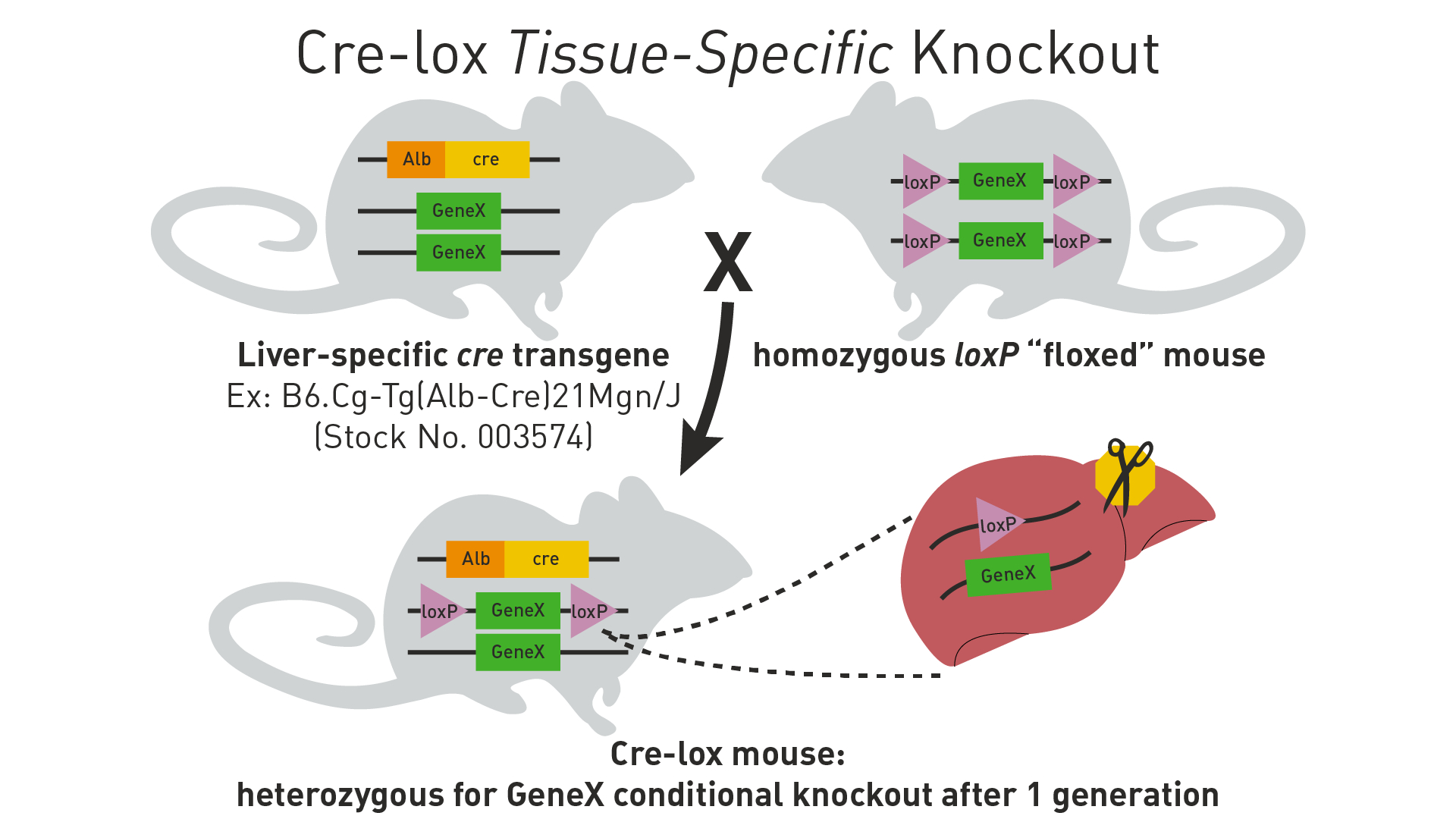
Describe the key concepts of tissue-specific conditional gene expression using the Cre-lox system
Introduction of loxP Sites: Scientists genetically engineer a mouse strain to have loxP sites flanking a specific gene of interest (this is called a "floxed" gene). This is done using techniques like homologous recombination in embryonic stem (ES) cells, which are then used to create transgenic mice. These loxP sites are not naturally occurring in the mouse genome and must be inserted at specific locations to target a particular gene.
Cre Recombinase Expression: Another mouse strain is engineered to express the Cre recombinase enzyme under the control of a tissue-specific promoter. This ensures that Cre recombinase is only produced in certain tissues or under certain conditions.
Breeding Strategy:
Male Mouse: Often carries the gene for Cre recombinase under a tissue-specific promoter. This means that only in certain tissues where the promoter is active, the Cre recombinase will be expressed.
Female Mouse: Typically carries the gene of interest flanked by loxP sites (the floxed gene).
Mating and Genetic Recombination: When these two mice are mated, their offspring have the potential to inherit both the floxed gene and the Cre recombinase gene. In the tissues where the Cre recombinase is expressed, it will cut out the floxed gene, leading to a tissue-specific gene knockout.
Existing Genetic Elements in Mice: The normal mouse genome does not contain the components necessary for the Cre-lox system (neither the loxP sites nor the Cre recombinase). These elements are introduced through genetic engineering.
Outcome of Mating: The progeny from this mating will be a mix. Some will inherit both the floxed gene and the Cre recombinase, leading to tissue-specific gene knockout. Others might inherit only one of the components (either the floxed gene or the Cre recombinase gene) or neither, depending on the pattern of inheritance.
Review data from Andersen 1997 (Dlx2 and cell migration). Compare and contrast neuronal migration along radial glia cells versus tangential migration in terms of cell types and contributions to cortical circuits.
Neuronal Migration Along Radial Glia Cells
Cell Types Involved: The document does not provide explicit details about radial glial cell migration. However, generally, radial migration is known to involve primarily excitatory neurons, including pyramidal neurons, which migrate from the ventricular zone to their final positions in the cortical plate.
Migration Pathway: Radial migration typically follows a straight path from the ventricular zone to the cortical surface, guided by radial glia fibers.
Contribution to Cortical Circuits: Radial migration is crucial for the formation of the layered structure of the cortex, with neurons positioning themselves in specific cortical layers based on their time of birth.
Tangential Migration
Cell Types Involved: The document discusses the migration of GABA-expressing interneurons from the lateral ganglionic eminence (LGE) to the neocortex. These interneurons are inhibitory neurons, which play a critical role in balancing the excitatory activity of pyramidal neurons.
Migration Pathway: Tangential migration does not follow the radial glia scaffold. Instead, it occurs along the plane of the neural tube, allowing cells to migrate over long distances horizontally across different brain regions.
Contribution to Cortical Circuits: Tangentially migrating interneurons are essential for establishing the inhibitory circuitry of the cortex. They integrate into existing circuits, providing critical inhibitory control and contributing to the functional diversity of the cortical network.
Key Differences
Direction of Migration: Radial migration is predominantly vertical, following the radial glia scaffold, while tangential migration is horizontal, independent of the radial glia.
Types of Neurons: Radial migration is associated with excitatory neurons, whereas tangential migration primarily involves inhibitory interneurons.
Role in Cortical Development: Radial migration is fundamental for establishing the layered structure of the cortex, while tangential migration contributes to the diversity and complexity of cortical inhibitory circuits.
Similarities
Importance in Cortical Development: Both types of migration are crucial for proper cortical development and the establishment of functional neural circuits.
Regulation by Genetic Factors: The migration processes are regulated by specific genetic factors, as indicated by the role of Dlx genes in tangential migration.
In summary, while radial and tangential migrations differ in their pathways, cell types involved, and specific contributions to cortical circuits, both are integral to the development of a functionally diverse and complex cerebral cortex.
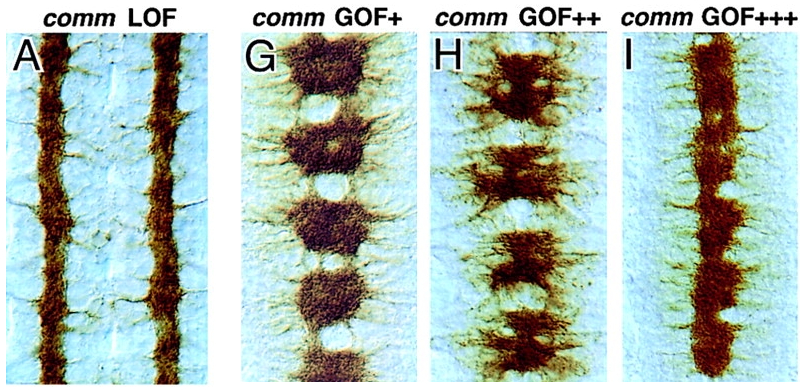
Review data in Kidd 1999 (Slit and Robo) and be able to diagram the expression patterns of Slit, Robo, and Comm. Be able to recognize images of mutants for these genes
Comm Protein ➡️
Blocks Robo Expression / Localization ➡️
Ignores Slit Repellent ➡️
Crosses the Midline ➡️
Degrade Comm ➡️
Express Robo ➡️
Bind Slit Repellent ➡️
Move away from the midline
Comm Protein Knockout ➡️
Robo Expression ➡️
Binds Slit Repellent ➡️
Never Crosses the Midline
Wild Type

Slit
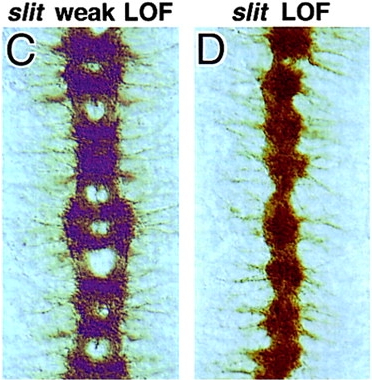
Roundabout ( Robo )
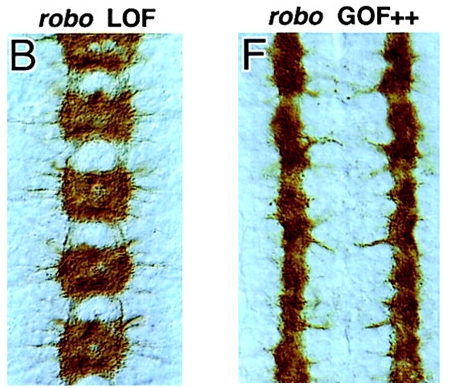
Commissurless ( comm )
Review data in Nakamoto 1996 (ephrins and Eph receptors) and understand the ligand-receptor binding partners described in the paper
ELF-1 = Ephrin-A2
Mek4 = EphA3
Sek = EphA4
retinal ganglino cells = express receptor on growth cone
gradient of receptor expression as you go across the retina
highly sensitive = lots of receptors = stop growing early
Eph Family Ligands and Receptors in the Retinotectal System: The document discusses several Eph family ligands and receptors expressed in the retinotectal system. For instance, Mek4, a receptor that binds ELF-1, shows matching expression and binding patterns, suggesting that ELF-1 and Mek4 could be complementary recognition labels in retinotectal mapping (Page 8).
ELF-1 and Retinal Axon Behavior: ELF-1 can act as a repellent, and models in which it acts only as an attractant can presumably be excluded. The document suggests that ELF-1’s action on retinal axon behavior refines the possible models of its function. For example, an ELF1–AP probe detects higher receptor levels on temporal than nasal axons, indicating at least one receptor with a temporal (high) to nasal (low) distribution (Page 9).
Gradient Complementarity and Chemoaffinity Theory: ELF-1 and Mek4 are expressed in gradients along matching axes in the chick retinotectal system, providing direct evidence for gradient complementarity predicted by the chemoaffinity theory. This supports the idea of complementary positional labels for retinotectal mapping (Page 1).
RAGS and ELF-1: RAGS, a ligand closely related to ELF-1, is reported to be in a gradient limited to the posterior tectum and has in vitro repellent activity. The activities of ELF-1 and RAGS seem consistent with their localizations, suggesting different roles in retinotectal development (Page 8).
Topographic Mapping and Eph Ligands/Receptors: The Eph family, including at least 7 ligands and 12 receptors, is known to be expressed in the nervous system. The properties of ELF-1 in the retinotectal system suggest that other Eph ligands could also have roles in neural map specification. The Eph family may have analogous functions in spatial patterning during development (Page 10).
In summary, the document provides a comprehensive overview of the roles of ephrins and Eph receptors in the retinotectal system, highlighting their importance in ligand-receptor interactions, spatial patterning, and the development of neuronal connections.
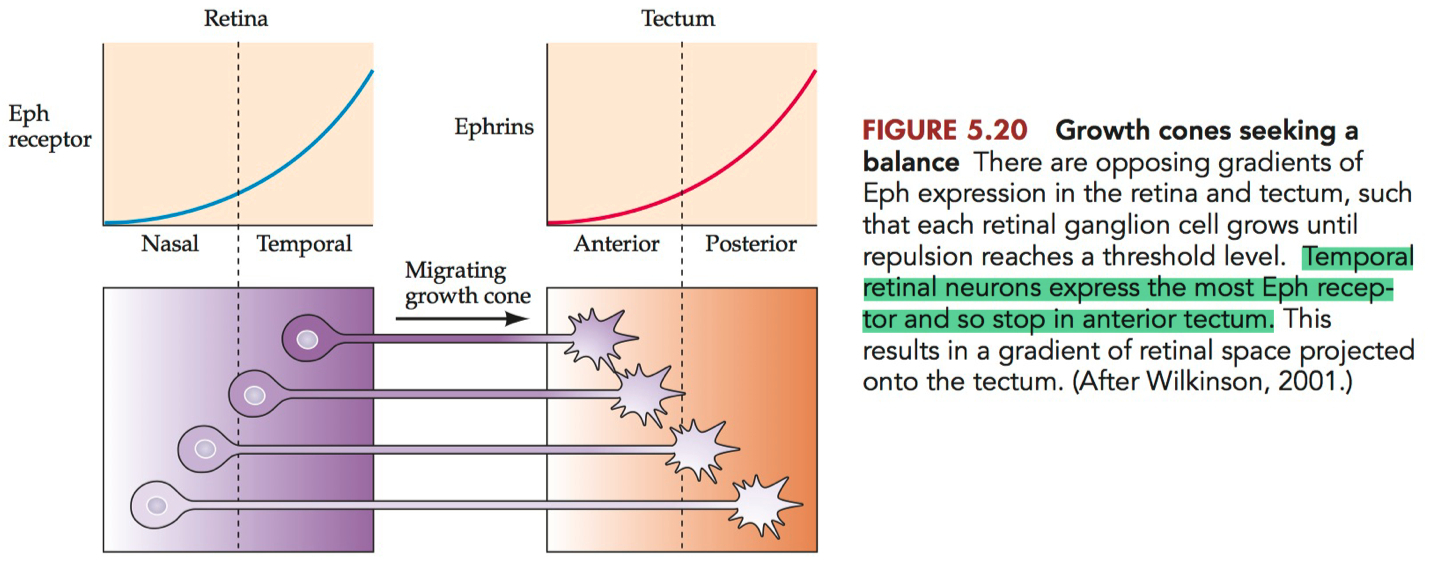
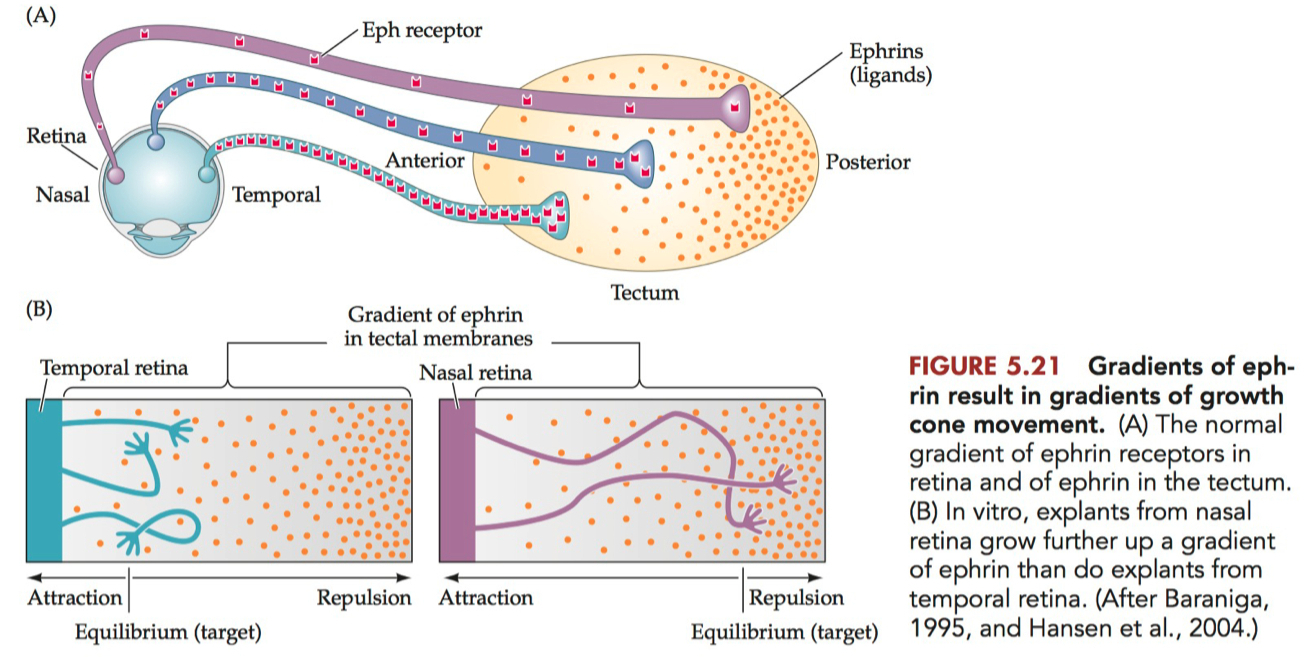
Diagram the gradients of ephrins and Eph receptors in the tectum and retina, respectively, and describe how these repellent gradients account for the topographic mapping of visual information onto the tectum
Diagram of Ephrins and Eph Receptors Gradients
Retina :
Eph Receptors: The retina primarily expresses Eph receptors, particularly EphA receptors. The concentration of these receptors is graded, with the highest levels in the temporal retina and progressively lower levels towards the nasal retina.
Ephrin Ligands: Lower or absent expression of ephrin ligands in the retina compared to the tectum.
Tectum :
Ephrin Ligands: The tectum, especially in species like birds and mammals, expresses ephrin ligands, primarily ephrin-A. The expression of ephrin-A is also graded, with the highest concentration in the anterior tectum and lower levels towards the posterior tectum.
Eph Receptors: Lower or absent expression of Eph receptors in the tectum compared to the retina.
How These Gradients Account for Topographic Mapping
The interaction between ephrin ligands and Eph receptors is primarily repulsive, and this repulsion is crucial for the proper mapping of retinal ganglion cells (RGCs) onto the tectum.
Retinal Ganglion Cell Axon Guidance: RGCs extend their axons from the retina to the tectum. The axons are guided in part by the interaction between Eph receptors (on the RGC axons) and ephrin ligands (on the tectal surface).
Gradient Interaction: When the RGC axons reach the tectum, they interact with the gradient of ephrin ligands. The strength of the repulsive interaction depends on the relative concentrations of Eph receptors and ephrin ligands.
Mapping Logic :
Axons from the temporal retina, with high levels of Eph receptors, are repelled more strongly by the high concentration of ephrin ligands in the anterior tectum. This repulsion directs these axons towards the posterior tectum.
Conversely, axons from the nasal retina, with lower levels of Eph receptors, are less repelled by the anterior tectum's ephrin ligands and thus innervate more anterior regions of the tectum.
Resulting Topographic Map: This gradient-based repulsion ensures that retinal axons are sorted along the anterior-posterior axis of the tectum in a manner that reflects their position in the retina, creating a topographic map.
This mechanism is a classic example of how molecular gradients can guide neural connectivity during development, ensuring that spatial information is preserved in the wiring of the nervous system.
Review data from Scheiffele 2000. Describe the roles of neurexin and neuroligin in synapse induction
Neuroligin Function in Synapse Formation:
Neuroligin, a postsynaptically localized protein, can trigger the de novo formation of presynaptic structures. When nonneuronal cells are engineered to express neuroligins, they induce morphological and functional presynaptic differentiation in contacting axons. This suggests that neuroligins are part of the machinery employed during the formation and remodeling of CNS synapses.
Neuroligin-1 and -2 are expressed during synaptogenesis in the cerebellum. The activity of neuroligin-1 requires specific sequences in its extracellular, acetylcholinesterase-homologous domain. The addition of soluble β-neurexin inhibits neuroligin activity, suggesting a direct involvement in the initial formation or stabilization of presynaptic structure.
Neurexin Role in Synapse Induction:
Neurexins were originally identified as receptors for the spider venom α-latrotoxin. They are hypothesized to generate specificity during synaptogenesis through thousands of variants generated by alternative splicing. The presynaptic localization of neurexins remains to be demonstrated, but neuroligin-1, a ligand of a specific splice variant of β-neurexins, has been clearly shown to localize to the postsynaptic compartment at excitatory synapses.
The interaction between neuroligin and neurexin is crucial for synapse formation. For instance, the addition of soluble β-neurexin to a coculture of neurons inhibits synaptic vesicle clustering in axons contacting target neurons, indicating that a splice site-specific ligand of β-neurexin, most likely neuroligin, is directly involved in the formation or stabilization of presynaptic structure.
Molecular Interactions and Specificity:
Neuroligin in a target neuron could bind β-neurexin in the presynaptic, axonal membrane. This interaction might lead to the recruitment of synaptic vesicles, potentially involving a series of protein-protein interactions. However, neuroligins are unlikely to specify neuronal cell types for synapse formation but are more likely components of a general synaptogenic machinery.
In summary, neuroligin and neurexin are integral to the process of synapse induction, with neuroligin primarily located postsynaptically and neurexin presynaptically. Their interaction is essential for the formation and stabilization of synaptic structures, highlighting their significance in the development of the nervous system.
Review data from Gautam 1996. Describe how agrin signaling initiates synapse development at the NMJ
Agrin Synthesis and Transport: Agrin is synthesized by motor neurons and transported along their axons. It is secreted from their terminals and retained in the basal lamina that occupies the synaptic cleft at the NMJ. Muscle cells also synthesize agrin, but the agrin purified from the brain is more potent at clustering acetylcholine receptors (AChRs) than that from muscle.
Role of Agrin in Synapse Formation: During synapse formation at the NMJ, a small patch on the muscle fiber surface differentiates into a complex postsynaptic apparatus. An early step in this process is the redistribution of AChRs within the myotube membrane, leading to their aggregation directly beneath the nerve terminal. This aggregation is crucial for synaptic organization and is induced by nerve-derived agrin.
Molecular Structure of Agrin: Agrin is a large protein (~200 kDa) containing several copies of sequences resembling protease inhibitors, EGF repeats, and laminin-like globular domains. The transcript of agrin is subject to alternative splicing, resulting in different isoforms with distinct expression patterns.
Agrin's Effect on AChR Clustering: Agrin induces the clustering of AChRs on cultured myotubes. This clustering is accompanied by the formation of extracellular matrix, membrane, and cytoskeletal components of the postsynaptic apparatus. The clustering mechanism involves both local aggregation and long-range dispersal of AChRs, mediated by agrin.
Agrin Deficiency and Synaptic Development: Studies on agrin-deficient mutant mice have shown that postsynaptic differentiation is profoundly impaired without agrin. In these mutants, nerve-associated AChR aggregates are fewer in number, smaller, and lower in receptor density. This suggests that agrin is a crucial organizer of postsynaptic differentiation, with the lack of nerve-derived z-agrin being largely responsible for these defects.
Bidirectional Signaling: The neuromuscular synapse formation involves bidirectional signaling between the nerve and muscle. While agrin from the nerve side plays a key role in organizing the postsynaptic apparatus, the muscle also provides signals that guide presynaptic differentiation.
Alternative Agrin Functions: Besides its role in synaptic differentiation, agrin might also influence other aspects of neuromuscular development, such as axonal branching patterns and muscle-nerve interactions.
In summary, agrin signaling is essential for the initiation and proper development of synapses at the NMJ, primarily through its role in clustering AChRs and organizing the postsynaptic apparatus. The absence of agrin leads to significant defects in synaptic development, highlighting its critical function in neuromuscular synaptogenesis.
adhesion :
"ligand" =
receptor = cadherin = post and pre synaptic ( specific homophilic binding )
allow synapses to take place ( induce assembly of synaptic machinery ) :
ligand = neuroligin = post-synaptic = expressed on soma and dendrites
receptor = neurexin = pre-synaptic = expressed on axon
maintaining terminal schwann cells and promoting AChR expression :
ligand = neuregulins = axon terminal
receptor = ErbB = post and pre synaptic ( nearby Schwann cells )
activation induces the Schwann cells to differentiate into terminal Schwann cells that cap the NMJ
AChR Clustering :
ligand = agrin = pre-synaptic
receptors = MuSK = post-synaptic
binding recruits rapsyn
intracellular protein that binds AChRs together
also anchors MuSK receptor to the muscle cytoskeleton
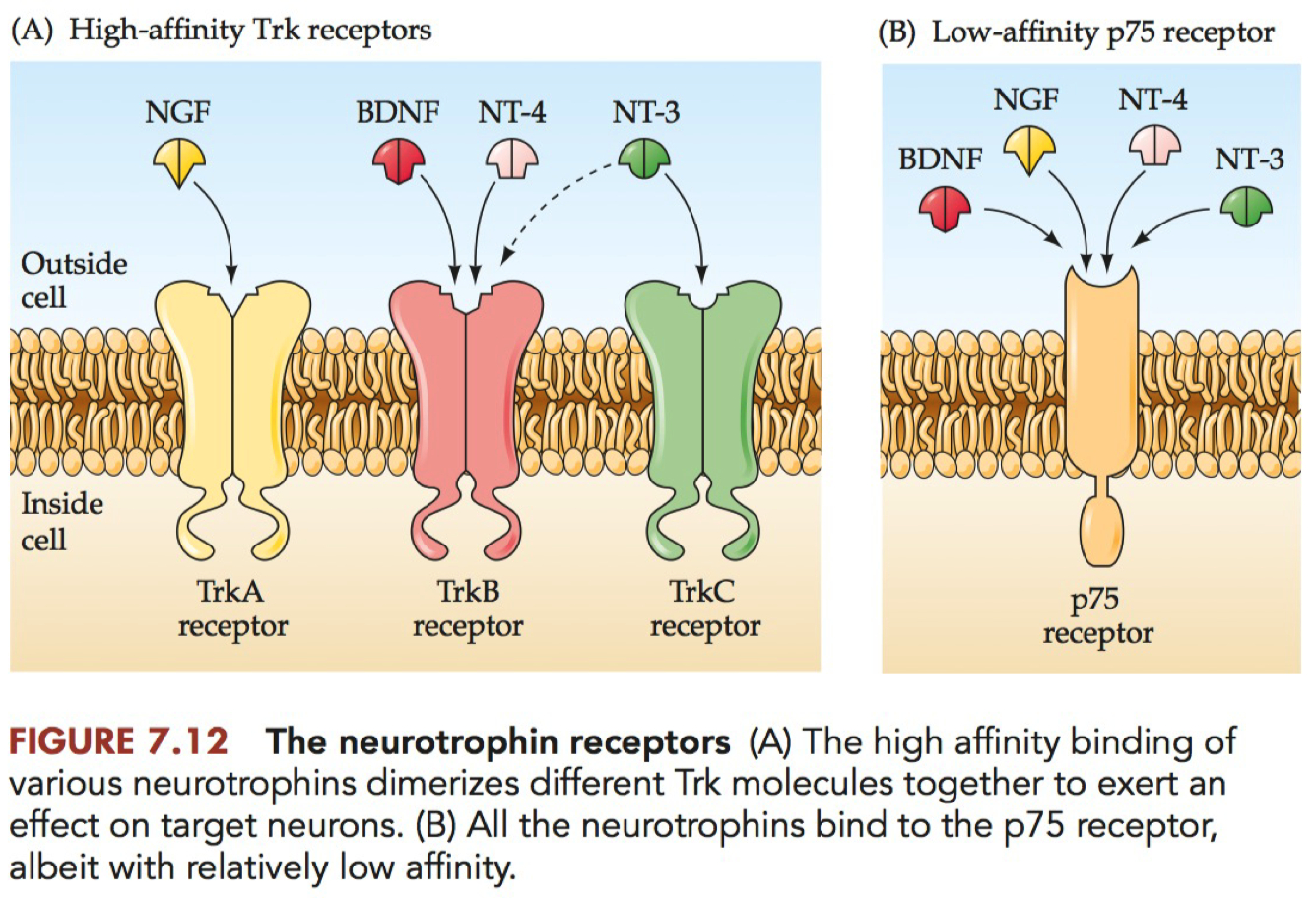
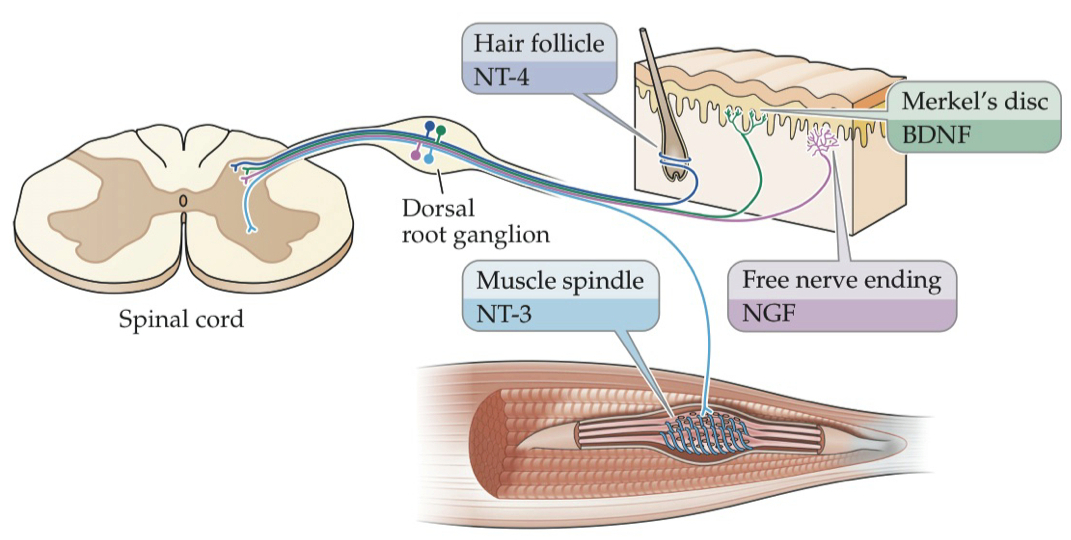
Match the list of neurotrophic factors with the Trk receptor(s) they bind with high-affinity
Contrast properties of AMPA and NMDA glutamate receptors and describe role of NMDA receptors in expression of LTP
AMPA Receptors = 𝛼 -amino-3-hydroxy-5-methyl-4-isoxazoleprepionic acid , a synthetic compound that binds one class of ionotropic glutamate receptors with high affinity.
fast kinetics
DON'T allow calcium
NOT voltage dependent
NMDA Receptors = N-methly-D-aspartate , a synthetic compound that binds with high affinity a class of ionotropic glutamate receptors that are known for imparting a Hebbian-like plasticity.
slow kinetics
allow calcium
voltage dependent
but it's pseudo voltage dependent
magnesium gets stuck in the pore
prevents current
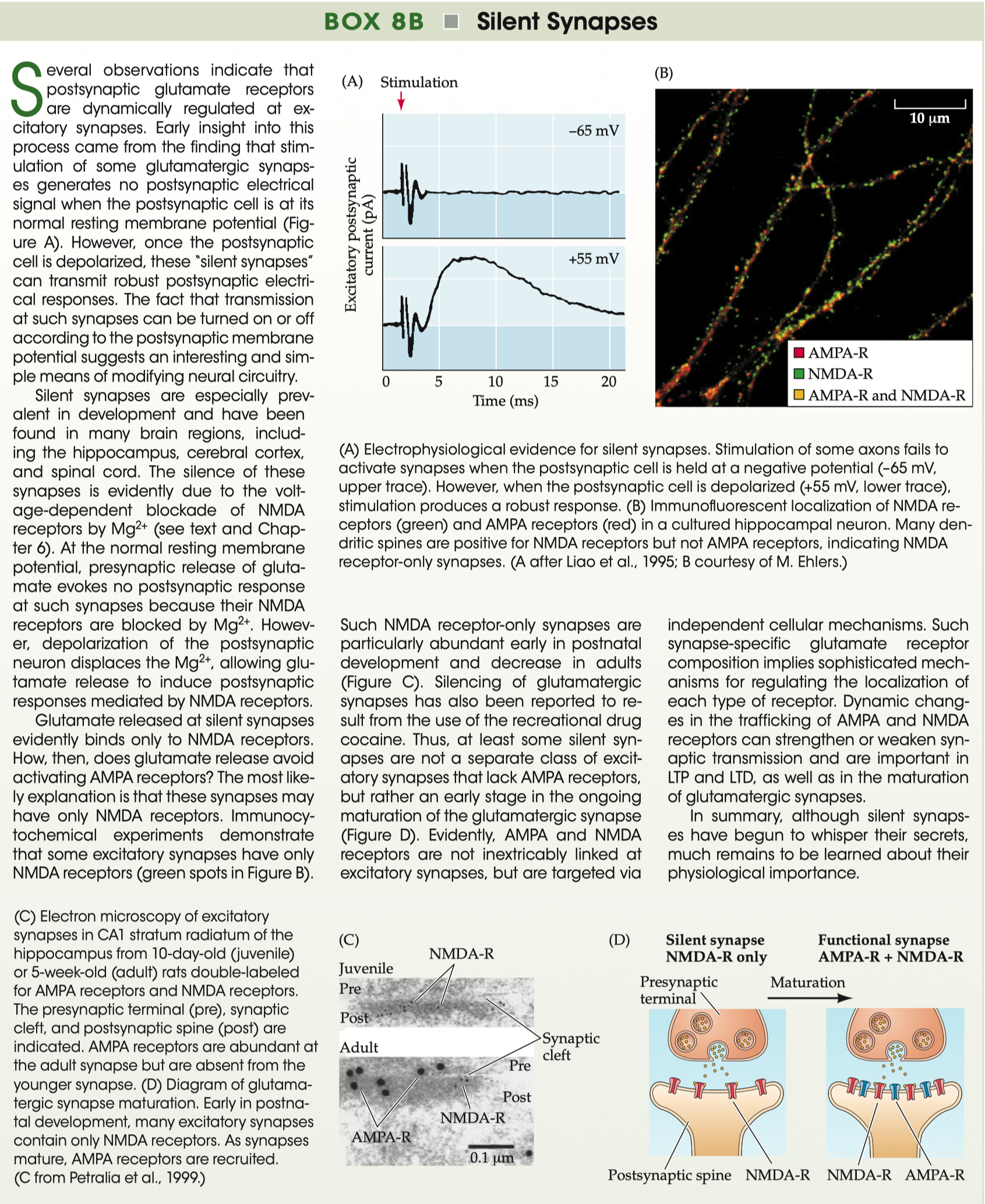
Review data on experiments that reveal the presence of silent synapses
Silent Synapses = glutamatergic synapses containing NMDA receptors, but not AMPA receptors
Neurotransmitter is released, but looks like “failure”
Silent Synapses :
first ones that get put into the membrane = NMDA receptors
if you stimulate pre-synaptic neuron
but channels are blocked by magnesium
Unmasking of Silent Synapses
during development , the silent synapses become unmasked
maturation eventually puts AMPA receptors into the membrane
now works at resting membrane potentials and depolarizing potentials
Describe the correlation between percent occupancy and axon diameter in the process of synaptic competition at the developing NMJ
Percent Occupancy: This term typically refers to the extent to which a particular neuron's axonal terminals cover or occupy the postsynaptic muscle fiber at the NMJ. During development, multiple axons from different neurons may innervate the same muscle fiber, each vying for synaptic space.
Axon Diameter: Larger axons have more surface area and can potentially form more synaptic contacts. The diameter of an axon can be indicative of its maturity and capacity to transport neurotransmitters efficiently.
Synaptic Competition: In the developing NMJ, there's a competition for synaptic sites between different neurons. Initially, a muscle fiber may be innervated by multiple axons. Over time, through a process of synaptic pruning, the muscle fiber will typically be innervated by a single axon. This competitive process is essential for the proper formation and refinement of neural circuits.
Correlation between Percent Occupancy and Axon Diameter: The correlation can be seen in the competitive process. Larger axons (with greater diameter) are often more successful in this competition, leading to a higher percent occupancy at the NMJ. The reasons for this are multi-faceted:
Resource Allocation: Larger axons might be more efficient in securing and allocating resources necessary for maintaining and strengthening synapses.
Signal Transmission Efficiency: Larger axons can transmit signals more efficiently, making their synaptic connections more reliable and effective.
Neurotrophic Factors: Synaptic competition is influenced by neurotrophic factors, which are crucial for the survival and growth of neurons. Larger axons might be more adept at securing these necessary factors.
Outcome of Competition: The outcome is that over time, the more successful axons (often larger ones) dominate the synaptic space on the muscle fiber, leading to an increased percent occupancy by these axons and the withdrawal of less successful, often smaller, axons.
This process is integral to the development of the nervous system, ensuring that the most efficient and robust connections are maintained while redundant or less effective ones are eliminated. It's a prime example of activity-dependent synaptic plasticity, where the use and effectiveness of synaptic connections determine their survival.
Describe the outcome of experiments where two eyes innervate the same tectal area in frogs and the role of visual experience and NMDA receptors in this process
Outcomes of the Experiments:
Tectal Innervation: In a normal frog, each eye projects to the contralateral (opposite side) tectum. However, when an additional eye is grafted, the neurons from both the original and the grafted eye compete to innervate the same tectal area. This leads to an overlapping of visual inputs from both eyes on the same tectal neurons.
Visual Map Formation: Normally, the tectum develops a topographic map of the visual field. However, with two eyes innervating the same area, this map can become disrupted or altered. The neurons in the tectum must sort out conflicting visual information from two different eyes.
Role of Visual Experience:
Activity-Dependent Refinement: The visual experience plays a crucial role in refining the connections. Neurons that fire together (experience correlated activity from the visual inputs) tend to strengthen their connections, while those that do not tend to weaken or lose their connections. This is in line with the Hebbian theory of synaptic plasticity.
Competition and Selectivity: There's a competitive aspect where more active or effective synaptic connections (those that better represent the visual environment) are strengthened at the expense of less effective ones. This competition can be influenced by the visual experiences the frog encounters during development.
Role of NMDA Receptors:
Synaptic Plasticity: NMDA (N-methyl-D-aspartate) receptors play a key role in synaptic plasticity. They are involved in activity-dependent strengthening and weakening of synapses.
Critical Periods: These receptors are particularly important during the critical periods of visual development, where the visual inputs are crucial for the proper mapping of the visual field onto the tectum.
Long-Term Potentiation (LTP) and Long-Term Depression (LTD): NMDA receptors are essential for these processes, which are believed to be the cellular basis for learning and memory. In the context of the frog’s visual system, they facilitate the adjustment and refinement of the visual maps in the tectum.
In summary, the experiments where two eyes innervate the same tectal area in frogs demonstrate the remarkable plasticity of the developing nervous system. They show how visual experience and molecular mechanisms, like NMDA receptor-mediated plasticity, interact to shape neural circuits. This research has broader implications for understanding how sensory experiences influence brain development and function.
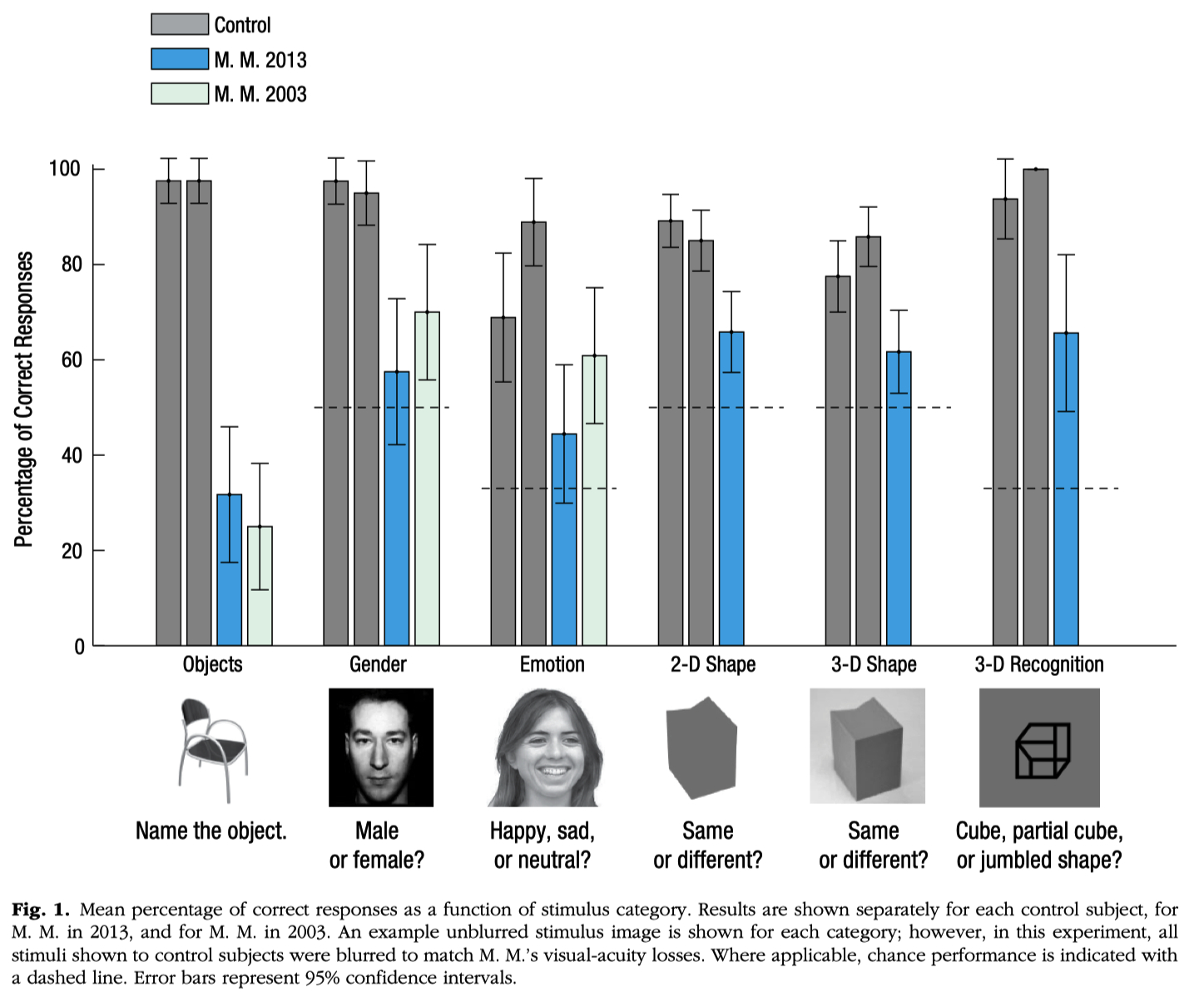
Review the story and data discussed in class about patient M.M. (blind man whose vision was restored later in life)
Molyneux Question = asks if a blind person, upon gaining sight, would be able to visually recognize objects previously known only by touch.
this question has implications for understanding the innate versus learned nature of sensory perception
Michael May :
https://sci.bban.top/pdf/10.1177/0956797614563957.pdf#view=FitH
blinded at age 3 due to a chemical explosion
at age 46, technical advances made it possible to restore vision in his right eye.
as soon as the bandages were removed , he could see
he could his wife's blue eyes and blond hair ( feature processing )
But even years later :
he cannot recognize her face ( configurational processing ) unless she speaks to him.
he can't recognize three-dimensional objects like cubes or spheres unless they are moving.
he can still ski, but he finds he has to close his eyes to avoid falling over.
on the slopes, seeing is more distracting than helpful