Definitions
Dorsal Root Ganglion = A collection of neuronal bodies embedded in vertebrate dorsal roots of the spinal cord that provides neurites to gather sensory information from the periphery and transmit it to the dorsal horn of the spinal cord.
Tropic effect = the capacity of a fact or to attract a growing neuronal process.
Trophic effect ( neurotrophic ) = the capacity of a fact or to prevent the death of a cell, as if "feeding" it.
Neurotrophins = the family of neurotrophic factors that includes NGF, BDNF, and the other neurotrophins, which are designated by number.
NGF = The first isolated and identified neurotrophic factor, critical for the developmental survival of DRG and sympathetic cells.
Brain-Derived Neurotrophic Factor ( BDNF ) = a factor , originally isolated from pig brains, that supports the developmental survival of DRG cells, but not sympathetic celIs.
NT-3 = a member of the neurotrophin family, discovered after NGF and BDNF.
NT-4 = the fourth-discovered member of the neurotrophin family.
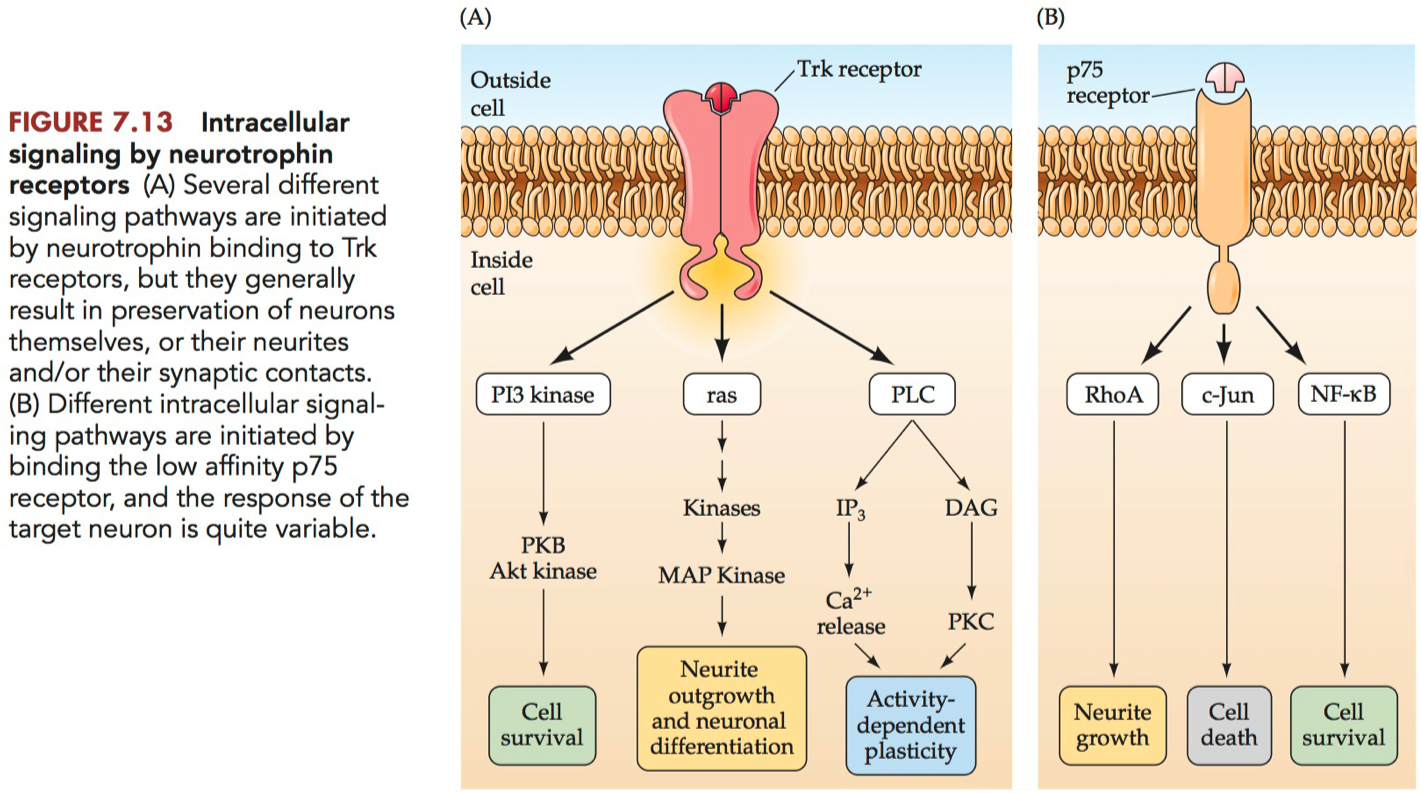
Tropomyosin receptor kinase ( Trk ) = a subfamily of the receptor tyrosine kinase ( RTK ) family that serve as receptors for neurotrophins.
TrkA = the first-identfied neurotrophin receptor, which has a high affinity for NGF.
TrkB = the second-identified neurotrophin receptor, which has a high affinity for BDNF and the other neurotrophins except NGF.
TrkC = the third-identified neurotrophin receptor, which has a high affinity for neurotrophin-3.
p75 = a low-affinity receptor for the neurotrophins.
"the periphery" = areas of the body that are distant from the central nervous system (CNS), which comprises the brain and spinal cord
generally refers to the limbs and muscles that are innervated by motor neurons originating in the CNS.
the number of muscles and the size of the areas to be innervated
Textbook Highlights
Hamburger worried that the change in motor neuron number might not have been in response to the limb bud's absence, but just a general response to tissue damage on that side of the embryo.
Maybe damaged limb tissue released chemicals that interfered with normal processes in the nearby spinal cord
Body regions with few muscles,
How does this matching of the number of motor neurons to muscles come about?
Deliberately manipulate the periphery, providing either fewer or more leg muscles on one side of the body in early embryos
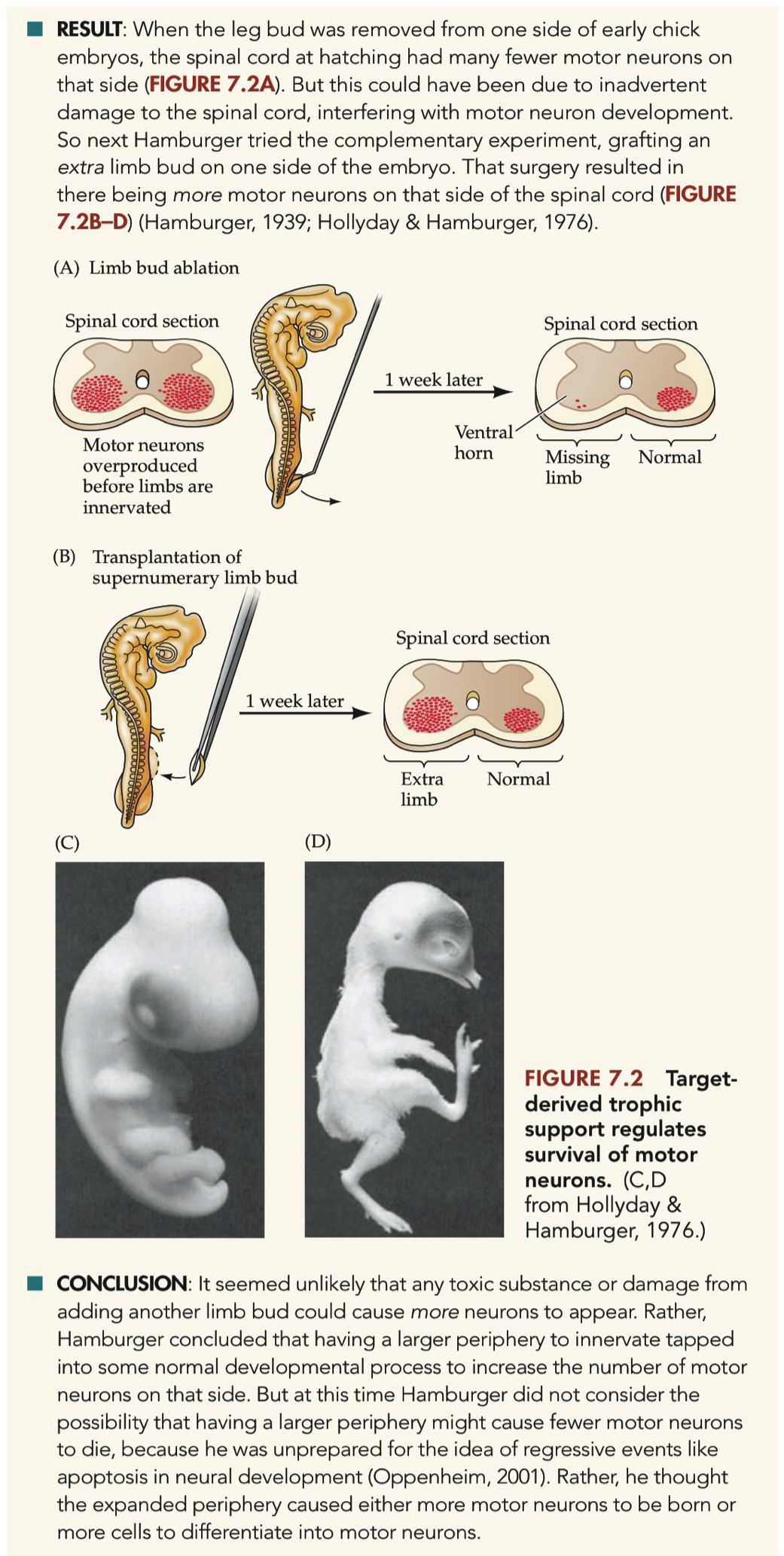
When the leg bud was removed from one side of early chick embryos, the spinal cord at hatching had many fewer motor neurons on that side
But this could have been due to inadvertent damage to the spinal cord,
grafting an extra limb bud on one side of the embryo resulted in there being more motor neurons on that side of the spinal cord
Hamburger concluded that having a larger periphery to innervate ,
tapped into some normal developmental process to increase the number of motor neurons on that side.
Hamburger thought the expanded periphery caused either more motor neurons to be born or more cells to differentiate into motor neurons
muscle mass / size
keeping track of the number of mitotic figures revealed that neurogenesis was complete quite early
but were the cells dying, or simply de-differentiating to become some other type of neuron?
we can also look for pyknotic cells as an indication of cells that were in the final stages of apoptosis.
but our chance of catching a glimpse of the pyknotic cell before it disappears altogether is low.
generally only useful if many cells are dying, increasing our chance of catching a fraction of those "in the act" of pyknosis.
sometimes there are indeed a lot of neurons undergoing apoptosis at the same time
the appearance of pyknotic cells helped persuade scientists, initially resistant to the idea that many neurons might die during normal development ,
that apoptosis is important for the formation of the nervous system.
on average about 30% of the neurons that arise early in life die before maturity
While the motor neurons' target cells have a strong effect on regulating their apoptosis ,
the cells that provide synapses onto the motor neurons have an effect, too.
it became clear that the size of the periphery somehow manipulated the extent of apoptosis in the nervous system ,
in order to match the number of neurons to the number of jobs available
How does the periphery influence cell death in the nervous system?
The answer to that question came
Levi-Montalcini repeated Hamburger's grafts of an additional limb bud in chicks
and found that these animals not only had additional motor neurons on that side of the spinal cord,
they also had more dorsal root ganglion (DRG) cells on that side of the body
Recall that DRGs contain the cell bodies of sensory fibers,
whose branched axons extend both to
the periphery to gather sensory information
and into the spinal cord to deliver that information to neurons in the dorsal horn.
Thus having more peripheral structures reduced apoptosis of both motor and sensory cells,
nicely matching the size of the nervous system to the size of the body.
But how?
they became convinced that the sarcoma was producing some chemical substance
that attracted sensory fibers and prevented their cell bodies in the DRG from dying.
explants of DRG cells exposed to fluid from the sarcoma grew much more abundant "halos" of neurites than explants from control fluid
By harvesting thousands of mouse salivary glands and using biochemical fractionation methods, scientists eventually identified a single protein, NGF
scientists noted that DRGs were not the only parts of the nervous system affected by NGF.
These sympathetic ganglia in chicks also swelled in response to the sarcoma fluid
suggesting that they also respond to NGF
Having established that NGF promotes outgrowth in neurons from DRGs and sympathetic ganglia
NGF has a tropic effect, encouraging process outgrowth
NGF also has a trophic effect, keeping DRG and sympathetic ganglion neurons alive during the period of cell death.
the next question was whether NGF could also prevent death of DRG cells in vivo.
Indeed, injections of NGF into the limb bud on one side of a chick embryo resulted in fewer degenerating DRG cells on that side
Still, this did not prove that the embryo normally uses that same substance, NGF, to regulate DRG cell apoptosis.
those DRG cells that had survived development in the absence of NGF were different they did not transport radiolabeled NGF from the periphery to their cell bodies, as most DRG neurons do in control animals
The researchers concluded that a subset of DRG neurons are NGF sensitive and need NGF to survive apoptosis and then, in adulthood, continue to respond to NGF.
Campenot
Those neurites growing in fluid with same cell body, growing in fluid without NGF regressed.
What's more, providing NGF to the neurites was enough to keep the cell alive, even if the fluid around the cell body was without NGF.
This result suggested that NGF was being retrogradely transported from cell processes to the cell body
Both the NGF and the receptor that binds it, which we'll discuss next, are internalized and retrogradely transported to the cell body
where the NGF alters gene expression to, among other things, prevent the cell from undergoing apoptosis.
For example, there was no effect of NGF manipulations on motor neurons, nor any visible effect on brain growth
it was obvious that not all neurons respond to NGF.
So scientists assumed that there must be other neurotrophic factors, including some normally produced by one group of brain cells to maintain innervation from other neurons
BDNF
unlike NGF ,
BDNF has no effect on sympathetic neurons
These three neurotrophins have distinct but overlapping effects on the survival and outgrowth of different classes of neurons
The four mammalian neurotrophins are each made from a larger propeptide, which itself appears to have no biological activity but which can be posttranslationally processed rapidly to produce one particular neurotrophin or another
The receptor for NGF was originally studied by cancer biologists
it is also a tyrosine receptor kinase (RTK)
The Trk receptors are said to be the high-affinity receptors for the neurotrophins
Neurotrophin binding of p75 activates one of several other pathways quite distinct from Trk receptors
So the response of a given cell to a neurotrophin is a function of the proportion of Trk versus p75 receptors it possesses, as well as the prior biochemical state of the cell when the neurotrophin arrives at the surface.
targets supply innervating neurons with a neurotrophic signal ,
that because it is in limited supply,
causes some of those innervating neurons to die,
resulting in the appropriate number of neurons surviving to adulthood.
Describe how removing a limb bud, or grafting on an extra limb bud, affects the number of motor neurons in a chick embryo.
Removal = reduction in motor neurons on ipsilateral side
initially thought to be a response to the absence of the limb bud, suggesting that the nervous system actively matches its size to the body part it innervates.
however, concerns were raised that the reduction in motor neuron number might not be a direct response to the limb bud's absence but rather a general response to tissue damage.
Addition = increase in motor neurons on ipsilateral side
suggested an active developmental process that increases motor neuron numbers in response to a larger periphery to innervate.
Mechanism of Regulation:
The document indicates that the size of the periphery manipulates the extent of apoptosis (programmed cell death) in the nervous system to match the number of neurons to the available "jobs" or target cells.
The mechanism by which the periphery influences cell death in the nervous system was explored through the study of sensory neurons and the role of nerve growth factor (NGF).
It was observed that having more peripheral structures reduced apoptosis of both motor and sensory cells, aligning the size of the nervous system with the size of the body.
Apoptosis and Motor Neuron Survival:
apoptosis is a characteristic process during development, with pyknotic cells (cells undergoing apoptosis) being a visible sign of this process.
the appearance of pyknotic cells coincided with the loss of motor neurons, indicating that apoptosis is an important mechanism in the formation of the nervous system.
Conclusion:
The experiments led to the conclusion that the periphery (the target area for innervation) plays a crucial role in regulating the survival and number of motor neurons during development.
The increase in motor neurons due to the grafting of an extra limb bud was seen as unlikely to be caused by any toxic substance or damage from the surgery, but rather as tapping into a normal developmental process that responds to the size of the periphery.
Fate switching/differentiation on the control side?
Decrease in Motor Neurons:
The removal of a limb bud from one side of early chick embryos resulted in significantly fewer motor neurons on that side of the spinal cord at hatching.
The addition of an extra limb bud led to an increase in motor neurons on that side of the spinal cord, suggesting a developmental process that increases motor neuron numbers in response to a larger periphery.
Fate Switching/Differentiation:
The concern that the reduction in motor neuron number might not be a direct response to the limb bud's absence but rather a general response to tissue damage was raised.
It was initially unclear whether the cells were dying or de-differentiating to become another type of neuron. However, further observations indicated that healthy-looking motor neurons and dorsal root ganglion cells were indeed disappearing on both the manipulated and unmanipulated sides of the body.
Apoptosis:
The appearance of pyknotic cells, which are indicative of cells in the final stages of apoptosis, coincided with the loss of motor neurons.
This suggested that apoptosis, rather than de-differentiation, was a significant factor in the regulation of motor neuron numbers during development.
Conclusion:
The experiments supported the hypothesis that the size of the periphery controls the number of motor neurons in the innervating regions of the spinal cord.
The findings imply that the periphery (the target area for innervation) plays a crucial role in regulating the survival and number of motor neurons during development, primarily through the mechanism of apoptosis rather than fate switching or differentiation.
Was there a factor from the limb that kept motor neurons alive?
Perhaps limb ablation has toxic effects
Limb-Derived Trophic Support:
The experiments showed that grafting an extra limb bud onto one side of the embryo resulted in more motor neurons on that side of the spinal cord.
This suggests that the limb provides a trophic factor that supports the survival and possibly the proliferation of motor neurons.
The conclusion from these experiments was that it seemed unlikely that any toxic substance or damage from adding another limb bud could cause more neurons to appear.
Instead, it indicated that having a larger periphery to innervate tapped into some normal developmental process to increase the number of motor neurons on that side.
Role of NGF (Nerve Growth Factor):
NGF was discovered to regulate apoptosis in sensory neurons, and it was shown that NGF promotes outgrowth in neurons from dorsal root ganglia (DRGs) and sympathetic ganglia.
It was also found that NGF has both tropic (guiding neuronal growth) and trophic (supporting neuron survival) effects on certain neuronal populations.
Injections of NGF into the limb bud on one side of a chick embryo resulted in fewer degenerating DRG cells on that side, indicating that NGF or a similar factor might be involved in preventing motor neuron death in vivo.
Apoptosis vs. Toxic Effects:
The document discusses apoptosis as an active process of cell death, rather than cells dying from "malnutrition" or toxic effects.
The doomed neurons actively begin the process of dismantling themselves, which is a regulated form of cell death rather than a toxic response.
The hypothesis that the size of the periphery controls the number of motor neurons was tested by manipulating the periphery, providing either fewer or more target cells for innervation. The results supported the idea that the periphery influences the survival of motor neurons, primarily through mechanisms like apoptosis rather than toxic effects.
Conclusion:
The presence of additional limb structures reduced apoptosis of motor neurons, suggesting that the limb provides a survival signal to motor neurons.
The document does not support the idea that limb ablation has toxic effects; rather, it indicates that the absence of limb-derived trophic support leads to increased apoptosis of motor neurons.
These findings underscore the importance of limb-derived factors, such as NGF, in the survival of motor neurons during development, and they highlight apoptosis as a key mechanism in the regulation of neuron numbers, rather than toxicity from limb ablation.

Evidence that Decline in Motor Neuron Cell Number is Due to Cell Death
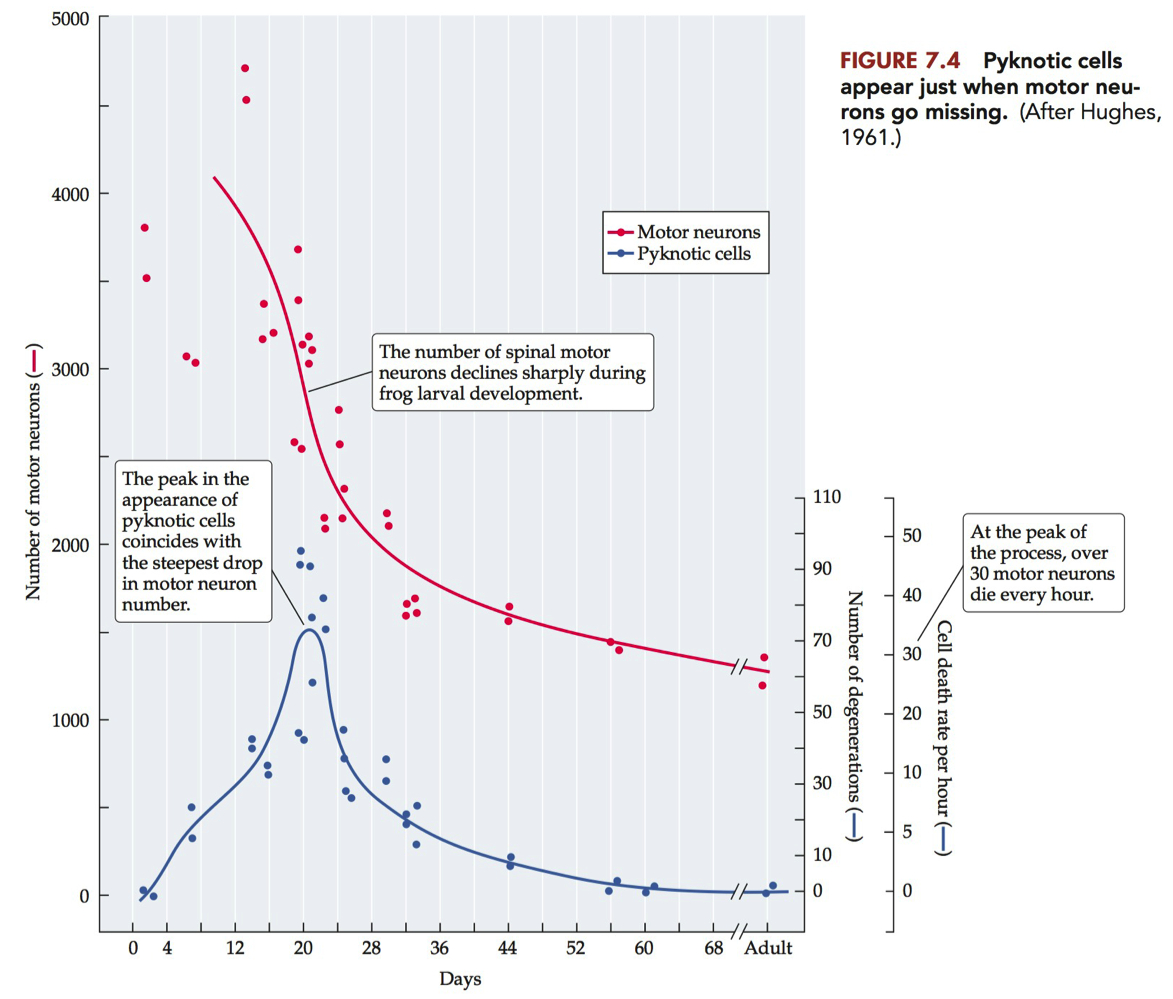
Analysis of motor neuron numbers in frog during development
Dying motor neurons become pyknotic
Condensed nucleus
Peak in pyknotic motor neurons corresponds with steepest decline in motor neuron numbers.
Describe a Campenot chamber and how experiments using this device demonstrated that NGF on distal neurites could promote survival.
Campenot Chamber Description:
The Campenot chamber is a device that allows for the separation of fluid compartments for a neuron's cell body, dendrites, and axon.
It consists of a Teflon "gasket" placed on top of cultured cells and sealed with silicon grease, effectively isolating the different parts of the neuron into separate environments.
Experiments and Findings:
The chamber was used to demonstrate that NGF has a local effect on growing neurites. Neurites that were exposed to NGF in their compartment continued to grow, while those not exposed to NGF regressed.
Neurites in the presence of NGF also exhibited a rapid influx of calcium ions (Ca2+), which promoted further growth.
Crucially, providing NGF to the neurites was sufficient to keep the cell body alive, even if the fluid around the cell body was devoid of NGF.
This indicated that NGF was being retrogradely transported from the cell processes to the cell body.
Implications for Neuronal Survival:
The retrograde transport of NGF to the cell body suggested that NGF altered gene expression to prevent the cell from undergoing apoptosis.
The experiments showed that NGF acts both locally at the site of neurites and also within the cell body to promote survival.
Conclusion:
The Campenot chamber experiments were pivotal in demonstrating that NGF provided to distal neurites could promote the survival of the neuron, even in the absence of NGF around the cell body.
This provided strong evidence for the retrograde transport of NGF and its role in neuronal survival.
These findings were significant in understanding the mechanism by which NGF influences neuronal survival and the importance of its transport within the neuron.
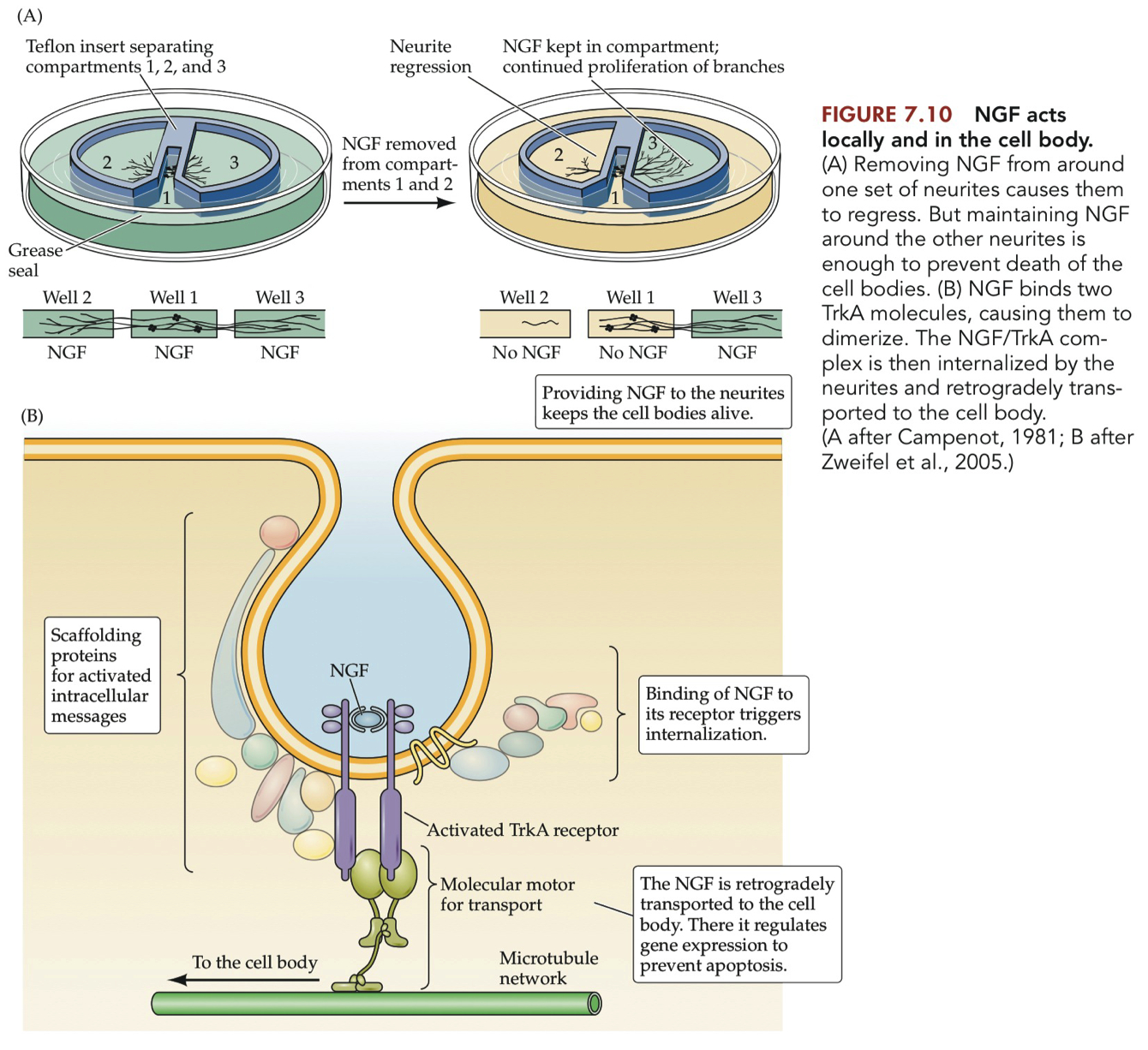
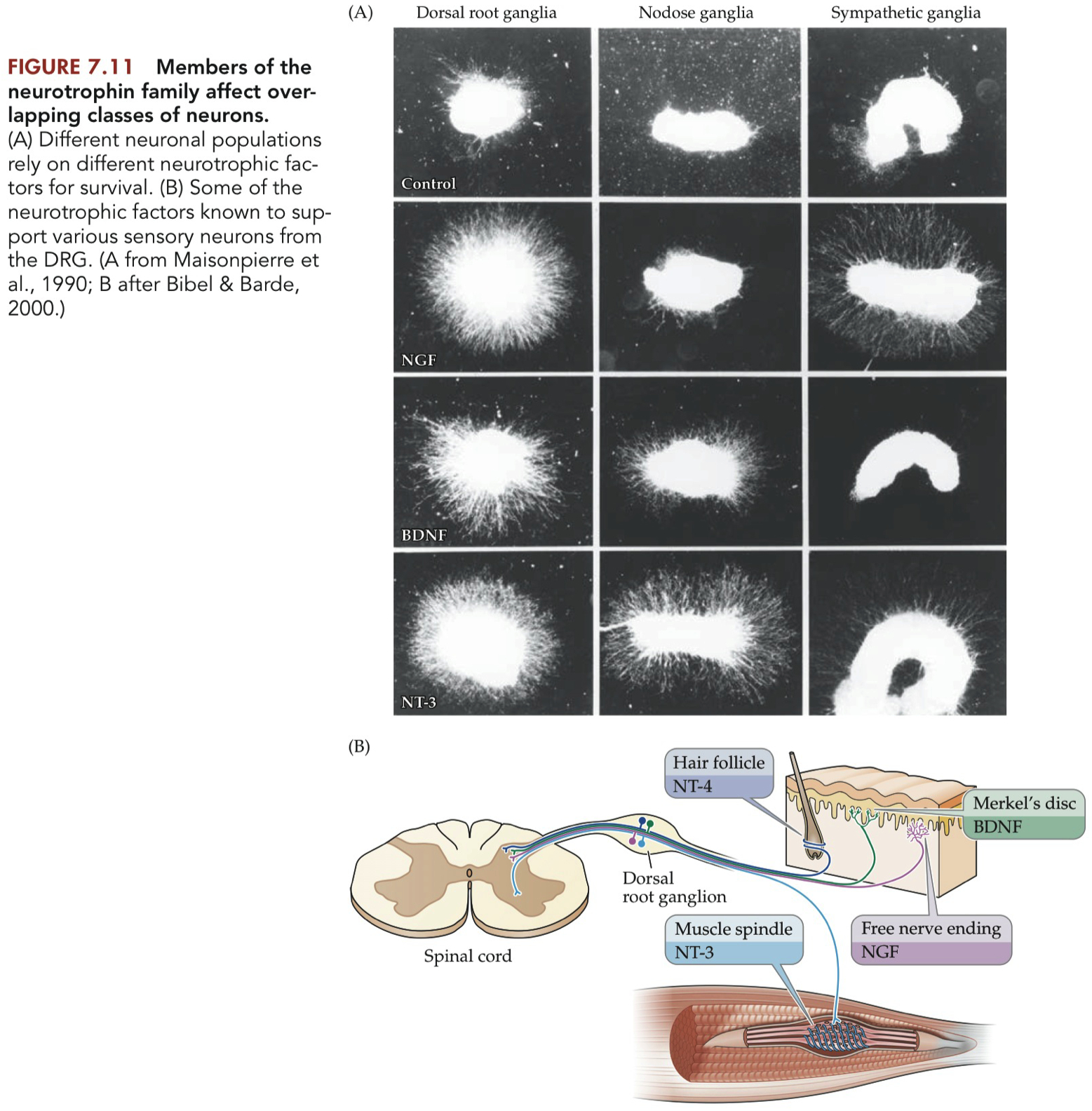
Figure 7.11
NT-3 is required for muscle spindle synapses
you can identify neurons in the dorsal horn by Trk expression
or by antibody labeling
Nodose = vagus nerve = afferent = sensory neurons from the heart
Sympathetic Ganglia = efferent
NGF does NOT promote nodose axon growth
2 different functions of neurotrophins
promot cell survival
or promote growth of neurites ( axon growth )
In the sympathetic ganglion , there are neurons that DON'T express TrkB
but they DO express TrackA
Match the list of neurotrophic factors with the Trk receptor(s) they bind with high-affinity. Describe the binding affinity of neurotrophic factors with the p75 receptor.
In general, neurotrophic factors such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5) have specific high-affinity interactions with Trk receptors:
NGF binds with high affinity to TrkA.
BDNF and NT-4/5 bind with high affinity to TrkB.
NT-3 binds with high affinity to TrkC, and to a lesser extent, can also interact with TrkB.
The p75 receptor, it is known to bind all neurotrophins with similar low affinity,
which is distinct from the high-affinity binding of neurotrophins to their specific Trk receptors.
P75
low affinity receptor
can trigger :
cell survival
OR cell death
Track Receptors
signal for :
cell survival
neuron outgrowth
List the sensory neuron types that depend on neurotrophic factors for survival during development.
Dorsal Root Ganglia (DRG) Neurons:
These neurons are sensitive to NGF (Nerve Growth Factor), which supports their survival.
BDNF (Brain-Derived Neurotrophic Factor) also supports the developmental survival of DRG cells.
Sympathetic Ganglia Neurons:
NGF is crucial for the survival of these neurons during development.
Other Sensory Neurons:
NT-3 (Neurotrophin-3) supports the survival of neurons associated with the nodose ganglia, hair follicles, and muscle spindles.
NT-4 (Neurotrophin-4) is also mentioned in relation to sensory neurons from the DRG, but the specific effect is not detailed in the provided excerpts.
These neurotrophic factors are essential for the survival of specific sensory neuron populations during development, ensuring that the nervous system is matched in size and function to the periphery it innervates.
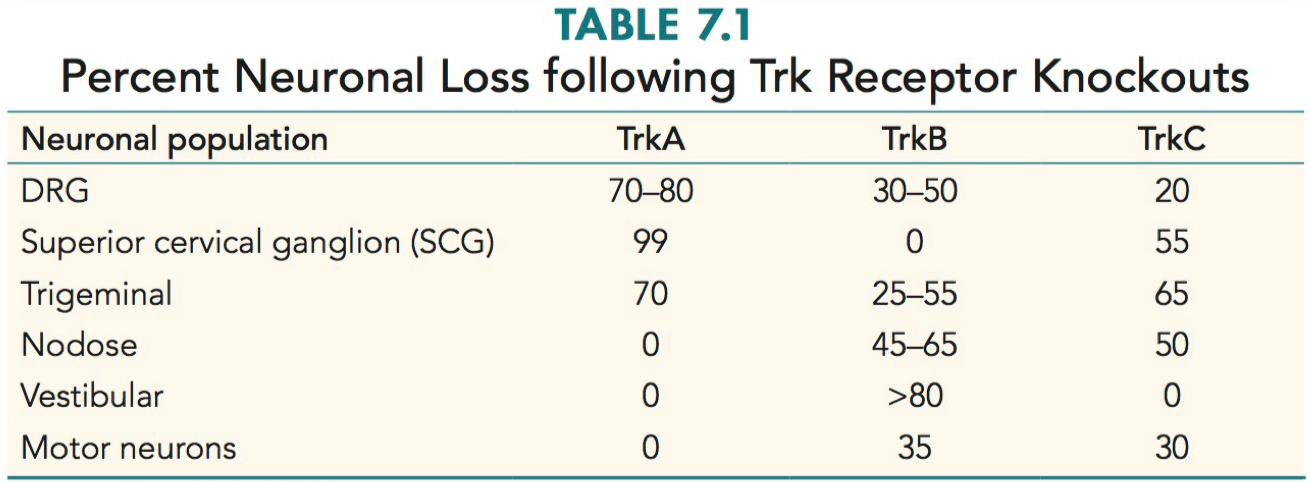
Predict the sensory neuron survival phenotypes of knockout mice for neurotrophic factors or their receptors.
TrkA Knockout Mice:
DRG Neurons: Substantial loss of sensory neurons, likely leading to impaired sensory processing.
Superior Cervical Ganglion: Almost complete loss, suggesting severe impairment in sympathetic nervous system functions.
Trigeminal Neurons: Significant loss, potentially affecting facial sensation and motor functions.
TrkB Knockout Mice:
DRG Neurons: Moderate loss, indicating partial sensory deficits.
Trigeminal Neurons: Variable loss, possibly affecting facial sensory and motor functions.
Nodose Ganglion: Moderate to significant loss, potentially impacting visceral sensory functions.
Vestibular Neurons: Major loss, likely leading to balance and spatial orientation issues.
Motor Neurons: Some loss, which could affect motor functions.
TrkC Knockout Mice:
DRG Neurons: Minor loss, suggesting relatively preserved sensory functions.
Superior Cervical Ganglion: More than half loss, indicating substantial sympathetic nervous system impairment.
Trigeminal Neurons: Major loss, likely affecting facial sensation and motor functions.
Nodose Ganglion: Half loss, potentially impacting visceral sensory functions.
Motor Neurons: Some loss, possibly affecting motor functions.
Crowley 1994
“Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons”
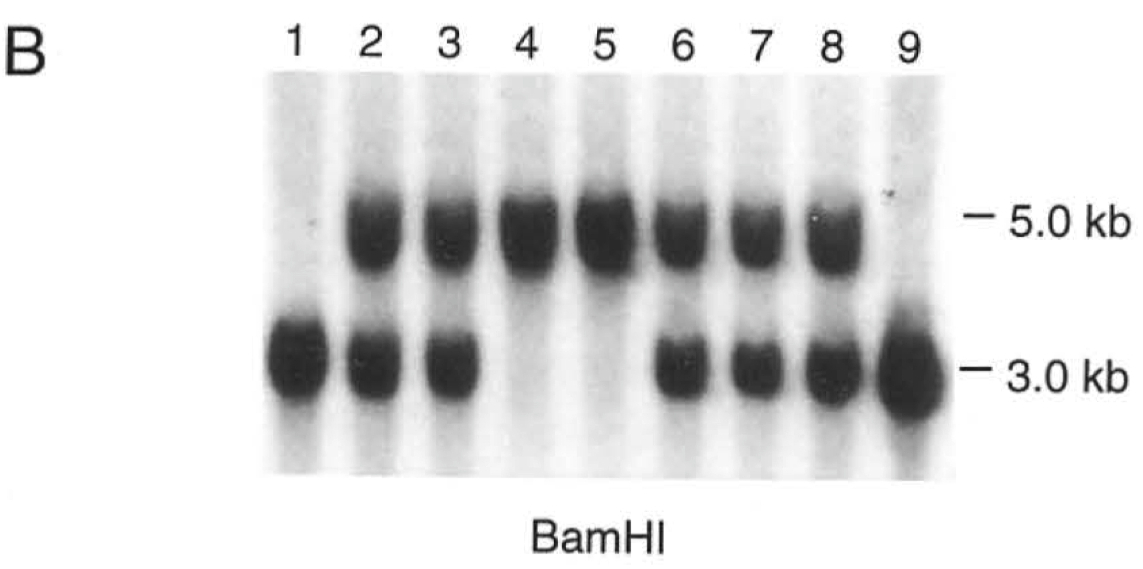
Figure 1B shows a Southern blot to confirm the genotype of a number of different offspring from matings between heterozygous parents. Do the numbers listed in the text of the frequency of different genotypes observed suggest any prenatal lethality from this mutation? Explain why or why not.
Genotype Frequencies in Offspring:
Homozygous (-/-): 24%
Heterozygous (+/-): 44%
Wild Type (+/+): 29%
Note: 3% were lost to cannibalism.
Analysis of Prenatal Lethality:
Expected Mendelian Ratios:
In a typical Mendelian cross between two heterozygous parents, the expected ratio of offspring genotypes is 1:2:1 (25% homozygous recessive, 50% heterozygous, 25% homozygous dominant).
Observed Ratios:
The observed ratios are 24% (-/-), 44% (+/-), and 29% (+/+).
Comparison with Expected Ratios:
The observed ratios are close to the expected Mendelian ratios.
A slight deviation is present but not significantly large.
Implications for Prenatal Lethality:
The closeness of the observed ratios to the expected Mendelian ratios suggests that there is no significant prenatal lethality associated with this mutation.
If prenatal lethality were a significant factor, a more pronounced deviation from the expected ratios would be observed, especially a lower percentage of homozygous (-/-) offspring.
Additional Observations:
Survival in Utero:
The ratios demonstrate that animals homozygous for the gene disruption are not compromised with regard to survival in utero.
Postnatal Observations:
Homozygous (-/-) mice are smaller at birth and have some developmental delays, but these do not indicate prenatal lethality.
In conclusion, the frequencies of different genotypes observed do not suggest any significant prenatal lethality from this mutation. The slight deviation from the expected Mendelian ratios can be attributed to factors other than prenatal lethality, such as postnatal viability or environmental factors.
What is the nodose ganglion and why would it be a useful comparison ganglion in these experiments?
What is the Nodose Ganglion?
The nodose ganglion is a type of sensory ganglion.
It is associated with the vagus nerve and contains the cell bodies of sensory neurons.
These neurons are involved in transmitting various types of sensory information, including visceral sensations.
Relevance in the Experiments:
Comparison with Other Ganglia:
The study examines the effects of nerve growth factor (NGF) deficiency on different types of ganglia, including the nodose ganglia.
The nodose ganglia did not show significant size differences compared to those of normal (+/+) littermates, indicating a lesser or no impact of NGF deficiency on these ganglia.
Contrast with Superior Cervical Ganglia (SCGs):
SCGs from NGF-deficient (-/-) animals were not visible upon dissection by postnatal day 14, indicating severe cell loss.
In contrast, the nodose ganglia displayed only rare instances of apoptotic neurons and were similar in appearance to those of normal mice.
Indicative of Selective Impact of NGF Deficiency:
This contrast suggests that NGF plays a selective role in the development of some sensory neurons but not others.
The nodose ganglia's relative normalcy in NGF-deficient mice indicates that these neurons might not be as dependent on NGF for survival or development as neurons in other ganglia, like the SCGs or dorsal root ganglia (DRGs).
Importance in Understanding NGF's Role:
Selective Influence on Neuronal Development:
The findings from the nodose ganglia provide evidence that NGF's influence on neuronal development and survival is selective.
It supports the hypothesis that not all sensory neurons are equally affected by NGF deficiency.
Comparative Analysis:
By comparing the effects of NGF deficiency on different types of ganglia, researchers can better understand the specific roles and mechanisms of NGF in neuronal development and survival.
In summary, the nodose ganglion serves as a crucial comparison point in these experiments to demonstrate the selective impact of NGF deficiency on different types of sensory neurons. Its relative normalcy in the context of NGF deficiency contrasts with the severe effects observed in other ganglia, thereby highlighting the specific and varied roles of NGF in neuronal development.
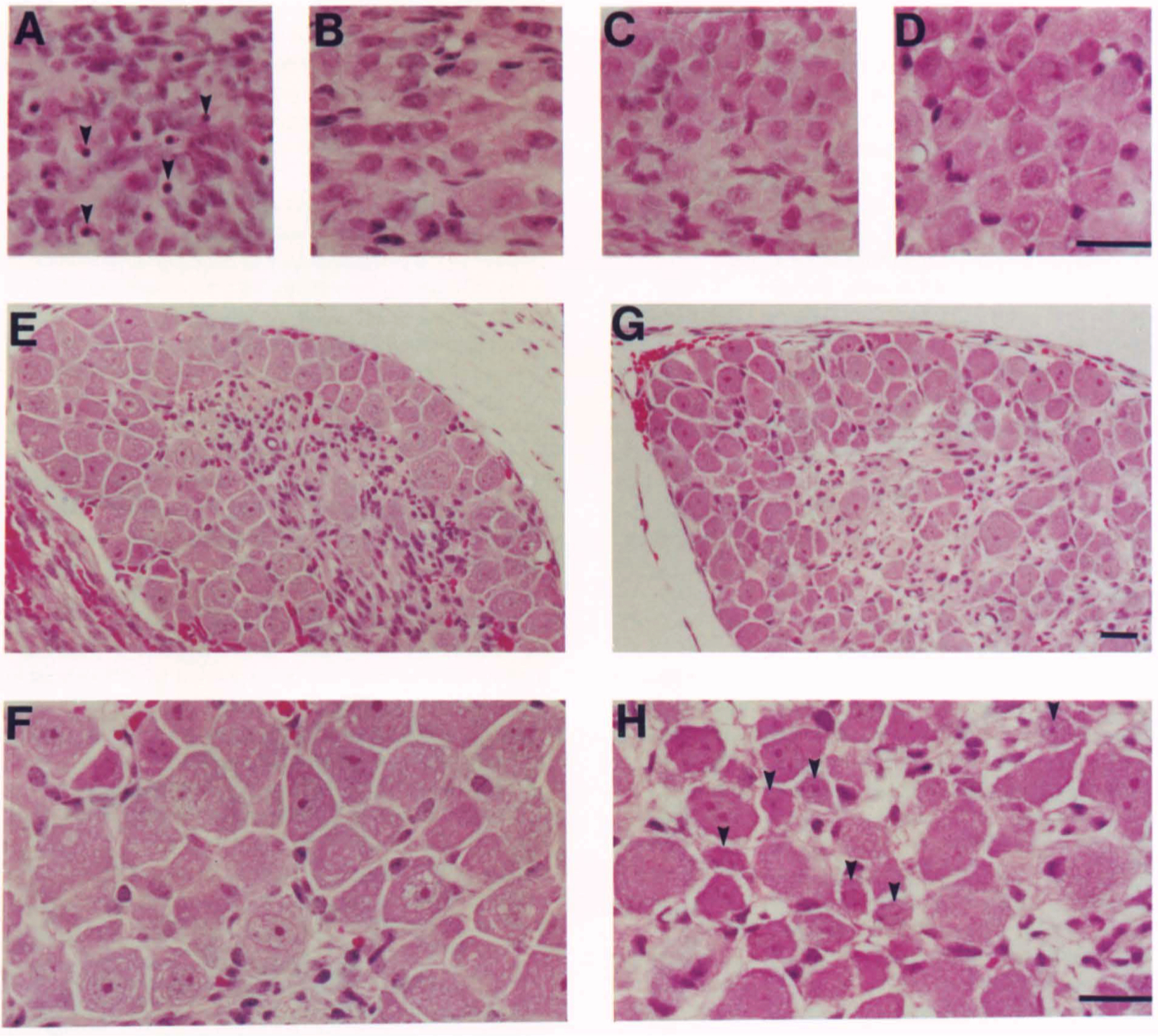
What is the take-home message of the histological stains of various ganglia shown in Figure 2?
Overview of Figure 2:
The figure presents histological appearances of sensory and sympathetic ganglia in 3-day-old mice.
It compares wild-type (B, D, G, H) and homozygous mutant (A, C, E, F) genotypes.
The ganglia examined include the Superior Cervical Ganglion (SCG), Nodose Ganglion, and the Fifth Lumbar Dorsal Root Ganglion (DRG).
Key Observations:
Superior Cervical Ganglion (SCG):
In the mutant mouse, the SCG shows numerous pyknotic nuclei, indicative of cell death (arrowheads in A).
This contrasts with the wild-type, where such features are not prominent.
Nodose Ganglion:
The nodose ganglion in the mutant does not show significant differences in appearance compared to the wild-type.
This suggests a lesser impact of the mutation on these ganglia.
Dorsal Root Ganglion (DRG):
The DRG of the wild-type animal shows numerous small neurons (arrowheads in H).
In contrast, the mutant DRG displays no small cells, indicating a significant impact of the mutation.
Implications:
Selective Vulnerability:
These observations suggest a selective vulnerability of certain types of neurons to the genetic mutation.
The SCG and DRG in mutants show significant changes, indicating a higher sensitivity to the mutation.
Role of NGF:
Given the nature of the mutation (NGF deficiency), these findings highlight the critical role of nerve growth factor (NGF) in the survival and maintenance of certain neuronal populations.
The differential impact on various ganglia suggests that NGF's role is more crucial in some neuronal types (like those in SCG and DRG) than others (like those in the nodose ganglion).
Conclusion:
The histological analysis in Figure 2 demonstrates the selective effect of NGF deficiency on different types of ganglia, underlining the importance of NGF in the development and survival of specific neuronal populations. The contrasting responses of these ganglia to NGF deficiency provide valuable insights into the varied roles and mechanisms of NGF in the nervous system.
One of the major findings in this paper is the selective loss of neurons in the sensory ganglia (DRGs). List the sources (Tables and/or Figure panels) of data that provide the evidence for this conclusion.
Figure 2 (E-H):
Visual inspection of sections through cervical or lumbar ganglia.
Observations include fewer neuronal profiles in the ganglia of (-/-) mice compared to (+/+) mice.
Notably, small profiles were rare in the ganglia of (-/-) mice.
Figure 3:
Figure 3A: Cell size histograms from lumbar DRGs of (+/+) or (-/-) mice, showing the absence of small cells (<15 µm in diameter) in the ganglia of (-/-) animals.
Figure 3B: In situ hybridization of cervical DRGs for neurotrophin receptors, indicating the absence of detectable trkA mRNA in ganglia from (-/-) mice, but the presence of cells expressing trkB and trkC.
Figure 4:
CGRP immunoreactivity in DRG neurons of 3-day-old mice of different genotypes.
The (-/-) animals showed a complete absence of CGRP-immunoreactive processes in the dorsal horn of the spinal cord and the skin, compared to (+/+) animals.
Table 2:
Neuronal cell counts from the fourth and fifth lumbar ganglia.
A 70% reduction in cell number was observed in (-/-) mice, with a marginally significant decrease in cell number in (+/-) animals compared to their (+/+) littermates.
These sources collectively provide comprehensive evidence for the selective loss of neurons in the sensory ganglia (DRGs), particularly in the context of NGF deficiency in the studied mice.
Why is the fact that DRGs from knockout (-/-) mice were "devoid of detectable trkA mRNA" a significant finding? Are the cells simply not expressing this gene or they missing in these DRGs?
trkA mRNA and Its Role:
trkA is the high-affinity receptor for nerve growth factor (NGF).
It plays a crucial role in the survival and maintenance of certain types of sensory neurons, particularly those responsive to NGF.
Implications of the Absence of trkA mRNA:
Selective Neuronal Loss:
The absence of detectable trkA mRNA in the DRGs of (-/-) mice suggests that neurons normally expressing this receptor are either missing or significantly reduced in number.
This aligns with the observed 70% reduction in DRG cell numbers in NGF null mutant mice.
Dependence on NGF for Survival:
The finding implies that DRG neurons expressing trkA are highly dependent on NGF for their survival.
In the absence of NGF (as in the knockout mice), these neurons likely undergo apoptosis or fail to develop properly.
Lack of Redundant Trophic Support:
The absence of trkA mRNA indicates that these neurons do not have redundant trophic support from other neurotrophins, making them particularly vulnerable to NGF deficiency.
Differentiation Between Non-Expression and Absence of Neurons:
Potential Non-Expression:
It's possible that some DRG neurons survive but down-regulate trkA expression to undetectable levels in the absence of NGF.
Neuronal Absence:
However, the significant reduction in neuron numbers and the lack of small neurons in the DRGs of (-/-) mice suggest that many of these neurons are indeed missing, not just non-expressive of trkA.
Overall Significance:
This finding is significant as it highlights the critical role of NGF in the development and survival of specific sensory neurons.
It provides insight into the molecular mechanisms underlying the development of the sensory nervous system and the specific requirements of different neuronal populations for survival and maintenance.
In summary, the absence of detectable trkA mRNA in the DRGs of knockout mice is a significant finding as it underscores the essential role of NGF and its high-affinity receptor trkA in the survival of specific sensory neurons. This absence likely reflects both the loss of neurons and the potential down-regulation of trkA expression in surviving neurons under NGF-deprived conditions.
Homozygous mutants show reduced responses in some assays to test pain, but not in others. Which tests demonstrate an effect and which do not? Why do you think this may be the case?
Tests Demonstrating an Effect:
Noxious Mechanical Stimulus Response:
Mice were tested for their response to a pinch applied to the tail.
Homozygous (-/-) mice very rarely responded to this noxious stimulus, in contrast to (+/+) and (+/-) mice, who reacted reliably.
The response in (+/+) and (+/-) mice consisted of vigorous squirming accompanied by vocalization.
The difference in response frequencies as a function of genotype was highly significant.
Tests Not Demonstrating an Effect:
Thermal Pain Response:
Mice were tested for responsiveness to thermal pain in two paradigms: the tail flick and hot plate test.
In the hot plate test, no significant differences in behavior were observed between the genotypes.
However, in the tail flick test, (+/-) animals displayed a small but significant prolongation of latency, indicating some degree of reduced sensitivity to pain.
Possible Reasons for Differential Responses:
Selective Neuronal Loss:
The selective loss of neurons in sensory ganglia, particularly those expressing trkA (the high-affinity receptor for NGF), in (-/-) mice could explain their reduced sensitivity to mechanical pain stimuli.
These neurons are likely involved in transmitting pain signals, and their absence or reduction would diminish the pain response.
Different Pain Pathways:
The differential response in thermal vs. mechanical pain tests suggests that these modalities might be mediated by different types of sensory neurons.
Neurons that respond to thermal stimuli may be less affected by the NGF deficiency, or other types of neurotrophins might compensate for NGF in these pathways.
Complexity of Pain Perception:
Pain perception is a complex process involving multiple types of sensory neurons, signaling pathways, and central processing mechanisms.
The NGF deficiency might affect some aspects of this system while leaving others relatively intact.
In summary, the study demonstrates that homozygous mutant mice show a marked reduction in response to mechanical pain stimuli but not to thermal pain stimuli. This differential response is likely due to the selective loss of certain types of sensory neurons and the complexity of the pain perception system, which involves multiple pathways and mechanisms.
Summarize the findings of this paper in one or two sentences. What experiments do you think would be important to try next?
The study demonstrates that the absence of a functional NGF gene in mice leads to severe cell loss in sensory and sympathetic ganglia, reduced viability, and marked changes in pain sensitivity. Specifically, there is a significant reduction in the number of sensory neurons in the dorsal root ganglia (DRGs), especially those expressing trkA, and a decreased response to noxious mechanical stimuli, but not to thermal pain.
For future experiments, the following could be important to explore:
Investigating Compensatory Mechanisms:
Examining whether other neurotrophins or their receptors compensate for the loss of NGF in sensory and sympathetic neurons.
This could involve studying the expression and function of other neurotrophin receptors like trkB and trkC in NGF-deficient mice.
Neurological and Behavioral Analysis:
Conducting detailed neurological and behavioral studies to understand the full spectrum of sensory deficits in NGF-deficient mice.
This could include more nuanced pain assays to dissect the types of pain (e.g., chronic vs. acute) affected by NGF deficiency.
Developmental Studies:
Investigating the developmental timeline of neuronal loss and dysfunction in these mice.
This would help in understanding at what stage NGF becomes critical for neuronal survival and function.
Therapeutic Interventions:
Exploring potential therapeutic interventions to rescue or mitigate the neuronal loss and sensory deficits in NGF-deficient mice.
This could involve gene therapy, neurotrophin supplementation, or other pharmacological approaches.
Ernfors 1994
Figure 1, how do the authors using the restriction enzyme BamHI to differentiate between the wild-type and mutant alleles in their Southern blots?
2 BamH1 sites that flank thee NT3 region
cut out a piece thats 15 kbp
introduced neomycin
Probe only binds that specific region of DNA
allows them to be certain the homologous recombination works
Figure 1C depicts genotype analysis for a cross between two heterozygous (+/-) parents. In your reading of the Southern blot shown in this panel, how many homozygous mutant (-/-) animals are found in this litter?
Wild Type = only has 1 band @ 15 kb
Heterozygous = has both bands
Homozygous / mutants / knockout = only 1 band @ 6.1 kB
Cell counts in six different ganglia and nuclei are presented in Table 1. What type of neurons are found in each of the ganglia/nuclei? Why do you think the authors chose these structures to analyze?
To see the difference between the sensory and sympathetic neurons
are their populations that depend on NT-3 ? and which ones don't ?
Trigeminal ganglion
affected
xxxxxxxxxx- Mesencephalic trigeminal nucleus (MeV)- affected
xxxxxxxxxx- Nodose ganglion- affected
xxxxxxxxxx- L4 (lumbar 4) DRG- affected
xxxxxxxxxx- Superior cervical ganglion- affected
xxxxxxxxxx- Facial nerve motor nucleus- NOT affected
xxxxxxxxxx- motor neurons are CNS from neural tube- CNS DOES NOT depend on NT-3 for survival- all of the other ones are PNS , from neural crest or placode- they DO depend on NT-3 for survival
Compare the data in Table 1 with Table 7.1 in the textbook. Are the changes in neuron number similar for NT-3 and TrkC mutants? What differences do you notice and what could that mean for the neurotrophic support of those types of neurons?
Table 7.1 = TrkC = lose 20%
in Table 1 in paper , they loose about 50%
why?
knockout NT-3 , loose half the cells
knockout TrckC = loose 20%
so TrkB , there is some low affinity binding of NT-3
See figure 7.12
they are binding more than 1 neurotrophin for TrkB
Figure 2A and 2B are micrograph cross-sections of the soleus stained for slow-tonic myosin heavy chain protein by immunohistochemistry. What is the significance of the lack of staining in the homozygous mutant animal? What do the authors conclude from this staining (see also Table 2)?
muscle spindles have a different type of myosin
Wild-Type = regular muscle fibers = lots of muscle spindles
Heterozygous = half
Knockout = none
Muscle Spindles = express TrkC , sensory neurons
just proprioceptors that are affected
What type of tissue is used in the DiI tracing shown in Figure 3? Is this tissue alive during the tracing process?
DiI = flourecent lipophilic molecule
stays inside of a membrane
dead tissue , fixed in paraformaldehyde
List the similarities and differences in the axon projections in the spinal cord in control and homozygous mutants in Figure 3.
Similarities :
layers of spinal cord , pain and touch fibers ( area 1 , 2 , 3 , 4 )
these are fine , and are present in the spinal cord
Differences :
C = proprioceptive neurons send axnos down , into the spinal cord
D = neurons are dead
axons are not present in the spinal cord
Please complete the table below for each of the different categories of sensory neurons evaluated in the DRG in Figure 4.
| Antigen | Type of Neuron it Marks | Status in NT-3 Homozygous Mutant |
|---|---|---|
| CA | sensory , proprioceptive | panel A / B = a few left in knockout |
| PV | proprioceptive | panel C / D = none |
| SP | pain / nociceptors | panel E / F = no change |
| CGRP | nociceptors | panel G / H = no change |
CA and PV are 2 different markers for proprioceptors
some are completely gone in the mutant
SP and CGRP mark 2 distinct types of nociceptors
they don't see any changes