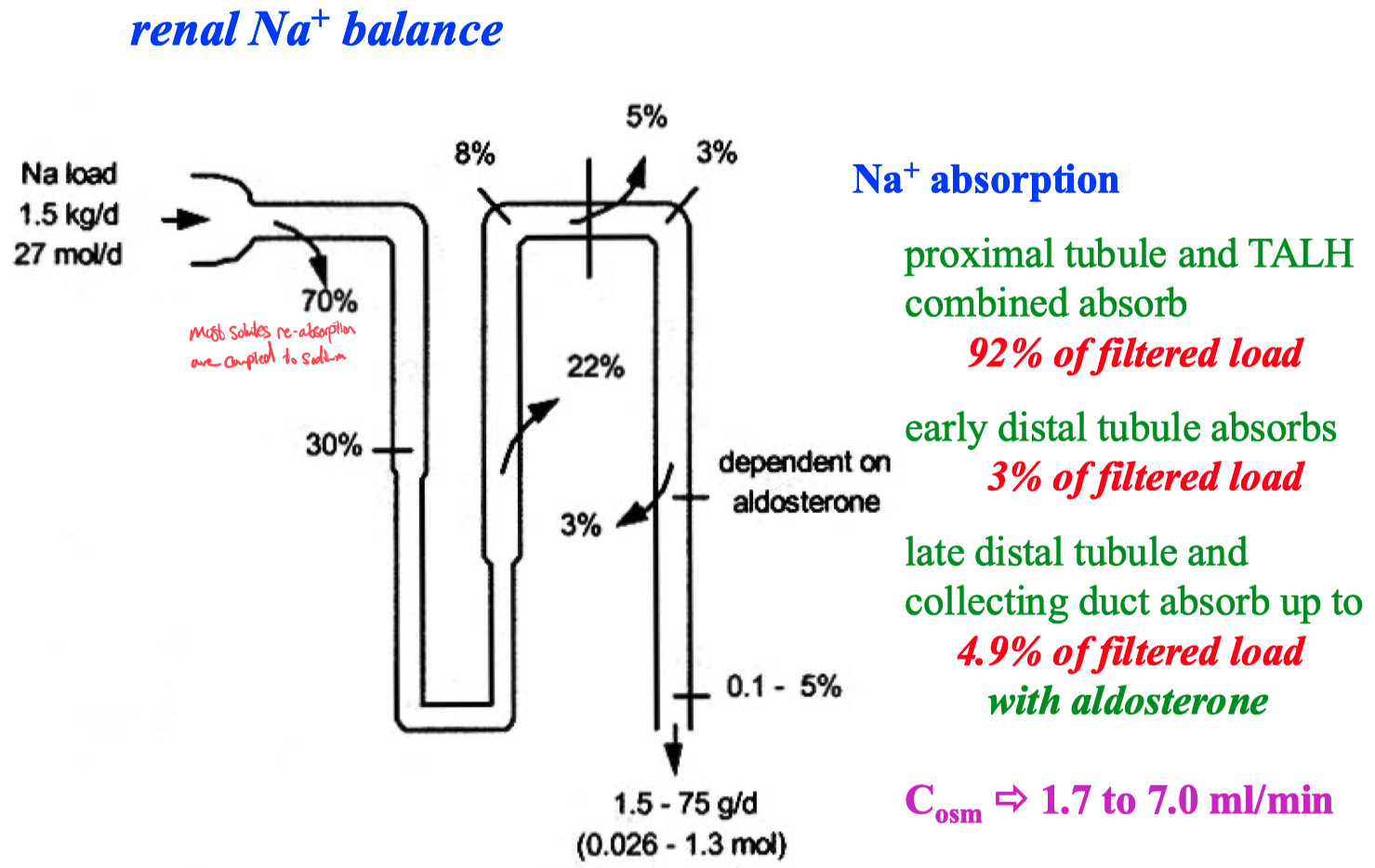
Aldosterone :
Huge ability to get rid of potassium
Because , aldosterone increases sodium absorption ,
this also drives potassium secretion
Intercalcated cells secrete acid ,
this turns on potassium pumps to re-absorb potassium
Topic 9
Breath Faster = export CO2
If we know how many cations there are , then there has to be a similar amount of anions if our solution neutral
aka , anion gap
There are a lot of acidic ( anions ) byproducts from normal metabolism
93% of filtered bicarbonate is absorbed in proximal tubule
We have to maintain the
acid = CO2
base = BiCarb
"New" BiCarb
at the end of proximal tubule , 1/2 the protons are on phosphates now
Ammonia's pKa = 9.3
its a very strong base , so by definition , its a much better proton trap
Transcytosis of Protons = protons from sulfate are moved to bicarbonate
Intercalcated Cells and Bacteria don't use Na \ K \ ATPase to maintain negative intracellular state
they use proton pumps instead
this allows the nephron to build up a much stronger gradient
more efficient
If you are willing to rip apart amino acids , you can get rid of as much acid as you want.
by-products of amino acid degradation create high amount of NH3
Ammonia = same size as potassium
it can travel through potassium channels
Example , Adaptation to Altitude ( acute effects )
Altitude Increase
Less Oxygen
Breath Faster
Offload CO2
Enter into Alkalosis ( basic ) state
Kidneys export more bicarb now
this happens in proximal tubule though
and since you are loosing osmoles ( not reabsorbing bicarbonate )
you don't reabsorb as much water now
so now you are also dehydrated
Absorbed = filtered - excreted
Volume Contracted = excrete more bicarbonate
Volume Expanded = stop secreting bicarb
Vomiting = loosing volume and acid , makes you enter into alkalosis state
Diarrhea = loosing volume and base , makes you enter into acidic state
RAAS System
Aldosterone
Students can describe glomerular filtration in the kidney.
Glomerular filtration occurs in the nephron’s glomerulus, where blood is filtered into the renal tubule.
The filtration barrier consists of capillary endothelium, the basal lamina, and podocyte slit diaphragms.
Plasma is filtered through these layers, forming the ultrafiltrate that excludes blood cells and plasma proteins due to size and charge barriers.
Students can predict changes in glomerular filtration rate due to arteriolar constriction.
The GFR depends on the pressure in the glomerular capillaries, which is controlled by the resistance of the afferent and efferent arterioles.
Constriction of the afferent arteriole reduces GFR by decreasing renal blood flow and glomerular pressure.
Constriction of the efferent arteriole initially increases GFR due to increased capillary pressure but can decrease GFR if severe constriction occurs
Students can calculate glomerular filtration rate and renal clearance.
GFR can be calculated using the formula:
Where
The renal clearance of a substance (
Renal clearance measures the effectiveness of the kidneys in removing a substance from the blood.
Students can describe renal tubule solute and water flow based on cellular transport mechanisms.
Different segments of the nephron utilize distinct transport mechanisms for solute and water flow.
For instance, in the proximal tubule, Na+ is absorbed with glucose and other solutes via Na+/glucose cotransporters.
Water follows passively due to the osmotic gradient.
In the loop of Henle , active NaCl transport occurs in the thick ascending limb ( TALH ) , which is water-impermeable, contributing to the dilution of the tubular fluid
Students can describe the morphological basis of tubule fluid concentration during high and low water intake.
During high water intake ( diuresis ) , water permeability in the distal tubule and collecting duct is low , producing dilute urine.
In contrast , during water scarcity ( antidiuresis ) , antidiuretic hormone ( ADH ) increases the water permeability of these segments , allowing for concentrated urine as water is reabsorbed.
The loop of Henle's countercurrent multiplication and urea recycling are critical in establishing the high osmolarity in the renal medulla necessary for this concentration process
Students can describe hormonal control of solute and water transport.
Hormones like ADH and aldosterone regulate water and solute transport.
ADH increases water reabsorption by inserting aquaporins in the collecting duct.
Aldosterone enhances Na+ reabsorption and K+ secretion in the distal tubule and collecting duct by increasing the number of Na+ channels and Na+/K+ pumps
Students can predict renal tubule response to perturbations in systemic acid/base balance.
The renal system compensates for systemic pH imbalances.
In acidosis, the kidney increases H+ secretion and promotes bicarbonate reabsorption through the action of intercalated cells ( Type A ).
Conversely, during alkalosis, Type B intercalated cells increase bicarbonate secretion and reduce its reabsorption
Misc
bulk of reabsorption in the intestine happens distally
this is opposite of the kidney nephron , where the bulk of water is reabsorped in the proximal tubule
most nutrient absorption occurs in the proximal intestine