Lack of Neurotrophin-3 Leads to Deficiencies in the Peripheral Nervous System and Loss of Limb Proprioceptive Afferents
https://www.sciencedirect.com/science/article/abs/pii/0092867494902135
https://sci.bban.top/pdf/10.1016/0092-8674%252894%252990213-5.pdf#view=FitH
Summary
Neurotrophin-3-deficient (NT-3-deficient) mice were generated by gene targeting. Mutant mice displayed severe movement defects of the limbs, and most died shortly after birth. Substantial portions of peripheral sensory and sympathetic neurons were lost while motor neurons were not affected. Significantly, spinal proprioceptive afferents and their peripheral sense organs (muscle spindles and Golgi tendon organs) were completely absent in homozygous mutant mice. This correlated with a loss of parvalbumin and carbonic anhydrase-posltlve neurons in the dorsal root ganglion. No gross abnormalities were seen in Pacinian corpuscles, cutaneous afferents containing substance P and calcitonln gene-related peptide, and deep nerve fibers in the joint capsule and tendon. Importantly, the number of muscle spindles in heterozygous mutant mice was half of that in control mice, indicating that NT-3 is present at limiting concentrations in the embryo.
Introduction
During development, neurons are produced in excess and their survival is dependent on neurotrophic factors secreted by the target cells of innervation . Neurotrophin-3 (NT-3) belongs to a family of structurally and fu notionally related neurotrophic factors, the neurotrophins, that also includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-4/5 (NT-4) . All four neurotrophins have been shown to promote the survival of neural crest-derived sensory neurons in culture , whereas BDNF, NT-3, and less efficiently, NT-4, but not NGF support placode-derived sensory neurons. Survival of sympathetic neurons is supported by NGF, and, to a lesser degree, by NT-3.
Specific subpopulations of neurons in a given ganglion appear to respond to one, or a selective set of, neurotrophins in culture. The neurotrophins also act sequentially to promote the survival of neurons during development. For example, while mouse trigeminal ganglion neurons respond to all four neurotrophins at embryonic day 12, only NGF is effective at later developmental stages. Similar results have been described for BDNF and NT-3 on vestibulocochlear neurons and for NGF and NT-3 on superior cervical ganglion neurons.
NT-3 mRNA is expressed in peripheral target tissues of sensory and sympathetic innervation during development in a cell-specific, partially overlapping pattern compared with the other neurotrophins . In addition, spinal cord motor neurons, a central target of dorsal root ganglion (DRG) neurons, express NT-3 mRNA embryonically. These findings suggest that NT-3 may be retrogradely transported from the targets to support the survival of neurons during development and that the spatial and temporal expression of NT-3 is precisely regulated to ensure the correct development of the nervous system. So far, only limited information is available on the role of NT-3 in vivo. It was recently shown, however, that NT-3 enhances sprouting and the survival of adult lesioned corticospinal nerves and locus coerulleus neurons and increases oligodendrocyte proliferation in the optic nerve during development.
Mice carrying a deletion in the NT-3 gene were generated by homologous recombination in embryonic stem (ES) cells to study the physiological functions of NT-3 during development. NT-3 was found to be required for specific populations of sensory and sympathetic peripheral neurons. Most significantly, the main components of the proprioceptive system, i.e., the muscle spindles and Golgi tendon organs, were completely missing in homozygous mutant mice, and the number of muscle spindles was reduced to 50% in heterozygotes.
Results
Generation of NT-3 Mutant Mice
ES cells were transfected with a targeting vector designed to replace the protein coding region of the NT-3 gene with a neomycin-resistance gene (Figure 1A). Independent ES cell clones (3) that had undergone homologous recombination (Figure 18) were injected into blastocysts. The animals resulting from the injection of all three clones showed extensive coat color chimerism and transmitted the ES cell-derived mutant allele to their offspring. They were intercrossed to generate homozygous mutant mice (Figure 1C). Mice heterozygous for the mutation displayed no overt behavioral abnormalities. Homozygous mutant mice, however, were reduced in size as compared with wild-type and heterozygous littermates, and the majority died shortly after birth. Those surviving postnatal day O (PO) often lived to the third postnatal week. Mutant mice displayed limb ataxia and an apparent inability to position the extremities properly when attempting to move. There was a striking tendency of all four limbs to intermittently stiffen in an extensor posture. This abnormality resembled the rigidity and cross-extensor reflex observed in limbs of experimentally deafferented animals. The phenotype was apparent directly after birth.
Deficiencies in Sensory and Sympathetic Ganglia
At autopsy, all peripheral ganglia were markedly smaller in the homozygous mutant mice as compared with controls. To quantitate neuronal loss, neurons were counted in serial sections of the dorsal root, trigeminal, nodose, and superior cervical ganglia and the mesencephalic trigeminal nucleus of P12-P16 mice (Table 1). The number of neurons was significantly reduced with 45 ± 12% remaining in the DRG, 36 ± 6% remaining in the trigeminal ganglion, 48 ± 5% remaining in the mesencephalic trigeminal nucleus, 53 ± 18% remaining in the nodose ganglion, and 47 ± 14% remaining in the superior cervical ganglion. It should be noted, however, that the values from our cell counts were not corrected for split nucleoli, which may result in oversampling of the neurons with two or more nucleoli. This could skew the actual value and lead to an over- or underestimation of the neuronal loss. The number of facial motor neurons was not significantly different from that of control mice (98 ± 9%). While the morphology of neurons in the ganglia of mutant mice was normal, mesencephalic trigeminal neurons appeared smaller in size as compared with controls. Thus, lack of NT-3 leads to a decreased number of neurons in all primary sensory ganglia examined as well as in the sympathetic superior cervical ganglion of homozygous mutant mice.
Mutant Mice Lack Peripheral Proprioceptive Afferents and Sensory Receptors
The loss of neurons in the DRG, sensory ataxia, and intermittent extensor rigidity of limbs suggested a deficiency in proprioceptive outflow from limbs. Limb proprioceptive sense organs include muscle spindles (innervated by type la and II afferents and fusimotor efferents), Golgi tendon organs (innervated by type lb afferents), and joint receptors. During development, afferent innervation by la neurons induces the formation of muscle spindles, and innervation by lb neurons induces the formation of Golgi tendon organs. Thus, lack of proprioceptive DRG neurons would be expected to cause defects in the corresponding peripheral sense organs.
Hindlimbs of PO homozygotes, heterozygotes, and controls were examined in 1-µm-thick serial transverse sections stained with toluidine blue. Control mice contained 11 .4 ± 0.6 (n = 5) muscle spindles in the soleus muscle, whereas homozygous mutant mice were completely devoid of spindles in all muscles, including the soleus (0 ± 0, n = 5) (Table 2). The loss of muscle spindles was revealed by the absence of encapsulated bundles of smallcaliber muscle fibers innervated by afferents. The muscles lacking spindles were also devoid of any expression of the slow-tonic myosin heavy chain isoform (Figures 2A and 2B), which is specifically expressed in the intrafusal muscle fibers of the spindles. Similar observations were made in P7 mice (n "' 2), indi• cating that the absence of spindles in homozygous mutant mice was not due to a delay of formation. Significantly, mice heterozygous for the NT-3 mutation contained approximately half the normal complement of spindles in the soleus muscle (5.7 ± 0.8, n = 6, Table 2). In addition to deficiency in muscle spindles, homozygous mutant mice were also devoid of Golgi tendon organs in the myotendonous junctions (Figure 2D, n = 5). Control mice had 3-4 Golgi tendon organs in each myotendonous junction of the soleus muscle as revealed by the presence of largecaliber lb sensory afferents and encapsulated formations (Figure 2C, n = 5). Thus, the mutation affected both type la and lb afferents. We do not know at this time whether type II afferents were also affected in muscle of the mutant mice. Electron microscopic examination of two mutant PO muscles, however, revealed no evidence of afferent nerve fiber-muscle contacts. In contrast with the spindles and Golgi tendon organs, the mutation did not alter the development of Pacinian corpuscles (Figures 2E and 2F, n = 5). Clusters of Pacinlan corpuscles Innervated by large-call• ber axons were observed in association with the periosteum of the fibula (Figures 2E and 2F). lmmunocytochemlstry was used to determine whether lack of NT-3 affected he innervation of cutaneous tissues, joint capsule, or tendon. No gross abnormalities were detected of cutaneous afferents containing substance P (SP) or calcitonin generelated peptide (CGRP), or of afferent nerve fibers in joint capsule and tendon as detected using the RT 97 antibody directed against phosphorylated neurofilaments (data not shown).
These results show that lack of NT-3 leads to the absence of muscle spindles, Golgi tendon organs, and afferent-muscle contacts but appears not to qualitatively affect Pacinian corpuscles, cutaneous afferents containing SP and CGRP, and deep nerve fibers in the joint capsule and tendon. Happlo insufficiency was revealed by the reduction of muscle spindles in heterozygous mutant mice to 50% of controls, indicating that NT-3 is produced at limiting concentrations in the embryo.
Mutant Mice Lack Central la Fibers In the Spinal Cord
To investigate whether the central branch of the proprioceptive la fibers was also affected in NT-3 mutant mice, the lipophilic carbocyanine dye, Oil, was used for retrograde tracing of DRG afferents in the spinal cord. The proprioceptive la afferents are part of the muscle-motor neuronmuscle circuit that mediates the stretch reflex and make monosynaptlc contacts with spinal cord motor neurons. In control mice, la fibers entered the dorsal horn, transversed the spinal grey matter as coarse bundles, and terminated in layer IX (Figure 3C, n = 4), where they synapsed with motor neurons. la fibers were all but missing In sections from the mutant mice, and only occasionally were fibers projecting ventrally to layer IX of the spinal cord detected (Figure 4D, n = 3). In contrast, no obvious change could conclusively be detected in the number or the architecture of fibers in the dorsal horn layers I-IV (Figures 3A and 3B). These results show that the central branch of the proprioceptive la fibers innervating spinal cord layer IX was absent in the NT-3 mutant mice.
Proprioceptive Neurons Are Absent in the DRG
We used immunocytochemistry to investigate whether the lack of proprioceptive sensory organs and central la fibers was caused by the absence of a specific subpopulation of DRG neurons. Sections of lumbar DRGs were incubated for the detection of carbonic anhydrase (CA) immunoreactivity. In control animals, a large number of L4 DRG neurons were CA immunoreactive (Figure 4A, n = 3). CAcontaining neurons were all but absent in the mutant mice, and only few weakly immunoreactive neurons could be found (Figure 4B, n = 3). Because the CA reaction product is present in large-diameter DRG neurons (30-33% of mouse lumbar DRG neurons) and is present in muscle spindle afferent terminals (Mayeux et al., 1991; Carr and Nagy, 1993), a loss of CA neurons is consistent with the notion that neurons innervating muscle are absent. Sections of DRG were also immunostained for parvalbumin (PV), another marker for proprioceptive neurons. Approximately 90% of the PV neurons are also positive for CA, and PV, which is localized to the large-diameter neurons, is present in approximately 14% of the rat lumbar DRG neurons (Carr et al., 1989a). Whereas a large number of neurons in the DRG from control mice showed strong PV immunoreactivity (Figure 4C, n = 3), no PV-containing neurons were detected in any DRG sections from the mutant mice (Figure 4D, n = 3). When DRG sections were immunostained for CGAP and SP, both of which are not colocalized with CA or PV, no obvious changes in the number or intensity of immunostained neurons were observed between control and mutant mice (Figures 4E-4H). These results show that lack of NT-3 leads to an absence of DRG neurons associated with proprioception, but seems not to visibly affect CGAP- and SP-positive neurons that are associated with other sensory modalities.
Discussion
We show that NT-3 is required for the development and survival of particular populations of peripheral neurons in vivo. Lack of NT-3 expression resulted in a decreased number of neurons in all sensory ganglia examined, in• cluding DRG, trigeminal ganglion, and nodose ganglion, as well as mesencephalic trigeminal nucleus neurons. The severe effects on peripheral sensory neurons seen in the NT-3 mutant mice are consistent with previous culture results showing that NT-3 promotes the survival of neurons from these ganglia. In addition to its survival activity, NT-3 is also a mitogen for chick neural crest cells in culture. Because our neuronal counts were performed at one postnatal timepoint, it is possible that the decrease of neurons in sensory ganglia is due to alterations in proliferation, excessive cell death, or both. However, preliminary observations from immunocytochemistry for neurofilament (160 kDa) on E10.5 embryos revealed no visible difference in the size of and nerve processes from the trigeminal, geniculate, petrose, nodose, or DRGs in mutant as compared with control embryos, suggesting that NT-3 Is not necessary at least for the initial formation of these ganglia. In contrast with the severe effect on sensory neurons, motor neurons were not affected by the mutation. This Is consistent with studies showing that NT-3 injected into neonatal animals does not effectively promote the survival of motor neurons after axotomy, but contrasts with culture results showing that NT-3, BDNF, or NT-4 can rescue embryonic motor neurons from cell death (Henderson et al., 1993). Neither BDNF nor NT-3 mutant mice show defects of motor neurons, but it is possible that other neurotrophic factors compensate for the lack of these factors in vivo.
The loss of neurons in the DRG and movement defects raised the possibility of a deficiency of proprioceptive sensation. Indeed, no muscle spindles or Golgi tendon organs were detected in the mutant mice. Consistent with this deficiency, the total number of DRG neurons was decreased, and neurons expressing CA and PV were absent. The latter neurons mediate proprioceptive sensation, suggesting that the lack of proprioceptive afferents was due to a loss of these DRG neurons. This agrees with a previous culture study in which NT-3 supported the survival of DRG neurons that innervate muscle. Although the loss of proprioceptive afferents and end organs most likely causes the movement defects, we can not exclude that central nervous system lesions also contribute to the mutant phenotype. The marked decrease of DRG neurons suggested additional sensory deficiencies in the periphery. While deep nerve fibers in the joint capsule and tendons, Pacinian corpuscle AP afferents, or cutaneous SP- and CG RP-containing afferents were not qualitatively affected, it cannot be excluded that there are quantitative differences. In addition, SP- or CGAP-negative neurons that subserve other sensory modalities may be affected, contributing to the loss of DRG neurons in the mutant mice.
Small-, medium-, and large-diameter DRG neurons express trkA, trkB, and trkC, respectively, suggesting that neurons of different modalities may depend on specific neurotrophins for survival, differentiation, or both . This is consistent with the observation that NGF supports selectively small-diameter DRG neurons projecting to spinal cord lamina I and II as well as A6 cutaneous afferents that mediate nociception and thermoreception. Thus, these observations together with our findings suggest that survival of DRG neurons of different sensory modalities depends on different neurotrophins in vivo.
What is the source of NT-3 that supports survival of the proprioceptive neurons during development? In situ hybridization experiments have shown that the spinal motor neurons express high levels of NT-3 mRNA at the time of muscle spindle induction, which coincides with the time when the central connections are made between the proprioceptive DRG neurons and the motor neurons of the ventral horn. This suggests that motor neuron-derived NT-3 may be crucial for the survival of the proprioceptive neurons. NT-3 is, however, also produced in the developing and adult muscle, implying that a peripheral source of the neurotrophin may also play a role in sustaining the sensory neurons. In addition to NT-3, BDNF could also support proprioceptive DRG neurons because BDNF mRNA has been detected in embryonic muscle. This neurotrophin seems, however, not to be a crucial survival factor for proprioceptive neurons because BDNF mutant mice display no overt deficiency in positioning their limbs, loss of muscle spindles, or loss of CA and PV DRG neurons (data not shown). This is consistent with previous results showing that NT-3 supports a greater number of the sensory neurons that innervate muscle in culture than BDNF does.
It has been postulated for a long time that the number of neurons in the developing vertebrate organism is regulated by neurotrophic factors, and one of the basic conjectures of this concept has been the hypothesis that these factors are present in limiting concentrations in the target fields of innervation. According to this hypothesis, competition for the limiting amounts of neurotrophic factors assures target innervation by the appropriate number of neurons, excessive neurons being eliminated by programmed cell death owing to their failure to attain sufficient amounts of survival factors. The observation that heterozygous animals, carrying a single functional NT-3 gene, have half the number of muscle spindles as compared with wild-type mice, carrying two functional gene copies, provides direct experimental evidence of a neurotrophic factor that is present in limiting amounts during development of the nervous system.
The survival of proliferating embryonic sympathetic neuroblasts is stimulated by NT-3. However, NT-3 promotes the survival of only a small population of neonatal sympathetic neurons in culture. Thus, the dramatic reduction of sympathetic neurons in the NT-3 mutant mice is likely due to the lack of this factor during formation of the ganglia and, perhaps to a lesser degree, the lack of it during the period of programmed cell death.
Our results should be of clinical interest because a variety of inherited and acquired peripheral neuropathies are associated with damage to the proprioceptive system. For instance, patients or experimental animals with sensory neuropathy suffer profound sensory ataxia and areflexia owing to the loss of proprioceptive sensibility caused by the degeneration of sensory endings in muscle spindles and large-diameter DRG neurons. Our results suggest that administration of NT-3 may promote the survival and regeneration of the proprioceptive neurons in such patients.
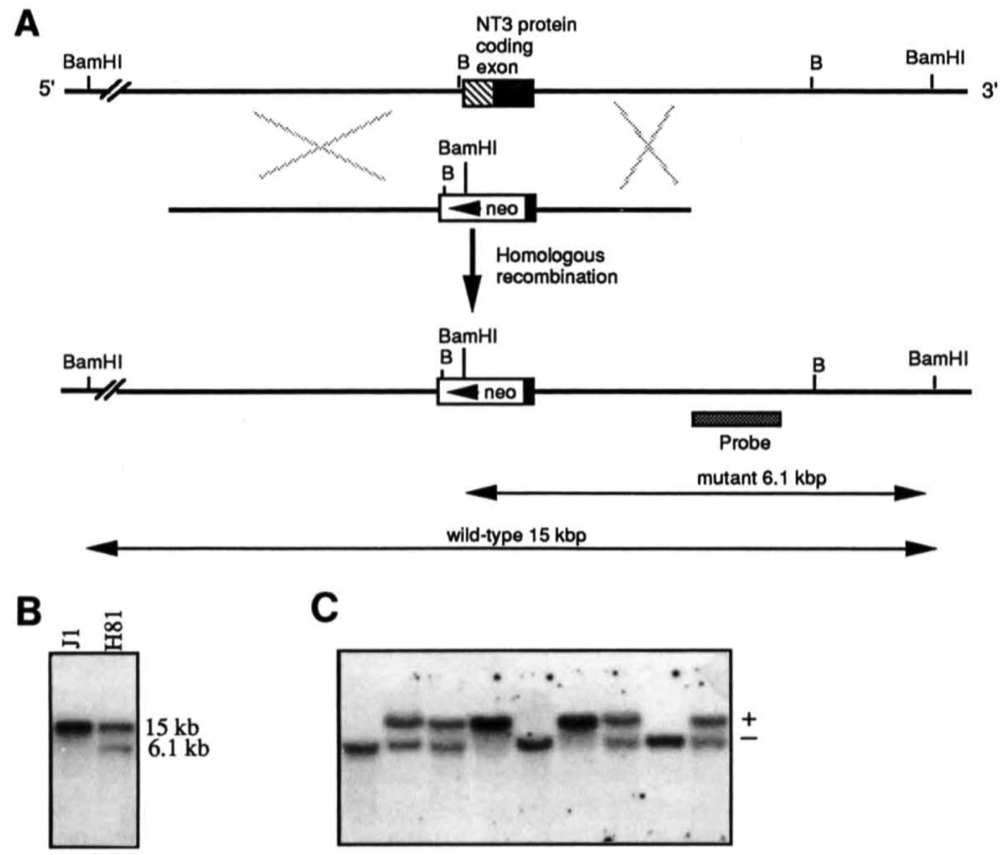
Figure 1. Targeted Inactivation of the NT-3 Gene in Mouse ES Cells and Generation of Mutant Mice
(A) Schematic diagram of the strategy used to target the NT-3 gene. Top line, genomic structure of the NT-3 gene. The entire coding sequences are contained within the exon represented by a box, prepro coding sequences are hatched, and the closed box represents coding sequences for the biologically active protein. Middle line, the 8.0 kb targeting replacement vector used for electroporation. A neo expression cassette, including the phosphoglycerate kinase promoter and polyadenylation signals, was inserted into a partially deleted coding exon of the NT-3 gene. The deletion left 97 amino acids in the C terminus. The ES cell line J1 was electroporated with the targeting vector, followed by selection with G418. G418resistant clones were expanded and analyzed by Southern blot analysis. Bottom line, representation of the structure of the inactivated NT-3 gene. B, Bgl II.
(B) Southern blot analysis of the parental and of a targeted ES clone. DNA from parental J1 ES cells and mutant clone H81 was digested with BamHI and hybridized to a genomic probe external to the 3' homologous arm. This probe hybridized to a 15 kb and a 6.1 kb fragment from wild-type and mutant alleles, respectively. (C) Southern blot analysis of the offspring from a NT-3 (+/-) x NT-3 (+/- )cross.Tail DNA was extracted, digested with BamHI, and analyzed as described in (B).
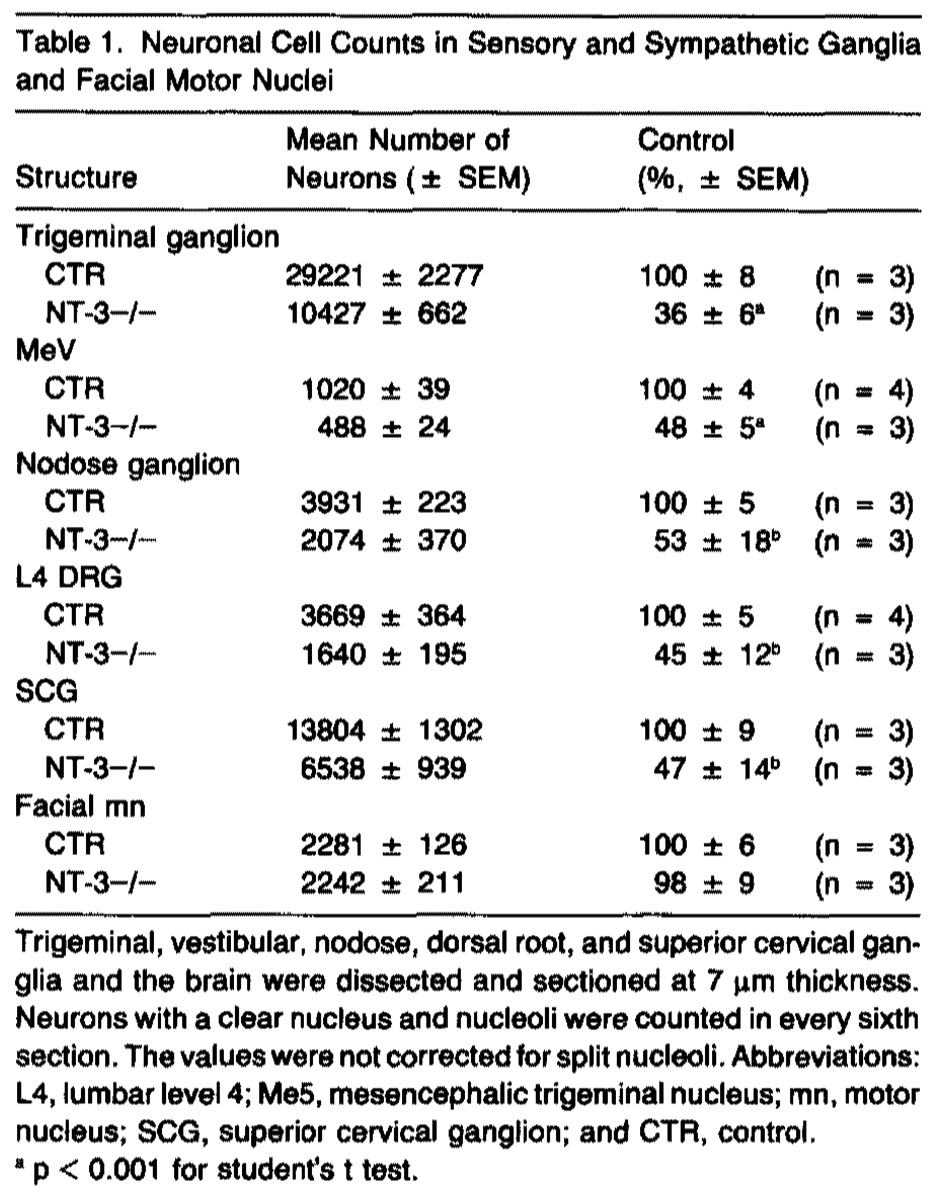
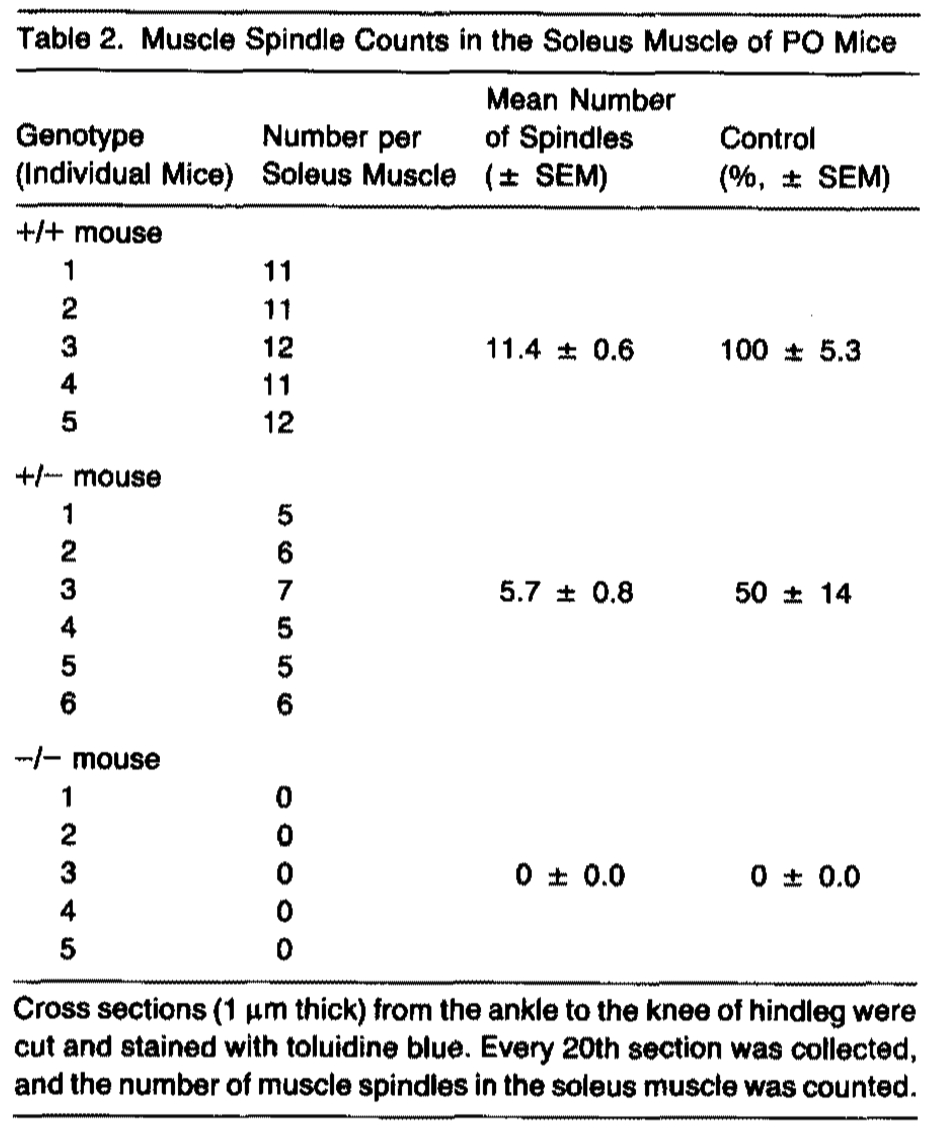
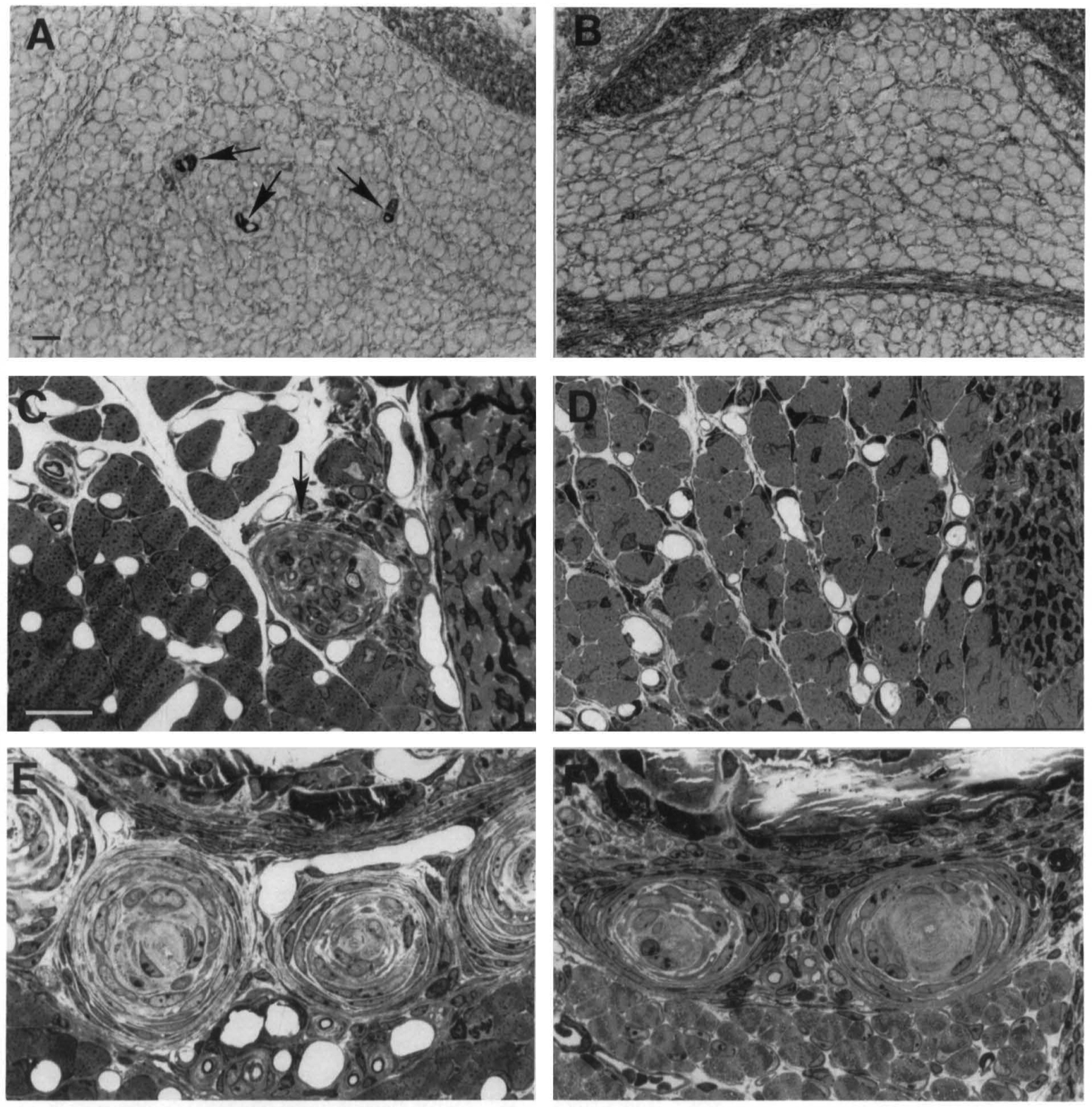
Figure 2. Absence of Some Sensory Organs in NT-3 (-/-) Mice
Control (A, C, and E) ancJ NT-3 mutant (B, D, and F) PO mice. (A and B) lmmunocytochemistry for slow-tonic myosin heavy chain reveals numerous muscle spindles in the soleus muscle of the control (A), but reveals an absence of muscle spindles in the homozygous mutant mice (B). Arrows in (A) indicate muscle spindles.
(C and D) High magnification of a semithin section through the distal myotendonous junction of the soleus muscle (note tendon at right). Arrow in (C) indicates a Golgi tendon organ with a large-caliber lb sensory afferent in a control. No Golgi tendon organs were seen in mutant mice (D). (E and F) Photomicrograph of a cluster of Pacinian corpuscles in the periosteum of the fibula. No disturbances of Pacinian corpuscles were seen in the homozygous mutant mice (F) as compared with controls (E). Note the sensory afferent at the center of each corpuscle. Scale bars are 20 µm.
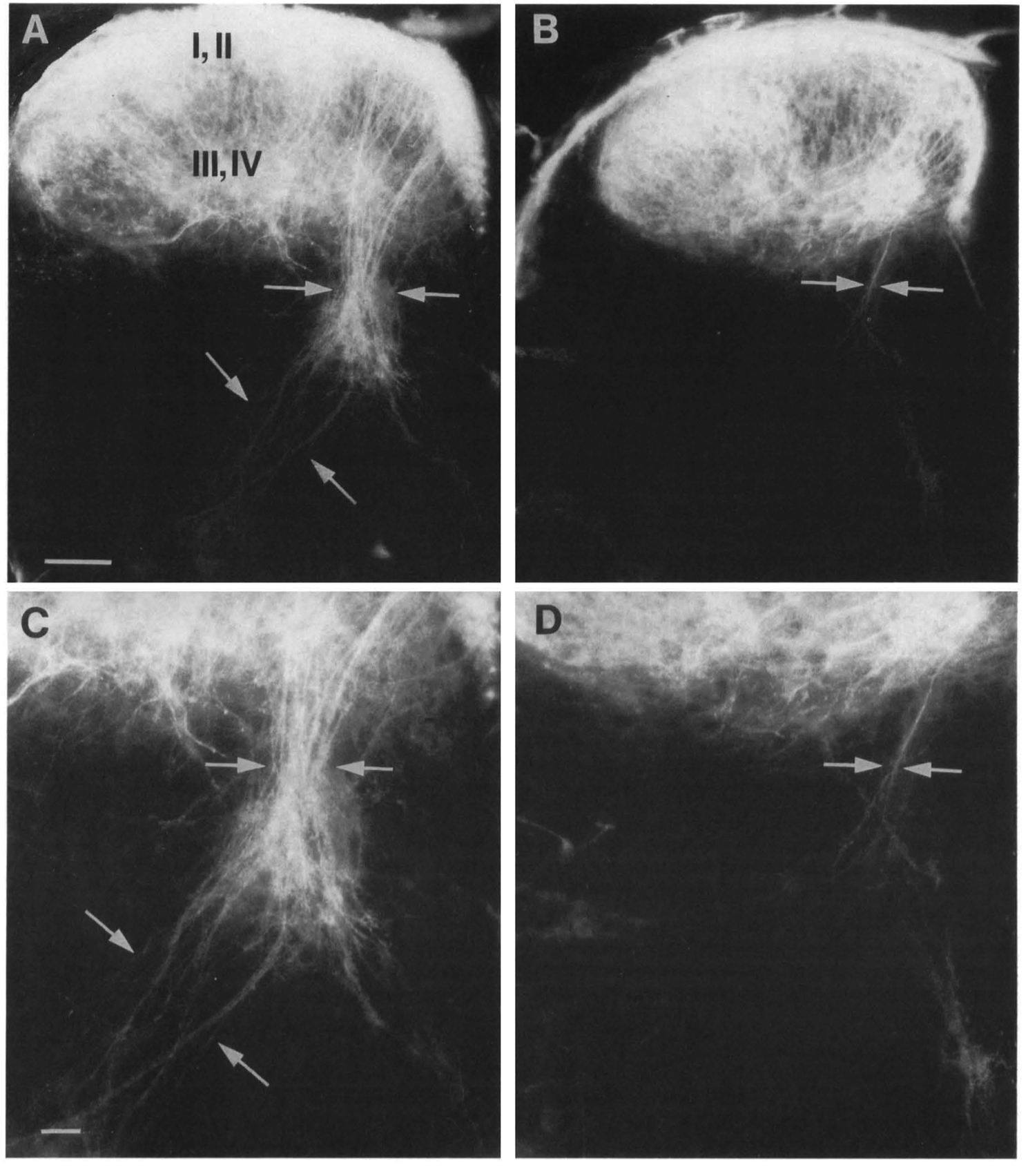
Figure 3. Oil Tracing of the Central Branches of DRG Fibers to the Spinal Cord Revealed a Loss of Central la Afferents in NT-3 (-/-) Mice
Control (A and C) and NT-3 mutant (Band D) PO mice.
(A and B) Low-power micrograph of a section through the cervical spinal cord. The la fibers (arrows) were all but missing in mutant mice (B) as compared with control mice (A). No abnormality could conclusively be detected in the central fibers terminating in spinal cord layers I-IV.
(C and D) Higher magnification of the la fibers. In control mice (C), the la fibers (arrows) transversed the spinal grey matter in coarse bundles and terminated in layer IX (lower left corner of photomicrograph). Occasionally, a few la fibers (arrows) were found remaining in the mutant mice (D). Scale bars are 45 µm.
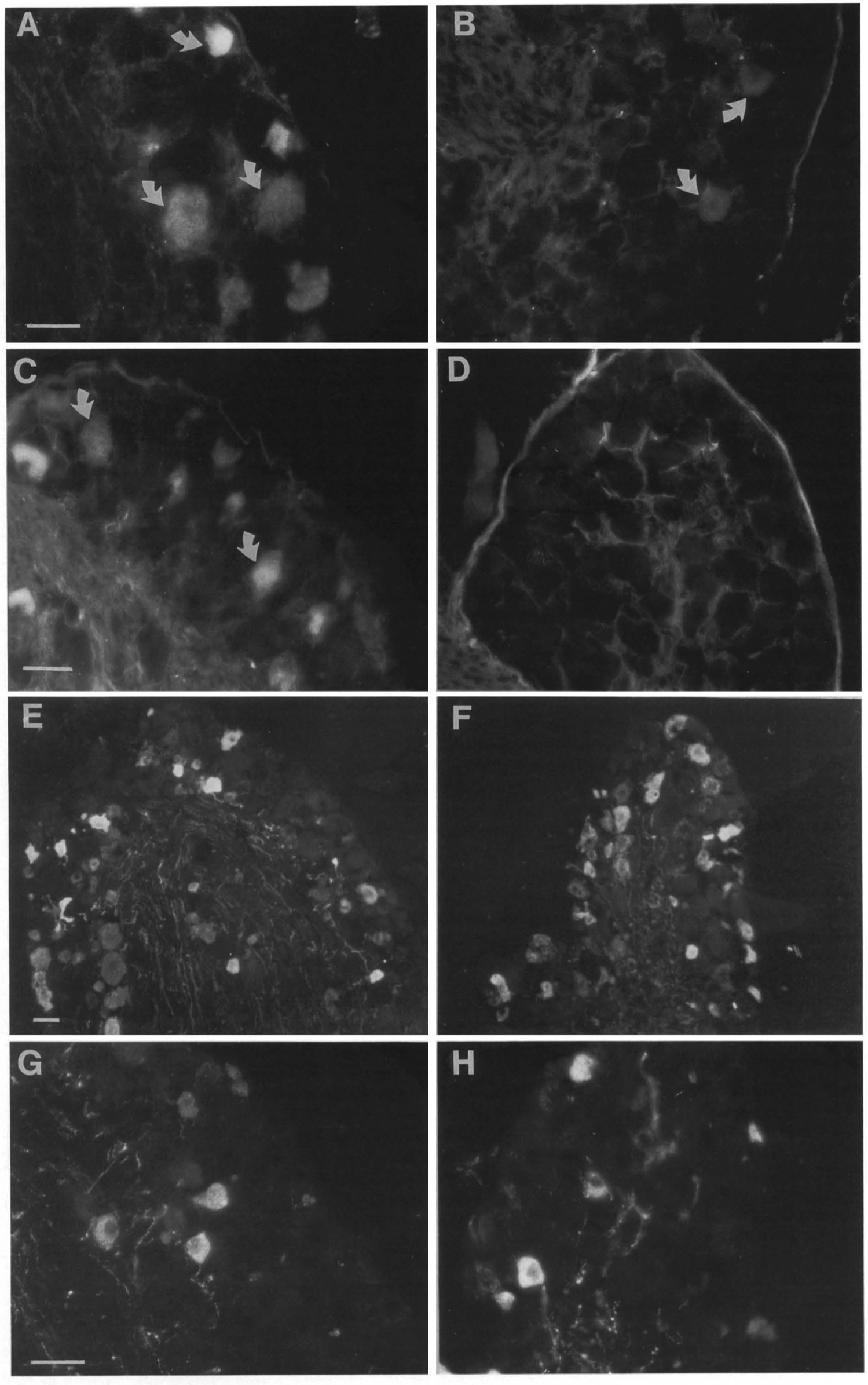
Figure 4. Loss of CA- and PV- but Not SP- and CORP-Containing Neurons in the DRG
Sections ofDRG from control (A, C, E, and G) and NT-3 (-/-)(B, D, F, and H) mice. (A and B) CA lmmunocytochemlstry; (C and D) PV immunocytochemlstry; (E and F) CGRP lmmunocytochemlatry; and (G and H) SP lmmunocytochemlstry. Whereas numerous CA- and PV-positive neurons were seen in each section from control mice (A and C), only a few CA-immunoreactive (B) and no PV-lmmunoreactive (D) neurons could be seen in DRG from mutant mice. No apparent difference In the number or intensity of CGRP-immunoreactive (E and F) or SP-lmmunoreactive (G and H) neurons could be seen in mutant as compared with the control. Scale bars are 40 µm.
Experimental Procedures
Gene Targeting, Generation of Mutant Mice, and Southern Blot Analysis
Cloned genomic ONA corresponding to the NT-3 locus was isolated from a phage library of strain 129 mouse ONA. A replacement targeting vector with a partially deleted NT-3 coding exon, containing 97 amino acids remaining in the C terminus, was constructed as described in Figure 1. The J1 ES cell line was derived and grown on embryonic fibroblast feeder cells as previously described (Li et al., 1992). Following electroporation of cells with 60 µg/ml of linearized targeting vector (Bio-Rad gene pulser at settings of 400V and 25 mF), cells were selected in 0.4 mg/ml of G418. Resistant colonies were trypsinized individually. Hall of the cells were expanded in a 96-well plate for freezing, and the other half were expanded in a 24-well plate for genotyping. Clones that contained the expected 6.1 kb BamHI fragment diagnostic for homologous recombinants were obtained at a frequency of 1 in 48. These ES clones were further analyzed by digestion with Bglll, generating a 4.5 kb wild-type and a 5 kb mutant fragment when probed as above. The membranes were rehybridized to a neo probe to verify a single integration. Of three ES clones injected into blastocysts, all contributed to the germline of recipient embryos. Chimeric mice were generated as previously described (Li et al., 1992). Chimeric males were bred to BALB/c females, and germ line transmission of the mutant allele was identified by Southern blot analysis of tail DNA from F1 offspring with agouti coat color.
Histological Methods
For immunocytochemistry, mice were perfused with 4% paraformaldehyde, dissected, and postfixed for 2 hr. Sections were cut at 7 µm on a cryostat and preincubated in dilution buffer (0.5 M NaCl, 0.01 M phosphate buffer [pH 7.3], 3% bovine serum albumin, and 0.3% triton X-100) for 1 hr, followed by overnight incubation with the indicated concentration of antisera in dilution buffer. After four washes in phosphate-buffered saline, sections were incubated for 2 hr with the appropriate rhodamine-conjugated secondary antiserum, washed three times for 10 min, and covered with glycerol-PBS (9:1) for viewing. The mouse monoclonal RT97 antibody (Wood and Anderton, 1981) was diluted 1 :500; the rabbit anti-SP antisera (lncstar) was diluted 1: 1000, the rabbit anti-CGRP antisera (Amersham) was diluted 1 :500; the rabbit anti-CA antisera (PerWistrand, Uppsala University, Sweden) was diluted 1:2000; and the mouse monoclonal anti-PV antibody (Sigma) was diluted 1 :300. Rhodamine-conjugated goat anti-mouse and goat anti-rabbit secondary antisera were used as secondary antisera. For immunocytochemical detection of slow-tonic myosin heavy chain, the hindlimbs were quenched in isopentane, cooled to -160°C with liquid nitrogen, and cut transversely into serial sections of 8 µm thickness from the ankle to the knee in a cryostat, The sections were immunoreacted with ALD 19 antibody using the avidin-biotin complex method, as previously described (Kucera and Walro, 1992).
For histology of muscle spindles, Golgi tendon organs, and Pacinian corpuscles, mice were perfused and the hindlimbs were excised and embedded into plastic. Serial sections of 1 µm thickness were cut from the ankle to the knee on an ultramicrotome and stained with toluidine blue. For the analysis of Golgi tendon organs, all sections were collected, and for analyses of muscle spindles and Pacinian corpuscles, 1 section was collected for every 20 sections cut.
D11 Tracing
PO mice were fixed for 6-12 hr in 4% paraformaldehyde. The cervical spinal cord with the dorsal root ganglia attached was dissected, the pia membrane was carefully removed, and the ventral roots were cut. The spinal cords were pinned down on sylgard gel petri dishes with insect pins and submerged in 4% paraformaldehyde. Individual C5C6 DRGs were injected with 0.25% Dil (1, 1'-dioctadecyl-3,3,3',3'tetramethylindocarbocyanine perchlorate, Molecular Probes) in dimethyl formamide using a 20-µm-thick glass pipette and were incubated for seven days at 37°C. The spinal cords were rinsed several times in PBS, followed by embedding in 2% agar at 50°C. Cross-sections (70 µm) of the spinal cord were cut on a vibratome and viewed with rhodamine filter on a Zeiss microscope.