Slit Is the Midline Repellent for the Robo Receptor in Drosophila
Summary
Previous studies suggested that Roundabout (Robo) is a repulsive guidance receptor on growth cones that binds to an unknown midline ligand.
Here we present genetic evidence that Slit is the midline Robo ligand; a companion paper presents biochemical evidence that Slit binds Robo.
Slit is a large extracellular matrix protein expressed by midline glia.
In slit mutants, growth cones enter the midline but never leave it; they abnormally continue to express high levels of Robo while at the midline. slit and robo display dosage-sensitive genetic interactions, indicating that they function in the same pathway. slit is also required for migration of muscle precursors away from the midline.
Slit appears to function as a short-range repellent controlling axon crossing of the midline and as a long-range chemorepellent controlling mesoderm migration away from the midline.
Introduction
The roundabout (robo) and commissureless (comm) genes in Drosophila were identified in a large-scale mutant screen for genes that control the decision by axons to cross or not to cross the CNS midline.
In robo mutant embryos, too many axons cross and recross the midline. robo encodes an axon guidance receptor of the immunoglobulin superfamily that is highly conserved in fruit flies, nematodes, and mammals.
For those axons that never cross the midline, Robo is expressed at high levels on their growth cones from the outset.
For the majority of commissural axons that do cross the midline (but only once), Robo is expressed at high levels on their growth cones after they cross the midline.
Transgenic rescue experiments reveal that Robo can function cell autonomously, further supporting the hypothesis that Robo is a growth cone guidance receptor. comm mutant embryos display the opposite phenotype in that no axons cross the midline.
Comm is a novel transmembrane protein.
The robo; comm double-mutant phenotype is identical to robo alone, suggesting that in the absence of Robo, Comm is no longer required to allow axons to cross.
Overexpression of Comm (i.e., the comm gain of function) leads to a phenotype nearly identical to the robo loss of function.
Taken together, these results suggest that Comm regulates Robo function by either controlling Robo levels or Robo signaling.
Further analysis revealed that Comm controls Robo expression; increasing Comm leads to a reduction of Robo protein.
These studies led to the model that Robo is a repulsive guidance receptor for an unknown midline ligand and that Comm downregulates the levels of the Robo receptor on commissural axons.
We argued that this midline repellent is likely to function in a short-range fashion, since growth cones that express high levels of Robo do not necessarily extend away from the midline, but rather extend longitudinally close to the midline.
One candidate ligand might be one of the two Drosophila Netrins that are expressed by midline glial cells.
However, our unpublished genetic analysis led us to believe that the Netrins are not Robo ligands.
What, then, is the midline Robo ligand?
We were also interested in answering a more general question.
If the midline, with its expression of Netrins, is such an attractive place, with mirror-symmetric commissural axons from both sides extending toward and entering the midline, why do growth cones ever leave the midline?
Why don’t these growth cones fasciculate with their contralateral homolog and extend longitudinally along the midline?
In a robo mutant, axons freely cross and recross the midline, but they do not stay at the midline.
However, when the midline cells are genetically deleted in single-minded (sim) mutants, all axons converge on the midline and do not leave it, forming a single large fused longitudinal tract at the midline.
This phenotype suggests that the midline cells normally express two repellent activities, one that controls crossing (and prevents recrossing) of the midline, and another that assures that axons do not stay at the midline.
One possibility is that the same molecule might serve both functions.
We hoped that the identification of the Robo ligand might shed some light on this issue.
In principle, if there is a one-to-one relationship between the Robo ligand and the Robo receptor, then we might expect the gene encoding the ligand to have the same mutant phenotype as robo.
But in our large-scale mutant screen, we screened most if not all of the genome, and although we recovered eight independent alleles of robo, we found no other gene whose mutant phenotype is identical to robo.
The likely explanation is that either there are two ligands for the one Robo receptor (in which case each ligand might have a weaker mutant phenotype than robo), or alternatively, there is one ligand but two receptors (in which case the ligand might be expected to have a stronger mutant phenotype than robo).
We already knew that there was a second Robo receptor expressed in the developing CNS , making the second alternative seem more likely.
In this paper we present genetic evidence that Slit is a Robo ligand.
Slit is a large extracellular matrix protein expressed by midline glia.
In robo mutant embryos, growth cones that normally do not cross the midline now do so.
In slit mutant embryos, these same growth cones enter the midline but never leave it.
Moreover, they continue to express high levels of Robo even while extending along the midline. slit and robo display dosage-sensitive genetic interactions, indicating that they are likely to function in the same pathway. slit is also required for migration of muscle precursors away from the midline.
Slit appears to function as a short-range repellent, controlling axon crossing of the midline.
However, the muscle phenotype suggests that Slit also functions as a long-range chemorepellent, controlling mesoderm migration away from the midline.
In a companion paper , we and our colleagues show direct binding between Slit and Robo in Drosophila and then go on to present data on the sequence, Robo binding, expression, and function of three mammalian Slits.
Results
High-Level Overexpression of Robo and Comm Generates Opposite Phenotypes
We previously reported that panneural transgenic overexpression of robo does not give a mutant phenotype due to posttranslational regulation of Robo protein.
However, we find that if the copy number of the UAS-robo transgene is increased, a robo gain-of-function phenotype is generated that is nearly identical to the comm loss-of-function phenotype in which axons do not cross the midline.
This result further confirms the proposed role of Robo as a repulsive receptor that prevents axons from crossing the CNS midline (Figure 1F).
We previously reported that overexpression of comm produces the complementary robo-like phenotype in which axons freely cross and recross the midline ( Figure 1G ).
This phenotype appears to be generated by Comm’s ability to negatively regulate Robo protein levels; increasing levels of Comm lead to decreasing levels of Robo.
If the copy number of the comm transgene is increased, a more severe phenotype results (Figure 1H).
The strongest comm gain-of-function phenotype has axons entering the midline but not leaving it, leading to a collapse of the CNS axon scaffold onto the midline (Figure 1I).
Even in the most extreme comm gain-of-function phenotypes, the midline cells are still present as assayed by a monoclonal antibody against Wrapper, a protein expressed specifically by midline glia.
The strongest comm gain-of-function phenotype is highly reminiscent of the slit loss-of-function phenotype The similarity between the comm gain-of-function phenotype and the slit loss-of-function phenotype led us to evaluate Slit as a candidate ligand for Robo.
Axon Guidance Defects in slit Mutant Embryos
slit mutations were first isolated in a screen for mutations affecting the pattern of the larval cuticle and were found to have defects in CNS formation and head involution.
Null alleles of slit show a characteristic collapse of the CNS axon scaffold (Figure 1D). We find that hypomorphic slit alleles show a less complete midline collapse of the CNS axon scaffold, with the CNS in some segments resembling a robo mutant (Figure 1C). The striking phenotype of slit mutant embryos is similar to sim mutants.
When the slit mutant was first characterized, the limited availability of markers for midline cells led to some uncertainty as to whether Slit might also control midline cell fate and differentiation (as does sim), raising concern as to whether the slit mutant axon phenotype was a primary or secondary consequence of the absence of Slit protein.
However, a more recent study showed that midline cell fate appears normal in slit mutants, suggesting that Slit might indeed directly control axon guidance.
This observation, coupled with the finding that the strongest comm gain-of-function phenotype resembles the slit loss-of-function phenotype, led us to examine slit mutant embryos for axon guidance defects.
We first examined the slit midline glia mutant phenotype (using anti-Sim and anti-Wrapper;).
Examination of the midline glia at stage 12 and early stage 13 reveals that their initial organization is essentially wild type (data not shown).
However, with time, as the axon scaffold collapses on the midline (see below), the glia become displaced and disorganized.
This later displacement of the midline glia appears to be a secondary phenotype.
We next analyzed the slit mutant axon phenotype using the 1D4 mAb (anti-Fasciclin [Fas] II) that at stage 13 stains a subset of growth cones (including aCC, pCC, vMP2, MP1, dMP2) and from stages 14–17 stains three major longitudinal axon tracts, including (from medial to lateral) the pCC pathway (pioneered by the pCC growth cone), the MP1 pathway (pioneered by the MP1 growth cone), and a third lateral pathway.
Previous analysis of the robo mutant with mAb 1D4 showed that the pCC growth cone, which normally projects anteriorly on its own side near the midline, projects across the midline, fasciculating with its contralateral homolog at the midline.
As a result, the pCC pathway, which normally projects longitudinally on its own side near the midline, projects back and forth across the midline in robo mutant embryos.
In slit mutant embryos, the pCC axon also aberrantly extends toward the midline (Figure 2A). However, unlike in robo, in slit mutant embryos the pCC axon does not leave the midline, and instead the axons of the two contralateral homologs fasciculate and extend anteriorly along the midline (Figure 2B). This phenotype becomes increasingly apparent for all CNS axons in older embryos (Figure 2D). In some segments in slit mutant embryos, the axon of the aCC motoneuron, which normally extends ipsilaterally away from the midline (robo mutants included), instead now crosses the midline and fasciculates with the axon of its contralateral homolog (Figure 2C). Interestingly, this axon behavior looks very similar to the wild-type behavior of the axons from the RP motoneurons whose cell bodies lie equally close to the midline but whose axons normally extend across the midline and fasciculate with their contralateral homologs before extending toward a nerve root and exiting the CNS.
Commissural axons such as SP1 are also unable to leave the midline (as visualized with anti-Connectin antibody; data not shown).
In addition, some neuronal cell bodies appear to be closer to the midline than in wild-type embryos, suggesting that slit has a role in controlling cell migrations as well as axon guidance.
The behavior of axons in the slit mutant and other genotypes is summarized in Figure 3.
Genetic Interactions between robo and slit
The axon guidance defects in slit mutant embryos are initially similar to those observed in robo mutants in that axons freely extend toward and enter the midline.
However, with time the slit phenotype becomes more severe because axons do not leave the midline.
The slit guidance phenotypes are consistent with the hypothesis that Slit is the Robo ligand.
To further test this model, we looked for genetic interactions between these two genes.
Dosage-sensitive genetic interactions between two loci are a good indicator that the two gene products are functionally related.
We examined the CNS of embryos transheterozygous for slit and robo, that is, carrying one mutant and one wild-type copy of each gene.
We examined whether the Fas II (i.e., staining with the 1D4 mAb) positive fascicles abnormally crossed the midline (particularly the most medial pCC pathway).
In either slit or robo heterozygotes, we observed few guidance defects in these pathways (Table 1; Figure 2F). However, depending upon the combination of alleles used, 26%–39% of the segments examined in embryos transheterozygous for slit and robo had Fas II–positive axons inappropriately crossing the midline (Table 1; Figure 2E). Such a dosage-dependent, transheterozygous phenotype is a strong indication that Slit and Robo function in the same pathway.
Table 1Transheterozygous Interactions of robo and slit
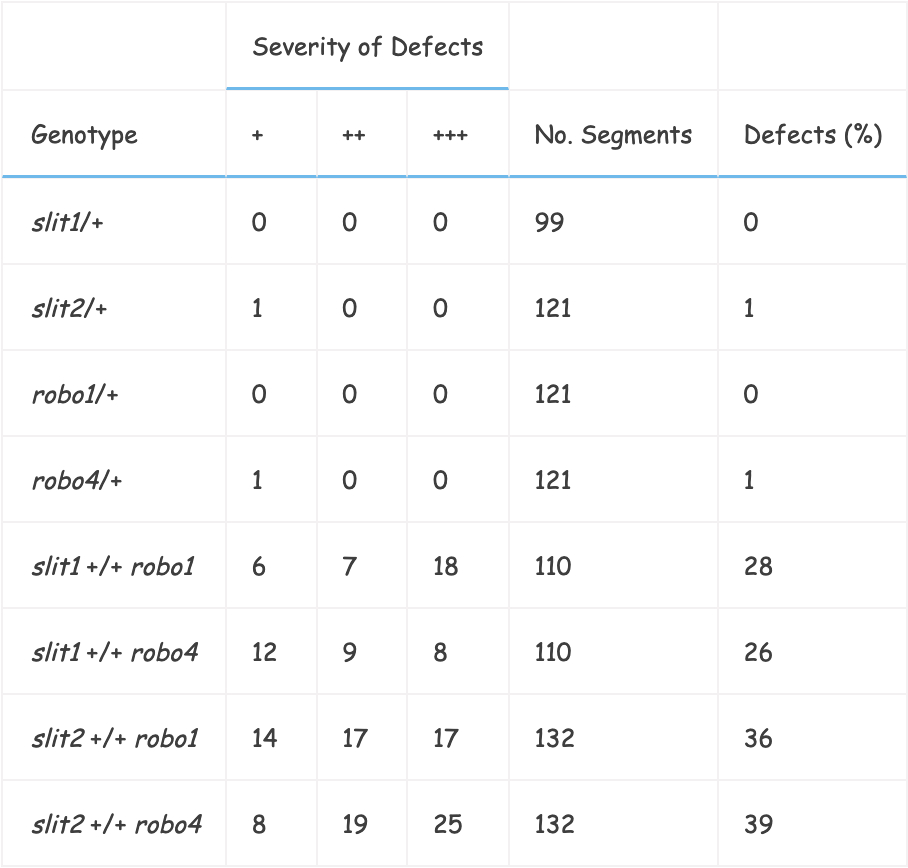
Stage 16/17 embryos in which the six Fas II–positive longitudinal fascicles (three on each side) were scored for axons crossing the midline.
Defects were subdivided according to whether the group of axons crossing the midline were less thick (+), the same size (++), or of greater thickness (+++) than a wild-type Fas II–positive fascicle.
The abdominal and thoracic segments were scored.
We also generated chromosomes doubly mutant for slit and robo.
The genetic distance between the two loci predicted recovery of the double mutant chromosomes at a frequency of 1 in 8; when null alleles of both slit and robo were used instead, the recovery rate was 1 in 35, indicating that removal of one copy of each locus decreases viability.
In a late stage wild-type embryo, the cell bodies of the RP neurons are readily visible between the two commissures (Figure 1E). In robo mutants, typically one or both RP cell bodies are obscured by the increased number of axons abnormally crossing in the commissures.
However, the longitudinal part of the scaffold always remains outside (lateral) of the RP cell bodies.
In slit mutants, this is not the case (Figure 1C and Figure 1D). We tested the effect of removing one copy of slit on the robo phenotype.
When the spacing of the longitudinal axons was examined, slit was found to dominantly enhance the robo phenotype, as judged by the presence of segments displaying greater medial constrictions than are ever seen in robo mutants alone (Figure 4B). In some instances, an RP cell body can be seen lateral to the axon scaffold.
If Slit is the Robo ligand, then the double robo slit mutant phenotype would be predicted to resemble that of a slit mutant alone (due to slit having the more severe phenotype).
Embryos homozygous for a recombinant chromosome carrying null alleles of both slit and robo were found to resemble the slit null phenotype (Figure 4C).
Robo and Slit Expression
The commissureless phenotype produced by high-level overexpression of Robo (Figure 1F) suggests that Robo responds to a repulsive cue at the CNS midline.
Slit is a large extracellular matrix protein secreted by the midline glia (Figure 5A).
Slit was reported to be transferred to axons (albeit at a low level).
The mAb we are using displays only a very low level of axon staining, making an analysis of putative transfer in robo mutant embryos inconclusive.
Robo is primarily localized to growth cones of the longitudinal portion of the axon scaffold (Figure 5B).
These expression patterns are consistent with Slit being the repulsive ligand for Robo because Robo-positive axons avoid areas of high Slit expression.
We stained slit embryos with anti-Robo mAb 13C9 and found that Robo-positive growth cones are now present at the midline (Figure 5C). Staining of the mature CNS in slit mutants reveals that Robo protein levels are unaffected (unlike in comm gain-of-function embryos), and thus Robo is expressed at high levels along the midline (Figure 5D). In wild-type embryos, Slit and Robo both localize to the muscle attachment sites in complementary dorsoventral gradients, further suggesting the possibility of a functional relationship (data not shown).
Cloning of a Full-Length Open Reading Frame slit cDNA
We isolated a slit cDNA encoding the complete open reading frame (ORF) from the LD 0–22 hr embryonic library (EST Project, G.
Rubin lab).
We sequenced the ORF and identified an additional leucine-rich repeat (LRR) that is absent from the cDNA previously published (16).
This additional LRR is between the second and third repeats in the first set of tandem LRR arrays.
This LRR is present in vertebrate homologs of slit (3).
In addition to the extra LRR, we identified eight amino acid differences.
All of the substitutions are in LRR regions, but none occur in highly conserved residues of the motifs.
Ectopic Expression of Slit
We used the GAL4-UAS system to misexpress slit in several tissues (2).
We initially showed that expression of slit at the midline can rescue a slit phenotype.
Using the slit promoter as the GAL4 driver in a homozygous slit mutant background, we achieved partial rescue of the CNS axon phenotype (Figure 4D) in which the commissures and longitudinal tracts are all present and separated from each other.
The typical delay of 1.5 hr in expression introduced by using the GAL4 system is probably responsible for the incomplete rescue.
Next we examined the effect of high-level overexpression of slit in all postmitotic neurons (elav promoter).
The resulting phenotype resembles the robo loss-of-function phenotype (Figure 6A). However, when individual axon fascicles are examined, we observe that the slit overexpression phenotype is stronger than the robo loss-of-function phenotype.
Staining with the 1D4 mAb shows that in addition to aberrant midline crossing by axons in the innermost pCC pathway as seen in robo mutants, the medial and lateral pathways are also disrupted, sometimes crossing the midline.
The same results were obtained using a different panneural promoter (scabrous).
These results suggest that when Slit is panneurally expressed throughout the CNS, growth cones are impaired in their ability to respond to Slit at the midline.
A similar effect is seen when Netrins are expressed panneurally in that the panneural overexpression phenotype resembles the loss-of-function phenotype.
In both cases (Slit and Netrins), these results support the notion that the localized distribution of the guidance signal is of crucial importance and that approximating an even distribution throughout the CNS is equivalent to no expression at all.
We overexpressed slit at the CNS midline using two different promoters (slit and sim) but did not observe a consistent phenotype.
We suspect that this is due to the commissural axons being highly efficient at downregulating Slit receptors on their surface to allow midline crossing.
In addition, we have preliminary evidence suggesting that levels of Slit protein at the midline are tightly regulated.
Finally, we expressed Slit on muscles (using 24B-GAL4) and examined the guidance and connectivity of motor axons.
The ISNb motor axons normally innervate muscles.
When their muscle targets abnormally express Slit, their innervation is greatly perturbed.
Most of these motor growth cones stall in the vicinity of these muscles and fail to innervate them (61%, n = 106; Figure 6B). This lack of innervation is reminiscent of what is observed when the chemorepellent Semaphorin II is ectopically expressed by the same muscles.
We examined the morphology of muscles ectopically expressing Slit with mAb FMM5 (anti-muscle myosin) and found them to be normal in attachment sites, size, and position relative to each other and to the epidermis (n = 110).
The motor axon phenotype was not suppressed by removal of robo activity, providing further evidence that there is more than one Slit receptor.
Robo2 is a potential candidate for mediating the motor axon response to ectopic expression of Slit.
Slit Is Required for Correct Muscle Migration and Patterning Near the Midline
After gastrulation in Drosophila, many myoblasts migrate laterally at least five to six cell body diameters away from the ventral midline.
This migration occurs over the dorsal surface of the neuroepithelium.
Later, some ventral body wall muscles extend back toward the midline ventrally under the developing CNS, normally attaching to the epidermis underneath the CNS at some distance from the midline (Figure 7A). In contrast, in slit mutant embryos many developing muscles are found near and at the midline, stretching across the midline dorsally over the CNS (Figure 7C). This defect is not seen in robo embryos, although very rarely a single muscle can be seen extending inappropriately dorsally across the CNS (Figure 7B), suggesting that Robo participates in this process in conjunction with at least one other receptor (possibly Robo2).
However, in robo mutant embryos the ventral muscles are frequently found attached closer to the midline than in wild type, suggesting that Robo may in part prevent muscles from extending too close to the midline.
When slit mutant embryos are rescued by slit-GAL4 driving UAS-slit, the ventral muscle pattern is restored to near wild type, confirming that Slit expression at the midline is required for migration of muscle precursors away from the midline.
Discussion
We previously reported that Robo appears to function as a repulsive axon guidance receptor on growth cones that responds to a putative midline ligand
In the present paper , we present genetic evidence that suggests that Slit is the midline Robo ligand ( summarized in Figure 3 )
In a companion paper , we and our colleagues present biochemical evidence showing that Slit binds to Robo. In the original large-scale mutant screen for genes controlling midline axon guidance , 8 alleles were recovered of robo , 2 alleles of comm , and 13 alleles of slit
At the time , because slit had such a similar axon phenotype to sim , which controls midline cell fate and survival , and because of the lack of good midline markers , there was some uncertainty as to whether slit also controlled midline cell fate and survival
As a result , we focused our initial attention on robo and comm , two genes that clearly control midline axon guidance
Nevertheless , there was always the lingering possibility that Slit might directly control axon guidance: Slit is a large extracellular matrix protein expressed almost exclusively by midline cells , some Slit protein is found on axons , and the slit mutant displays a striking axon pathway phenotype
With the advent of better markers for midline cells , 19 showed that midline cell fate and differentiation are relatively normal in slit mutant embryos , thus suggesting that Slit might indeed control axon guidance. The key result that led us to the insight that Slit is likely to be the Robo ligand came from a further analysis of Comm
We previously reported that overexpression of Comm produces a robo-like phenotype in which axons freely cross and recross the midline
In the present paper , we report that if the copy number of the comm transgene is increased , a more severe phenotype results in which axons enter the midline but fail to leave it , leading to a midline collapse of the CNS axon scaffold
The strongest comm gain-of-function phenotype is highly reminiscent of the slit loss-of-function phenotype and led us to evaluate Slit as a candidate Robo ligand. The genetic analysis presented here provides strong support for the notion that Slit is the midline Robo ligand
One way to test the hypothesis that two proteins directly interact in a ligand–receptor fashion is to test for dominant genetic interactions between the genes encoding them
In most cases , reducing gene dosage by one copy ( thus reducing protein by 50% ) has little phenotypic effect
However , simultaneously reducing the dose of two genes whose protein products function together may sufficiently impair their combined function such that phenotypes appear
Such a “transheterozygous” phenotype has been demonstrated for various ligand–receptor pairs in Drosophila , including Delta and Notch , and more recently , in the field of axon guidance , the transmembrane Semaphorins and their Plexin A receptor. In either slit or robo heterozygous mutants alone , we observed few midline guidance defects
However , depending upon the combination of alleles used , in embryos that are transheterozygous for both slit and robo ( i.e. , carrying one mutant and one wild-type copy of each gene ) , 26%–39% of segments have midline axon guidance defects
This transheterozygous genetic interaction is a good indicator that Slit and Robo function in a common pathway. Transgenic experiments reveal that Robo can function in a cell-autonomous fashion consistent with the role of a receptor , whereas ectopic expression experiments reported here show that Slit can function in a non–cell autonomous fashion consistent with the role of a ligand ( e.g. , muscle expression repelling motor axons )
Taken together with the transheterozygous genetic interaction , these data strongly suggest that Slit is the midline ligand for the Robo receptor in Drosophila
In a companion paper , we and our colleagues present biochemical data supporting the same conclusion: Slit-AP binds COS cells expressing Robo , AP-Robo ectodomain binds cells expressing Slit , and AP-Robo binds Slit attached to protein A–sepharose beads.
Slit Must Have More Than One Receptor
Given the conclusion that Slit is the Robo ligand , the fact that the slit mutant phenotype is stronger than the robo phenotype suggests that Slit must have more than one receptor controlling midline guidance in Drosophila
In robo mutants , axons freely cross and recross the midline , while in slit mutants they enter the midline but do not leave it
Clearly , in the absence of Robo some other growth cone receptor must respond to Slit and assure that growth cones do not linger at the midline , even though it still allows them to cross the midline
A good candidate for a second Slit receptor is Robo2 , a closely related receptor that is also expressed by developing CNS neurons
Since the comm single-dose gain of function generates a robo-like phenotype by downregulating Robo protein , and since the comm double-dose gain of function generates a slit-like phenotype , it follows that Comm is likely to also control the level of expression of Robo2 or whatever other Slit receptor controls midline guidance. This model leads to two clear predictions , both of which should be relatively straightforward to test in the future
First , we would predict that the double mutant combination of robo and robo2 should generate a phenotype that resembles slit
Second , we would predict that Comm also regulates Robo2
Preliminary evidence shows that overexpression of Robo2 can produce a comm-like phenotype )
Moreover , when expressed in tissue culture , Robo2 binds Slit
Both of these results lend support to this model.
Slit Can Function as Both a Short-Range and Long-Range Repellent
The primary location of Slit expression in Drosophila is at the ventral midline of the developing CNS. Midline Slit expression begins during gastrulation.
Slit is also expressed at certain attachment sites of muscle to epidermis and by cardioblasts of the dorsal tube.
Slit is a large extracellular matrix protein, and consistent with its size and location, most Slit protein is detected immediately adjacent to the midline glia that make it.
However, in the more mature embryonic CNS in Drosophila, some Slit protein (as detected with antibodies against a carboxy-terminal fragment) is observed at a distance from the midline, and in particular associated with the surface of axons.
Immunoelectron microscopy confirmed this localization of Slit protein on lateral axons even though there is no evidence that any cells in the CNS other than midline glia make and secrete Slit.
On the one hand, the companion paper shows that Slit binds to Laminin, which might retain it in the extracellular matrix and help assure its localization as a short-range signal.
On the other hand, that paper also shows that Slit is proteolytically processed, raising the question of whether certain fragments of Slit can diffuse for a longer distance than the whole protein.
Furthermore, a third companion paper shows that the N-terminal proteolytic fragment of Slit2 in mammals can have a different long-range function as a positive regulator of sensory axon growth and branching.
These data suggest that Slits are likely to be multifunctional guidance molecules.
The axon guidance defects seen in robo mutant embryos in Drosophila suggest that the primary function of Slit in controlling Robo-mediated midline guidance is as a short-range repellent.
Growth cones that express high levels of Robo do not extend away from the midline, but rather they avoid entering and crossing the midline.
For example, the pCC growth cone expresses high levels of Robo, and it extends anteriorly near the edge of the midline.
In a robo mutant, the pCC growth cone freely crosses and recrosses the midline; in a slit mutant, the pCC growth cone enters the midline and does not leave it.
Although it is possible that Slit might also function as a long-range chemorepellent during axon guidance in Drosophila, causing some growth cones to extend some distance away from the midline, at present the strongest genetic evidence in Drosophila is for a short-range function.
This is in contrast to its function during mesoderm migration and muscle formation.
After gastrulation in Drosophila, many myoblasts migrate laterally away from the ventral midline.
The ventral body wall muscles normally attach to the epidermis underneath the CNS but stay some distance from and do not cross the midline.
In contrast, in slit mutant embryos, many developing muscles are found near the midline, stretching across the midline dorsally over the CNS. The slit mutant muscle defects are nearly identical to those seen in sim mutant embryos in which the midline cells are missing (10).
In contrast, in slit mutants, the midline cells are present but do not secrete Slit into the extracellular environment. 10 used genetic analysis of sim to show that after gastrulation the midline cells are required for the migration of muscle precursor cells away from the midline.
Many of these mesodermal cells normally migrate at least five to six cell body diameters away from the midline.
In the sim mutant, the precursors do not migrate away from the midline, presumably due to the absence of a midline-derived long-range chemorepellent.
Moreover, in the sim mutant the muscle precursors that extend ventrally toward the midline are not prevented from crossing the midline, presumably due to the absence of a midline-derived short-range repellent.
Rather, when these misplaced muscle precursor cells undergo myogenesis, they form abnormal contacts with each other that freely extend across the dorsal midline of the CNS. We found that slit mutant embryos display the exact same midline mesoderm phenotypes as do sim mutant embryos.
This suggests that Slit is both the long-range chemorepellent controlling mesoderm migration away from the midline and the short-range repellent preventing muscles from crossing the midline.
The Robo receptor appears to play only a minor role in the ability of Slit to direct the long-range migration of muscle precursors away from the midline.
Either Robo2 or some other Slit receptor must function as the major muscle receptor for Slit-mediated long-range chemorepulsion.
Why Do Growth Cones Leave the Midline?
At the outset we asked the question, if commissural growth cones are so attracted to Netrin, if the highest concentration of Netrin is at the midline, and if when growth cones arrive at the midline they meet their homologs from the other side for which they have a high affinity, why do these growth cones ever leave the midline?
Although we do not yet fully understand the mechanism, the answer to this question has something to do with the qualitatively different ways in which growth cones respond to Slit.
For growth cones near the midline that do not cross it, Slit forms a strong repulsive barrier.
But for growth cones that do cross the midline, Slit cannot be such a strong repellent, rather functioning in a more subtle fashion, somehow preventing them from lingering at the midline and driving them across.
In the absence of Slit, growth cones enter the midline but do not leave it, extending in a single fused longitudinal tract at the midline.
Thus, Slit must be part of the anti-linger mechanism.
One thing is certain: the ability of Slit to form a repulsive barrier requires the Robo receptor.
Any growth cone that expresses high levels of Robo cannot cross the midline.
So in a robo mutant, growth cones freely cross and recross the midline, but they do not stay at the midline.
Two inferences follow from these observations.
First, there must be at least one additional Slit receptor that controls midline guidance, and at present Robo2 is the best candidate.
Second, because Slit appears to have two different functions (one as a midline repulsive barrier and the second as a midline anti-linger signal), it follows that either Robo2 signals differently from Robo, or alternatively, that the low levels of Robo2 alone (or Robo2 and Robo together) on growth cones crossing the midline give rise to a qualitatively different response as compared to high levels of Robo.
Whether we are dealing with two qualitatively different negative responses, or alternatively, quantitative differences in a common repulsive mechanism, is not yet clear.
Teasing this mystery apart in the future should shed some light on how growth cones make stereotyped and divergent decisions at complex choice points.
Slits Are Likely to Have Other Functions
In the Drosophila embryo, Slit and Robo colocalize at muscle attachment sites.
We do not yet know whether this coordinated expression of ligand and receptor represents an attractive or a repellent function.
In vertebrates, experimental evidence supports the conservation of the repulsive function for Slits at the midline.
However, other experiments show that a fragment of Slit2 functions to promote axon branching and elongation of sensory axons.
Additional experiments will be required to determine whether other Slits can also function as positive regulators of axon growth and/or branching.
Experimental Procedures
Genetic Stocks
slit1 and slit2 are both null alleles; slitE-158 is a hypomorphic allele created by insertion of a P element into the 5′ region of the gene. slit1 and slitE-158 were obtained from S.
Artavanis-Tsakonas. slit2 was obtained from the Bloomington Stock Center.
The slit1 robo5, slitE-158 robo4, and slitE-158 robo5 chromosomes were generated by recombination.
A twist allele on the slit1 chromosome was removed during this process.
UAS-robo, UAS-comm, and sca-GAL4 are Goodman laboratory stocks. elav-GAL4 was obtained from A.
DiAntonio. slit-GAL4 was obtained from C. Klämbt.
RNA Localization and Protein Immunocytochemistry
RNA localization was performed as described in 20.
Immunocytochemistry was performed as described in 7.
The anti-Slit mAb C555.4c was used at a dilution of 1:50 in PBS with 0.1% saponin.
The anti-muscle myosin mAb FMM5 was used at a dilution of 1:10.
Transformation Construct
A cDNA containing the slit ORF was isolated from the LD cDNA library (EST Project, G. Rubin lab).
The ORF was sequenced and found to have an additional LRR between the second and third LRRs in the first set of tandem arrays.
The 5′ AsnI overhang of an AsnI–DraI fragment containing the ORF was filled in with the Klenow fragment of E. coli DNA Polymerase I, and the fragment was cloned into the EcoRV site of pcDNA3 (Invitrogen).
An Asp718–XbaI fragment containing the slit ORF was dropped out of the pcDNA3 construct and cloned into the Asp718–XbaI sites of pUAST.
Five transformant lines were generated and mapped using standard techniques.