Mice Lacking Nerve Growth Factor Display Perinatal Loss of Sensory and Sympathetic Neurons yet Develop Basal Forebrain Cholinergic Neurons
Summary
Homologous recombination was utilized to generate mice with a deletion In the coding sequence of the nerve growth factor (NGF) gene. Animals homozygous for NGF disruption failed to respond to noxious mechanical stimuli, and histological analysis revealed profound cell 1018 In both sensory and sympathetic ganglia. Within dorsal root ganglia, effects of the mutation appeared to be restricted to small and medium peptidergic neurons. These observations confirm the critical dependence of sensory and sympathetic neurons on NGF and demonstrate that other neurotrophins are not able to compensate for the 1018 of NGF action on these cells. Examination of the central nervous system revealed that, in marked contrast with neurons of sensory and sympathetic ganglia, basal forebraln cholinergic neurons differentiate and continue to express phenotypic markers for the Ute span of the null mutant mice. Thus, differentiation and Initial survival of central NOF-responsive neurons can occur In the absence of NGF.
Introduction
Nerve growth factor (NGF) is the first described member of a family of proteins known as the neurotrophins, which are believed to influence the differentiation and survival of neurons in the developing nervous system (LeviMontalcini, 1987; Thoenen and Barde, 1980; Thoenen, 1991). Overwhelming evidence for the role of NGF in promoting the survival of neurons of sympathetic and sensory ganglia comes from a series of in vitro and in vivo studies initiated over 40 years ago (see Levi-Montalcini, 1987; Hamburger, 1993). During the past decade, an increasing body of evidence indicates that NGF may also play a role in the brain to support the survival or function of the basal forebrain cholinergic system (see Hefti et al., 1989), a neuron population that undergoes profound atrophy in Alzheimer's disease. Thus, NGF has been proposed to play a critical role in the survival of selected populations of developing neurons in both the peripheral and central nervous system.
While there is compelling evidence that NGF is cai,llble of exerting dramatic influences on developing sympathetic, sensory, and basal forebrain cholinergic neurons (Chun and Patterson, 19TT; Hamburger et al., 1981; LeviMontalcini, 1987; Hefti et al., 1985; Hartikka and Hefti, 1988), the dependence of these neurons on endogenous NGF for survival remains to be definitively established. Sympathetic, sensory, and basal forebrain cholinergic neurons have all been demonstrated to respond in vitro to neurotrophins other than NGF (Leibrock et al., 1989; Rosenthal et al, 1990; Maisonpierre et al., 1990; Berkemeieret al., 1991; Ernforset al., 1990; Knusel et al., 1991). Most studies designed to investigate the role of endogenous NGF have been limited to the peripheral nervous system and have relied on the blocking activity of NGF antibodies, which have not been examined for cross-reactivity within the neurotrophin family. Thus, a role for NGF in the development of the central nervous system has not been demonstrated, and while it is widely accepted that NGF plays a role in the survival of sympathetic and sensory neurons, the extent to which other neurotrophins exert redundant trophic influences remains to be established.
To study the effects of NGF depletion on both central and peripheral neurons, we have used homologous recombination in embryonic stem (ES) cells to disrupt the NGF gene. Here, we report that mice that are homozygous for the disrupted allele display severe perinatal cell loss in the dorsal root and sympathetic ganglia yet develop basal forebrain cholinergic neurons that survive for the life span of the mice.
Results
Disruption of the Mouse NGF Gene In AB1 ES Cella
To inactivate NGF expression, a targeting vector was constructed in which 150 bp of NGF exon IV was removed and replaced with a neo resistance gene cassette. The deletion includes the splice acceptor of exon IV and 130 bp of NGF coding sequence including the start codon, the signal sequence, and part of the precursor region of NGF. In addition to the deletion of these sequences, the presence of a stop codon downstream of neo in the NGF reading frame precludes the possibility that a truncated NGF protein could be generated from the disrupted allele. The neo expression cassette (Crowley et al., 1993) is driven by a cytomegalovirus (CMV) immediate early gene promoter and enhancer element and lacks a polyadenylation signal. Polyadenylation is derived from genomic sequences after integration of the targeting vector. This replacement-type vector contains 11 .2 kb of NGF sequence, 9. 7 kb 5' and 1.5 kb 3' of the neo cassette. The counter-selectable marker herpes simplex virus thymidine kinase (Mansour et al., 1988) was added upstream of the NGF homology to reduce the number of colonies that arise from nonhomologous integration of the targeting vector (Figure 1A).
The targeting vector was linearized and then introduced into AB1 ES cells by electroporation. ES cell clones resistant to both G418 and FIAU were screened for the NGF gene disruption by Southern blot analysis using a probe to exon IV of NGF. Positive clones were confirmed by Southern analysis using a probe derived from NGF sequences outside of the targeting vector (data not shown). In this way, 576 clones were analyzed, and 11 clones that had a disrupted NGF allele were identified. FIAU counterselection reduced colony number 6.5-fold and yielded a targeting efficiency of about 1/50 after positive and negative selection.
Mice Heterozygous for NGF Gene Disruption Grow and Breed Normally
Into blastocysts derived from C578U6 female mice, 11 NGF-targeted AB1 clones were injected. Mosaic males were mated to C57BU6 females, generating agouti offspring from six of the cell lines, indicating germline transmission of the ES cell genome. Animals that are heterozygous for the NGF gene disruption (+/-) exhibit normal growth characteristics and could not be distinguished from their normal littermates by observation of their appearance or spontaneous behavior.
Heterozygous NGF-targeted mice obtained from one of the clones were identified by Southern analysis and were bred to obtain mice with both alleles disrupted. Analysis of F2 litters by Southern blotting (Figure 18) revealed that 24%, 44%, and 29% of the offspring were found to be homozygous (-/-), heterozygous, (+/-), or wild type (+/+) for the disrupted gene, respectively (the remaining 3% were lost to cannibalism). These ratios demonstrate that animals homozygous for the gene disruption are not compromised with regard to survival in utero. To verify that the phenotype observed in (-/-) mice was a result of the gene deletion, a limited number of F2 mice were produced from a second ES clone. From both ES clones, (-/-) animals displayed growth, survival, and nociceptive responses that were indistinguishable.
Mice with Both NGF Alleles Disrupted Are Born Live, but Fall to Thrive
On average, (-/-) mice are smaller at birth than (+/-) or (+/+) animals, ranging from 75%-95% of the mean weights of their (+/-) and (+/+) littermates. Observations during the first days of life demonstrated that (-/-) animals display largely normal patterns of spontaneous motor activity. During the first week of life, (-/-) animals were observed to display normal righting and placing reflexes, to seek to maintain contact with their littermates and mother, and to locate successfully and attach to a nipple. However, (-/-) animals did not appear successfully to ingest normal quantities of milk. In spite of efforts to increase food intake by reduction of litter size and through the use of surrogate mothers, some (-/-) animals displayed no evidence of food intake, failed to gain weight, and died (or were sacrificed) within the first 3 days of life. Other (-/-) animals were able to ingest some food and survived 1-4 weeks.
Homozygous animals that survived the first days of life gained weight more slowly than their littermates and were developmentally delayed in many aspects, including hair growth and eye opening. Some animals surviving the first week displayed mild gait abnormalities and developed a tremor, which was most evident during locomotion. All (-/-) animals surviving until eye opening were observed to display a marked ptosis (Figure 1C), a well-documented consequence of removal of the sympathetic innervation to the eye. Upon death, (-/-) animals were found to contain air, gas, or both throughout their digestive tract. Analysis of a series of hematoxylin and eosin stained sections through the thoracic and abdominal cavities of a litter of mice sacrificed on their day of birth revealed no obvious histological abnormalities in any major organs of (-/-) mice.
Homozygous NGF Null Mutant Mice Exhibit Severe Cell Loss In Sympathetic Ganglia
Superior cervical ganglia (SCG) dissected from early postnatal (-/-) animals were markedly smaller than those removed from (+/+) littermates (Table 1, p = .01, t = 4.18, 4 df), and by postnatal day 14, the SCGs from (-/-) animals were not visible upon dissection. In contrast, inspection of the nodose ganglia revealed that the size of these ganglia did not differ significantly from those of their (+/+) littermates (Table 1).
Consistent with the hypothesis that NGF deficiency results in the loss of sympathetic neurons by increasing naturally occurring cell death, pyknotic nuclei were abundant in the SCGs from 3-day-old (-/-) animals (Figure 2A). Pyknotic nuclei were far fewer in number in the SCGs from either (+/+) (Figure 2B) or (+/-) animals (data not shown). Nodose ganglia from (-/-) animals displayed only rare instances of apoptotic neurons and, with the exception that the neuronal profiles appeared to be somewhat reduced in size, were similar in appearance to those of (+/+) mice (Figures 2C and 20).
Homozygous NGF Null Mutant Mice Exhibit Selective Cell Loss In Senaory Ganglia
Dorsal root ganglia (DRGs) from 3-day-old animals were examined with regard to neuronal size and number. Visual inspection of sections through the cervical or lumbar ganglia revealed that the ganglia from (-/-) mice contained many fewer neuronal profiles and that, in contrast with the ganglia from (+/+) mice, small profiles were rare (Figures 2E-2H). Neuronal cell counts from the fourth and fifth lumbar ganglia revealed a 70% reduction in (-/-) mice (Table 2). The (+/-) animals displayed a marginally significant decrease in cell number as compared with their (+/+) littermates (Table 2). Cell size histograms from lumbar (Figure 3A) or cervical (data not shown) DRGs revealed a complete absence of small cells (<15 µmin diameter) in the ganglia of (-/-) animals. The histograms of the ganglia from (-/-) animals display no evidence of cell loss in the large cell range (>25 µm). Pyknotic nuclei were rare in DRGs from mice of all three genotypes.
In situ hybridization revealed that, in contrast with DRGs from (+/+) and (+/-) animals, ganglia from (-/-) mice were devoid of detectable trkA mRNA (Figure 3B). However, DRGs from (-/-) animals did contain cells expressing trkB and trkC (Figure 3B), and, although quantitation was not attempted, the numbers of cells expressing trkB and trkC did not appear to differ in the (+/+), (+/-), or (-/-) animals.
lmmunostaining with antibodies to substance P and calcitonin gene-related peptide (CGRP), markers expressed in small to medium DAG neurons (see Willis and Coggeshall, 1991), were used to characterize tissues from animals of all three genotypes. Results with both antibodies were qualitatively similar, but as fewer cells are labeled with the substance P antibody, only the CGRP data are shown (Figure 4). Staining of DRGs from (+/+) animals with the CGRP antibody produced an abundance of darkly stained perikarya. While ganglia from (+/-) mice displayed numerous CGRP-immunoreactive perikarya (Figure 4b), the immunoreactive cells gave the appearance of being both less numerous and more lightly stained than the ganglia of (+/+) mice (Figure 4a). In contrast with DRGs from both (+/+) and (+/-) mice, ganglia from (-/-) animals showed only a few weakly stained cells (Figure 4c).
The CGRP antibody was also used to examine both peripheral and central processes of primary sensory afferents. Staining of the spinal cord and skin from (+/+) animals revealed a dense plexus of immunoreactive fibers in the superficial laminae of the dorsal horn of the spinal cord and stained axons coursing through the dermis into the epidermis, respectively (Figures 4d and 4g). In keeping with the observations on DRGs, CGRP-immunoreactive axonal processes were completely absent in both the dorsal horn of the spinal cord as well as in the hairy skin of (-/-) mice (Figures 4f and 4i). Although no quantitation was performed, staining of the dorsal horn and skin of (+/-) animals (Figures 4e and 4h) appeared to reveal somewhat fewer CGRP-immunoreactive fibers in both, relative to (+/+) animals.
Mice carrying the Disrupted NGF Allele Display Decreased Responslveneu to Pain
To assess the pain sensitivity of mice carrying a deletion in the NGF gene, litters of newborn mice were tested (blind to their genotype) for their responsivity to a noxious mechanical stimulus. Mice later revealed to be of the (+/+) or (+/-) genotype reacted reliably to a pinch applied to the tail (Table 3). The response consisted of a vigorous squirming accompanied by vocalization and was exhibited by all (+/+) and (+/-) mice. In marked contrast with (+/+) and (+/-) mice, (-/-) mice very rarely responded to the noxious stimulus (Table 3). Differences in the frequencies of mice responding to tail pinch as a function of genotype were highly significant (x, 2 = 58, 4 df, p < .0001). Results of the pinch test conducted on litters prior to their first ingestion of milk were similar to those obtained later in life, indicating that the differences between (-/-) mice and their littermates may not be explained by differences in the nutritional status of the animals.
Mice of the F1 generation that were either wild type or heterozygous for NGF gene disruption were tested for their responsiveness to thermal pain in two paradigms, the tail flick and hot plate test. While no differences in behavior were observed on the hot plate test (Table 4, p > .20, t = 1.21, 139 df), the(+/-) animals were observed to display a small but significant prolongation of latency in the tail flick test.
Basal Forebraln Chollnerglc Neurons Differentiate and Maintain Their Phenotype for the Life Span of NGF Null Mutant Mice
Forebrains of homozygous NGF mutant mice ranging in age from 3 to 28 days of postnatal life were examined for expression of several markers of basal forebrain cholinergic neurons: mRNA for trkA (high affinity NGF receptor), immunoreactivity for p75 (low affinity NGF receptor), immunoreactivity for chollneacetyltransferase (ChAT), and acetylcholinesterase activity. As in the (+/+) mice, neurons expressing these markers were observed in the medial septum, the diagonal band of Broca, and the nucleus basalis magnocellularis of the (-/-) mice (Figure 5). Further, cells expressing trkA mRNA and ChAT were observed in similar patterns within the striatum of both mutant and wild-type mice (Figures SA and 5B and insets in Figures SC and 5D). Cholinesterase positive projections to the cortex and hippocampus were apparent in the brains of older wild-type (data not shown) and mutant mice (Figures SF and 5G), and the apparent density of these projections did not differ appreciably with genotype. Cholinergic neurons and their projections were observed to maintain expression of their phenotypic markers in the oldest surviving mutant mice (Figures 5F-5H).
Owing to the limited number of brains available from NGF null mutant mice and the difficulty of matching size or developmental stage of the mutant and wild-type mice, no attempt was made to quantitate the size or numbers of cells expressing cholinergic markers. Visual inspection of cholinesterase-stained sections from wild-type and homozygous mutant mice revealed qualitatively similar staining in animals of the two genotypes. However, staining of sections from brains of the mutant mice forChAT immunoreactivity, p75 immunoreactivity, or trkA mRNA revealed that cells of the mutant mice appeared smaller and more lightly stained than those of the wild-type mice at all ages examined.
Discussion
The actions of NGF on the developing neurons of sympathetic and sensory ganglia were first described over 40 yearsago(Levi-Montalcini, 1987; Hamburger, 1993). With the recent discovery that NGF is part of a family of structurally related molecules exhibiting overlapping sets of activities in vitro, a resurgence of Investigation on the role of NGF in the developing nervous system has begun, placing a special emphasis on the selectivity of effects. In the current study, we have employed homologous recombination in ES cells to generate mice that have both NGF alleles inactivated. Our results indicate thatthe absence of a functional NGF gene leads to severe cell loss in sensory and sympathetic ganglia and reduced viability of the affected mice. These results demonstrate a critical role for NGF in the development of some peripheral neurons and indicate that other neurotrophins do not compensate for the loss of NGF action on these cells. In marked contrast with NGFresponsive neurons of the peripheral nervous system, cholinergic cells of the basal forebrain were found to differentiate and maintain their phenotype for weeks in the absence of endogenous NGF.
Acceptance of a role for NGF in preventing cell death of sympathetic neurons has been afforded in large part by the demonstration that treatment of rodents in early postnatal life with NGF or antibodies to NGF has marked effects on cell number within the superior cervical ganglion (Levi-Montalcini and Angeletti, 1966; Levi-Montalcini and Booker, 1960). Our observations on mice lacking a functional NGF gene confirm a critical role for NGF in the development of SCG neurons. The presence of numerous pyknotic nuclei in neurons of postnatal day 3 (-/-) SCGs and the decrease in size of the ganglion between day 3 and 14 of life argue strongly that NGF deprivation via gene deletion exerts a major effect on the cell death of sympathetic neurons. These findings are consistent with earlier antibody deprivation studies suggesting that sympathetic neurons of the SCG are critically dependent on the presence of NGF during the early postnatal period (LeviMontalcini and Booker, 1960; Gorin and Johnson, 1979, 1980). However, we cannot rule out possible additional effects of the NGF gene disruption on proliferation or differentiation of SCG neurons.
With respect to sensory neurons, immunoneutralization experiments have indicated a role for NGF in promoting the survival of some cells of a neural crest-derived origin (Gorin and Johnson, 1980; Otten et al., 1980; Hamburger, et al, 1981; Johnson et al., 1980, 1983; Carroll et al., 1992). While antibody block has consistently lead to cell loss in ganglia derived from a neural crest origin, the degree of cell loss reported has varied greatly. Variability in the effectiveness of the antibody blocking experiments may be explained by differences in the developmental window covered by the treatment, by differences In the titers of antibodies to NGF, by the presence or absence of cross-reactivity of NGF antibodies with other members of the neurotrophin family, or by some combination of these effects. In the present study, animals homozygous for NGF null mutation were observed to exhibit 70% cell loss in the fourth and fifth lumbar DRGs. This number matches quite well with the results obtained in a recent antibody deprivation study employing an NGF antibody that exhibits no cross-reactivity to other neurotrophins (Ruit et al, 1992). Consistent with the proposed independence of placodally derived sensory neurons on trophic support by NGF (Da• vies and Lindsay, 1985; Johnson et al., 1980), nodose ganglia in mice homozygous for NGF disruption showed no signs of degeneration.
Our observations indicate that NGF plays a selective role in the development of some, but not all, sensory neurons of neural crest origin. Taken with the 70% reduction in DRG cell numbers of the NGF null mutant mice, the absence of DRG neurons expressing mRNA for trkA, the high affinity NGF receptor (Kaplan et al., 1991; Klein et al., 1991), suggests that all DAG neurons that express an NGF receptor during development are dependent on NGF for survival and are not subject to redundant trophic influences. However, we cannot rule out the possibility that some DAG neurons survive but down-regulate trkA expression to undetectable levels in the absence of NGF. The expression of trkB and trkC mRNA in the remaining DAG neurons underscores the earlier suggestion that DAG neurons surviving under conditions of NGF deprivation may be responsive to the actions of other neurotrophins (Carroll et al., 1992).
Taken together, our findings that NGF null mutant mice display a lack of small DAG neurons and a severe depletion of neurons expressing CGRP or substance P immunoreactivity suggest that NGF exerts a critical influence on the development of virtually all small peptidergic DRG neurons in the mouse. In keeping with the loss of small neuropeptide-immunoreactive DRG neurons and with previous observations from antibody deprivation studies (Johnson et al., 1983), animals that were either homozygous or heterozygous for NGF disruption were observed to display decreased responsiveness to painful stimuli.
In contrast with the marked degree of cell loss observed in sympathetic and sensory ganglia of neonatal NGF null mutant mice, many cells of the basal forebrain cholinergic system were found to differentiate and maintain their phenotype for the maximal life span of these mice, i.e., 4 weeks. Cholinergic cells and their projections were apparent in the oldest surviving animals, indicating that differentiation and survival of basal forebrain cholinergic neurons during the first weeks of postnatal life can occur in the absence of NGF. As the homozygous mutant mice are not viable past weaning, we have not been able to ascertain whether NGF is necessary for survival of basal forebrain cholinergic neurons at later timepoints. Consistent with in vitro studies on the ability of NGF to up-regulate ChAT expression in cultures of embryonic basal forebrain (Hefti et al., 1985; Hartikka and Hefti, 1988) and with a single report that NGF antibody treatment down-regulates ChAT in neonatal rats (Vantini et al., 1989), NGF null mutant mice appeared to display lighter ChATstaining in the basal forebrain than was observed in wild-type littermates.
Similar observations pertain to the pattern of labeling for both p75 immunoreactivity and trkA hybridization in the forebrain of homozygous mutant mice. Thus, our results suggest that while NGF is not required for survival or maintenance of the phenotype of cholinergic forebrain neurons during early postnatal life, this factor may regulate the functional status of these cells.
Animals homozygous for the NGF gene deletion were not found to be viable beyond the first few weeks of life. Owing to the lack of apparent changes in other organ systems, we speculate that the reduced viability may be attributed in part to ineffective ingestive behavior, which is secondary to deficits in the sensory nervous system. As a detailed histological analysis of nonneuronal tissues from the NGF mutant mice has not been conducted, we cannot rule out the possibility that pathological changes outside the nervous system contribute to the lack of viability.
Mice that are (+/-) for NGF gene disruption display a mild phenotype. These animals grow normally and cannot be distinguished from wild-type mice upon observation of their appearance or spontaneous behavior. Observations of the CGRP-immunoreactive processes in the skin or spinal cord gave the impression of a reduction in staining intensity, and blind observers were able to identify slides correctly as (+/-) versus (+/+) with approximately 80% accuracy. By examining responsiveness to heat-induced pain in a relatively large number of mice, the (+/-) animals were found to display a slight but significant reduction in 1 of 2 tests. These observations both confirm that the (+/-) mice show a subtle but statistically demonstrable phenotype and suggest that they may provide a useful model for studying the effects of partial NGF deprivation in healthy adult mice.
Our observations on the consequences of NGF gene inactivation indicate that NGF exerts a selective influence on developing sympathetic and sensory neurons and demonstrate that these actions of NGF are not compensated for by the actions of other neurotrophins. These findings underscore the critical dependence of some neurons on trophic influences during development and indicate that, in at least some cases, there is no redundancy in the actions of homologous trophic factors. In contrast with the dramatic effects of NGF on the survival of sensory and sympathetic neurons in the perinatal period, many basal forebrain cholinergic neurons survive the first weeks of postnatal life in the absence of NGF. Whether these cells are supported by the actions of other trophic factors during early postnatal life and whether they gain NGF dependence at a later age await further study.
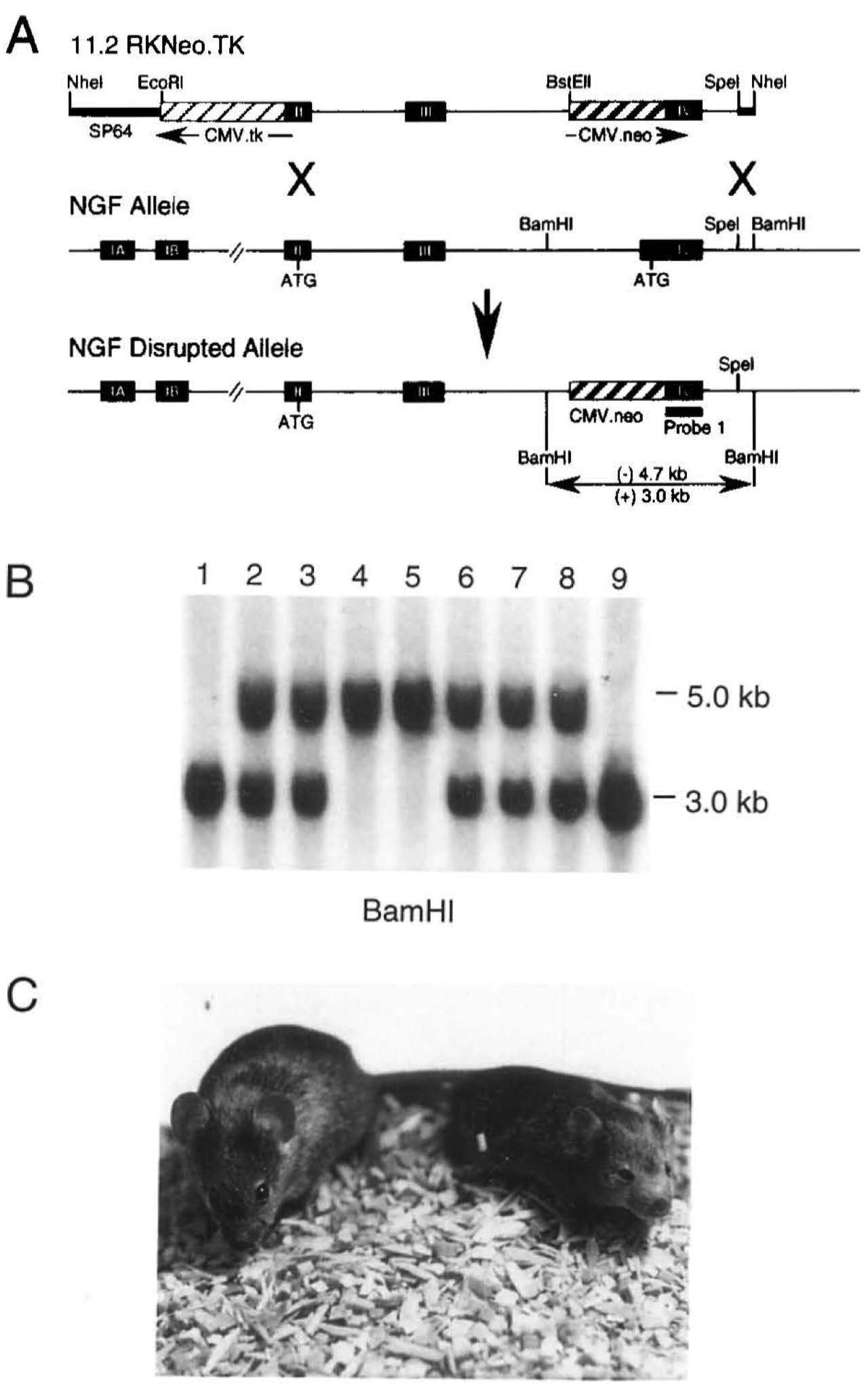
Figure 1. Targeted Disruption of the NGF Gene in Mice
(A) Targeting vector p11 .2RKNeo.TK, the NGFwlld-type allele, and the NGF disrupted allele after homologous recombination. Arrows indicate the direction of transcription relative to NGF transcription. The expression cassettes for CMV-Neo and CMV-TK are Indicated by hatched boxes, and NGF exons are indicated by closed boxes. In the disrupted allele, the replacement of 150 bp of NGF exon IV with 1.9 kb of CMVNeo results in a BamHI fragment that is 4.7 kb versus 3.0 kb from the wild-type allele.
(B) Genotype of an F2 litter analyzed by Southam blot analysis using a probe derived from exon IV. Lanes 1 and 9 are homozygous (+/+) for the wild-type allele. Lanes 2, 3, 6, 7, and 8 are heterozygotes (+/-) and lanes 4 and 5 represent animals homozygous for the NGF gene disruption.
(C) Wild-type (left) and homozygous mutant (right) NGF-knockout mice at 23 days of age. The mutant animal Is smaller than its llttermate and displays marked ptosis (drooping of the upper eyelid).
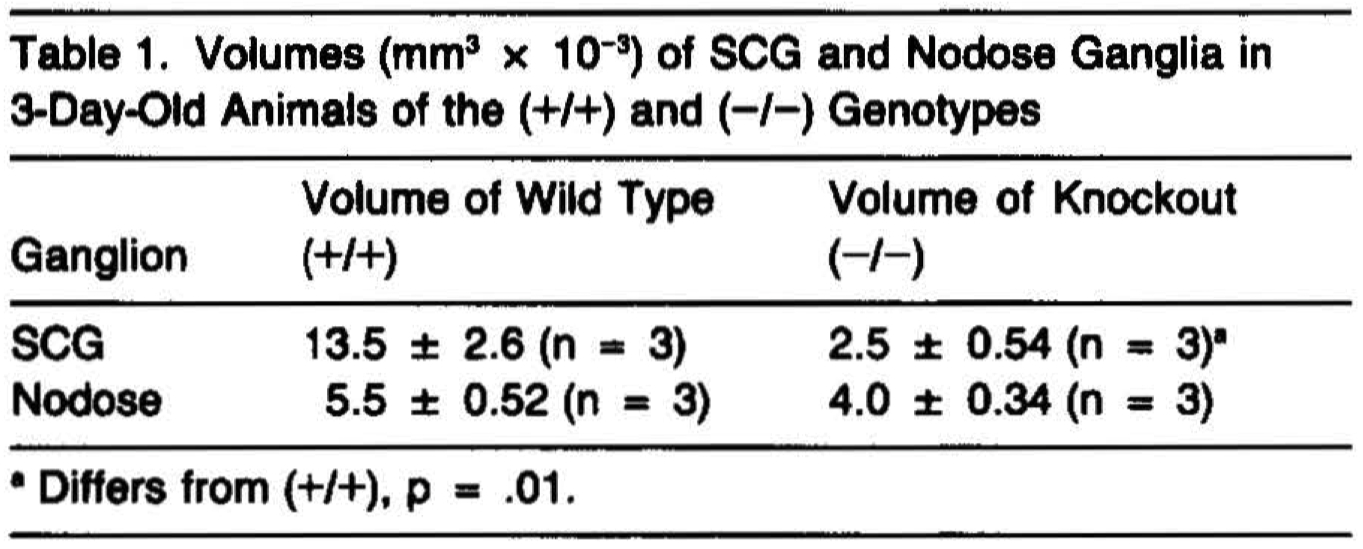
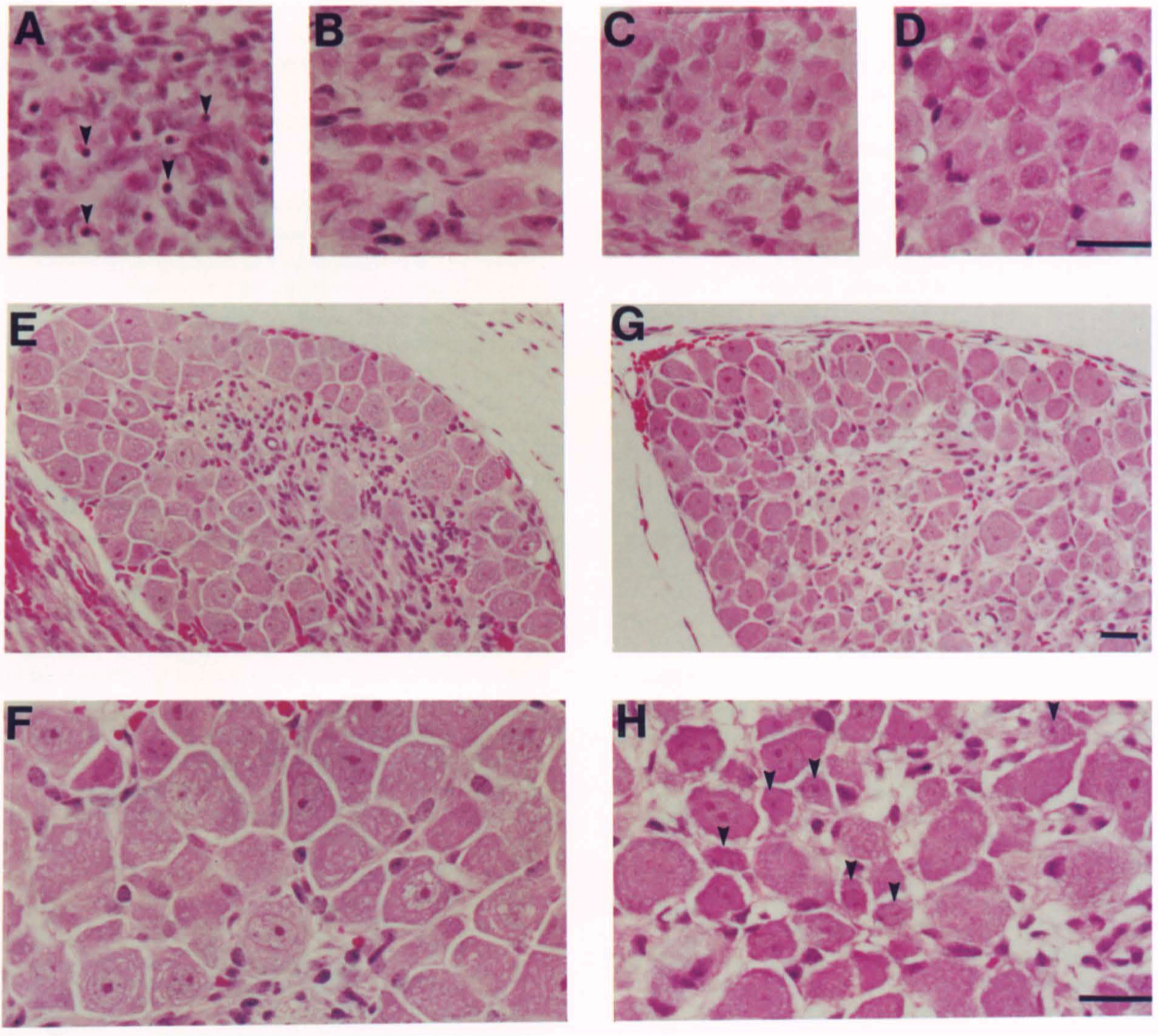
Figure 2. Histological Appearance of Sensory and Sympathetic Ganglia
SCG (A and B), Nodose Ganglion (C and 0), and Fifth Lumbar DAG (E-H) of 3-Day-Old Mice of Wild-Type (B, D, G, and H) and Homozygous Mutant (A, C, E, and F) Genotype. The SCG of the mutant mouse shows numerous pyknotic nuclei (arrowheads In A) Indicative of cell death. DAG of the wild-type animal show numerous small neurons (arrowheads In [HD, while that of the mutant displays no small cells. Scale bars • 50 µm for panels A-0, F, and H, while E and Gare at half power.
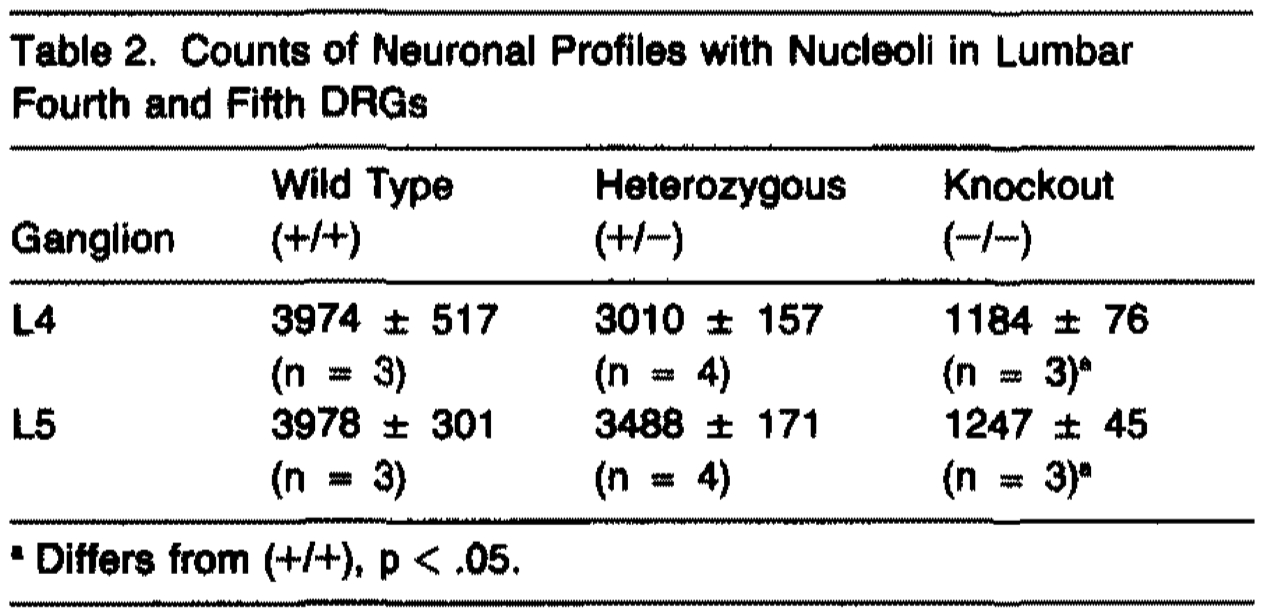
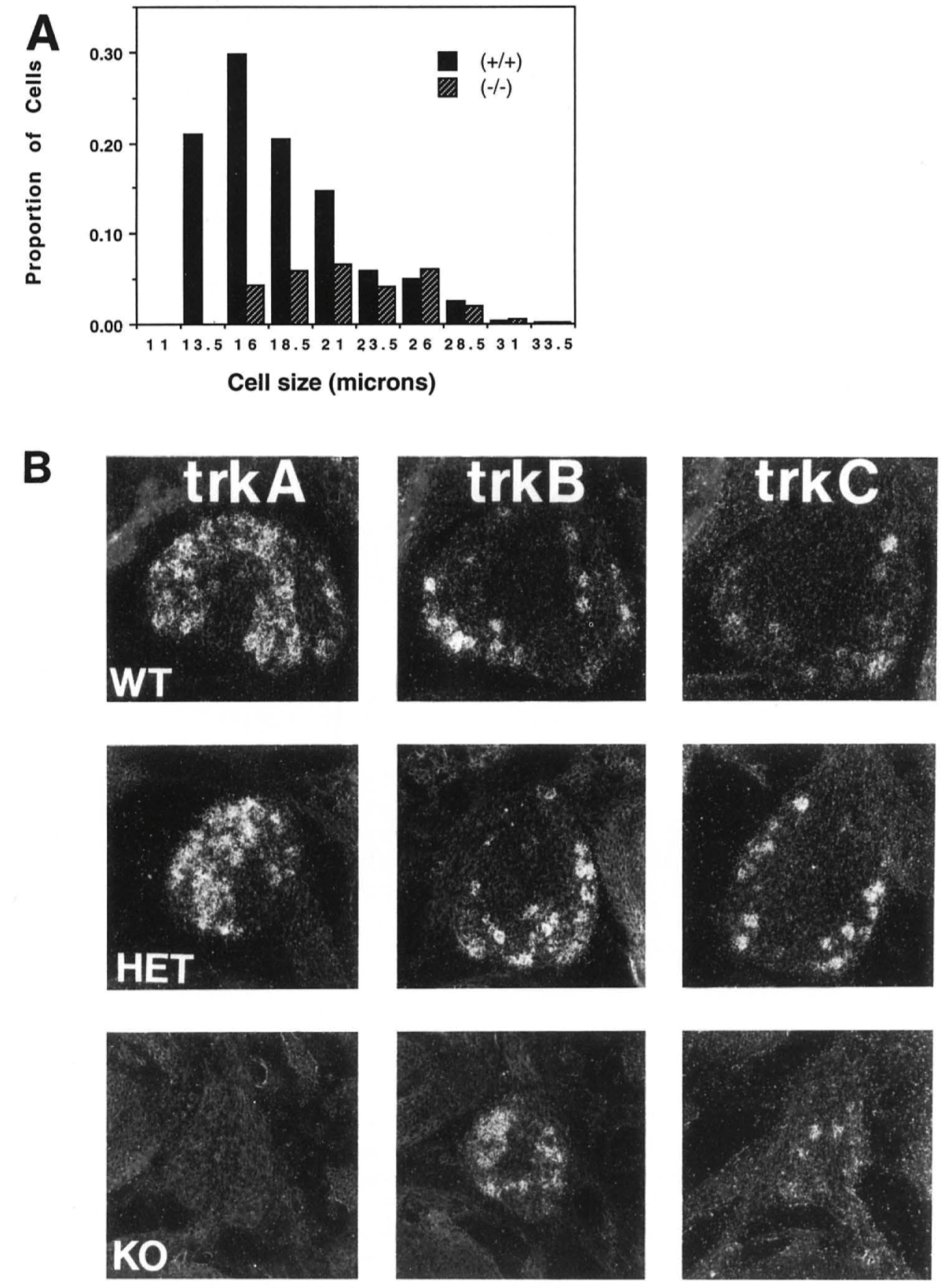
Figure 3. NGF Disruption Affects a Subpopulation of DRG Neurons
(A) Cell size histograms from lumbar DRG of (+/+) or (-/-) mice. The number of cells falling Into each size category is expressed as a percentage of total cells present in the wild type ganglia.
(B) In situ hybridization of cervical DRG for neurotrophin receptors. Ganglia from (+/+) or (+/-) mice contain cells expressing all of the known tries. DRG neurons remaining In (-/-) mice do not express NGF receptor (trlrA), but do express receptors for other members of the neurotrophin family (trkB and trl<C).
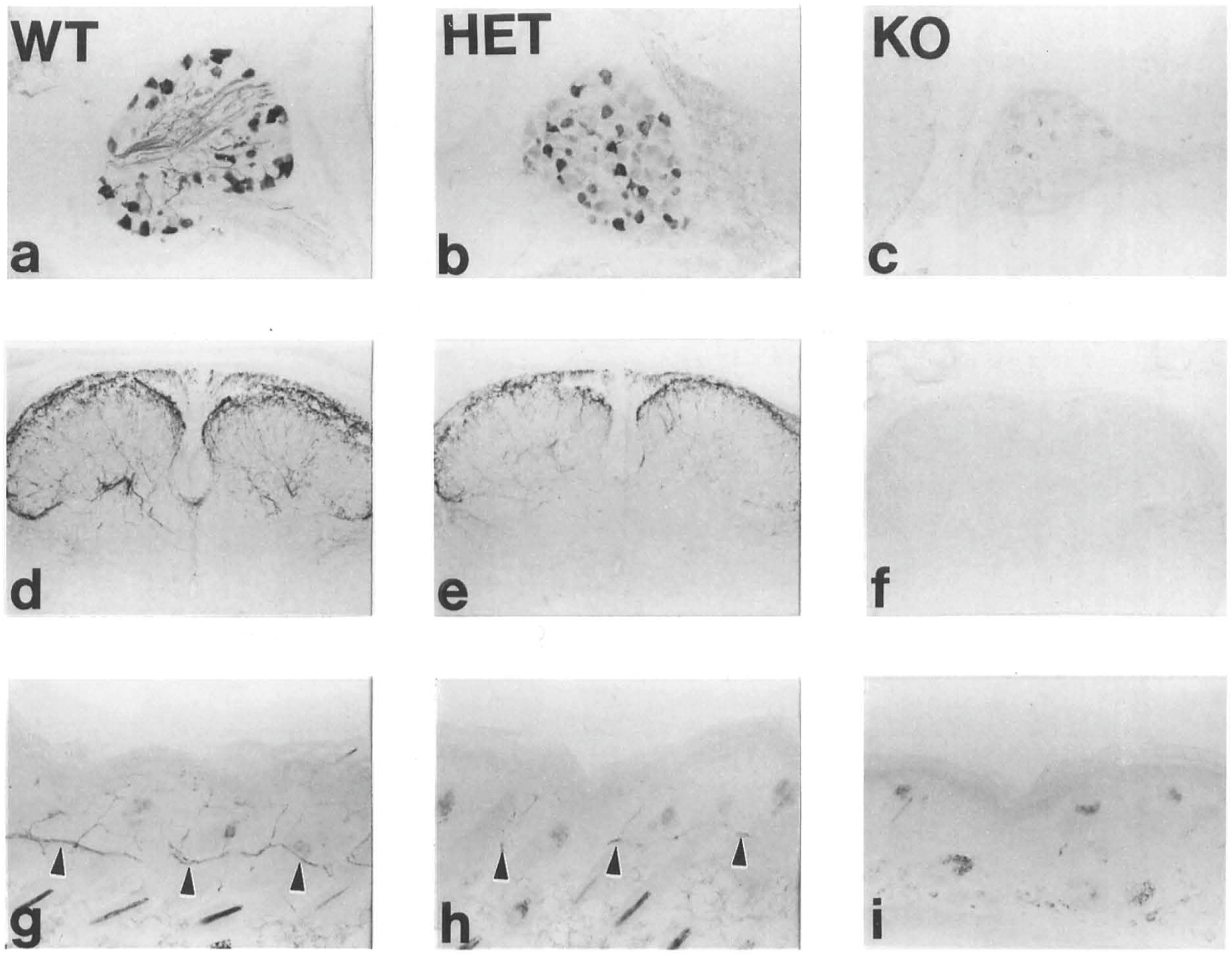
Figure 4. NGF Disruption Markedly Affects CGRP•lmmunoreactive Sensory Neurons
CGRP immunoreactivity in DAG neurons of 3-day-old mice of(+/+) (a, d, and g), (+/-) (b, e, and h), or(-/-) (c, I, and i) genotype. The(-/-) animals show a complete absence of CGRP-immunoreactive processes in the dorsal horn of the spinal cord (f) or the skin (i) as compared with similar regions from (+/+) animals (d and g). The number and intensity of immunoreactive fibers in both dorsal horn and skin of the (+/-) animals showed a moderate reduction.
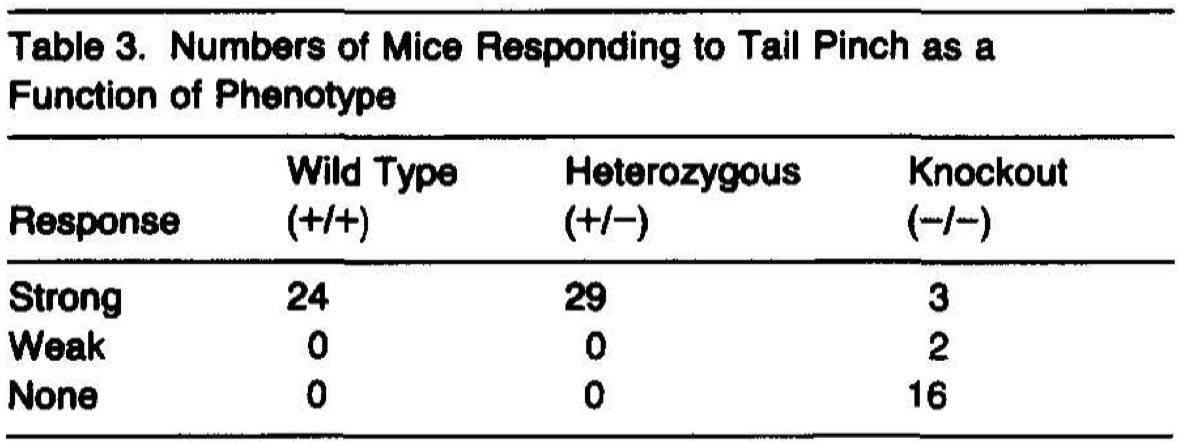
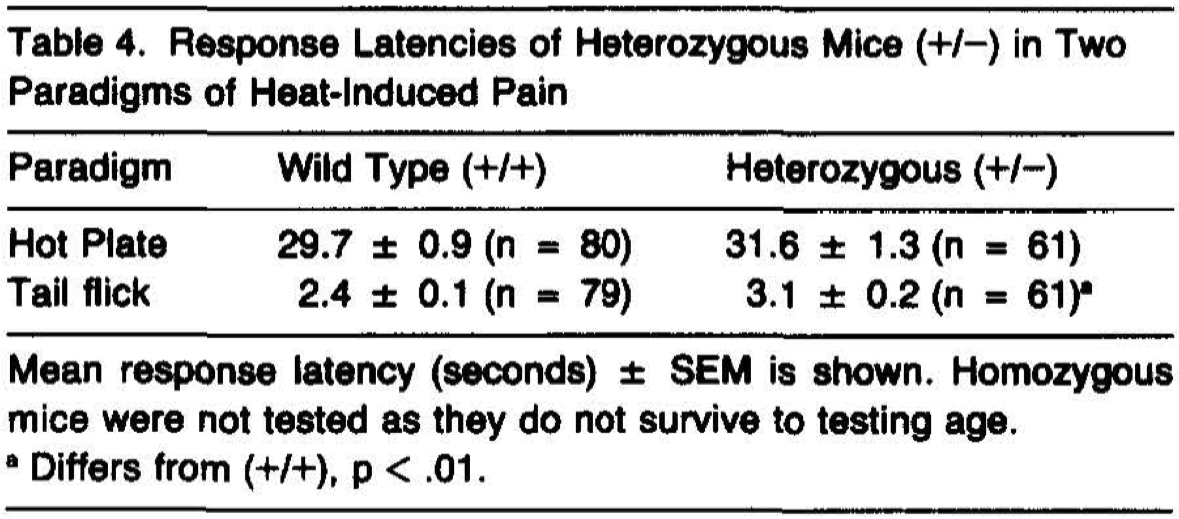
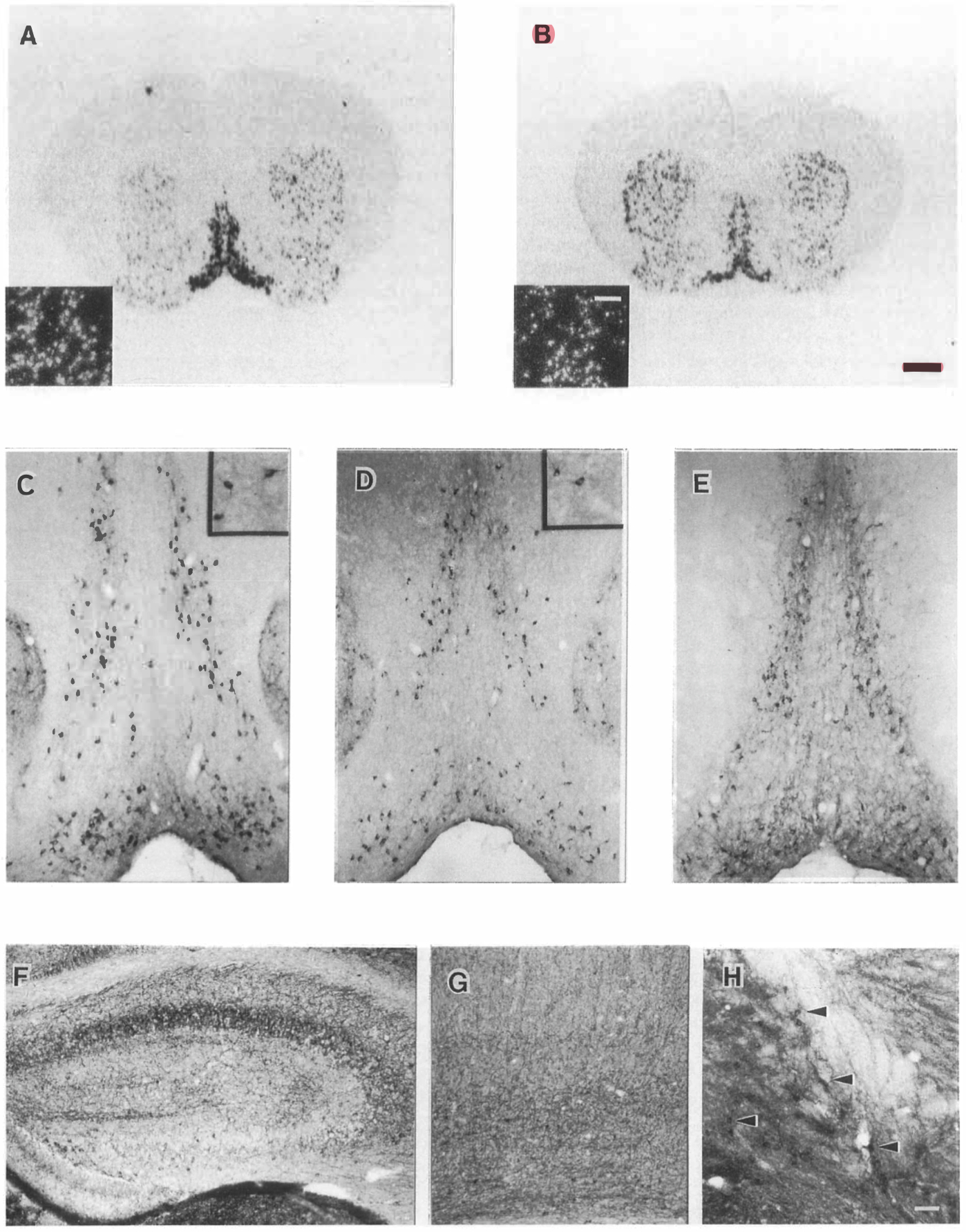
Figure 5. Basal Forebraln Chollnerglc System In (-/-) Mice
In situ hybridization for trkA in 14-day-old (+/+)(A) and (-/-) (B) mice show a similar pattern in the septal-<liagonal band region and striatum. (A) and (B) are the same magnification (scale bar, 1 mm). Insets show dark field emulsion autoradlography of septal-<liagonal band neurons(scale bar, 200 µm). ChAT-immunoreactive neurons are apparent in medial septum of 28-day-old (+/+)(C) and (-/-)(D) mice, as well as in the striatum of mice of both genotypes(Insets are at 2x magnification). p75 lmmunostalnlng is also apparent in medial septal neurons of a 28-day-old (-/-) mouse(E). Acetylchollnesterase staining is apparent In a 28-day-old(-/-) mouse In both fibers within target areas of the basal forebraln chollnerglc system, including hippocampus (F) and cortex (G) as well as in perikarya(arrowheads) of cholinergic nuclei such as nucleus basalis(H). Magnification for (C-H) Is denoted by the bar, representing 200 µm, in (H).