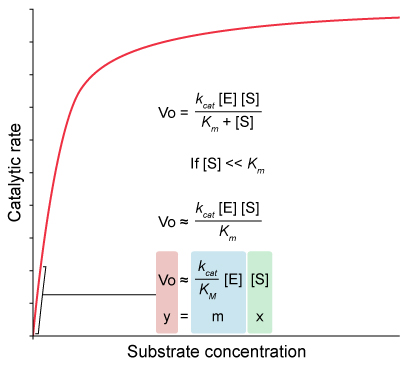
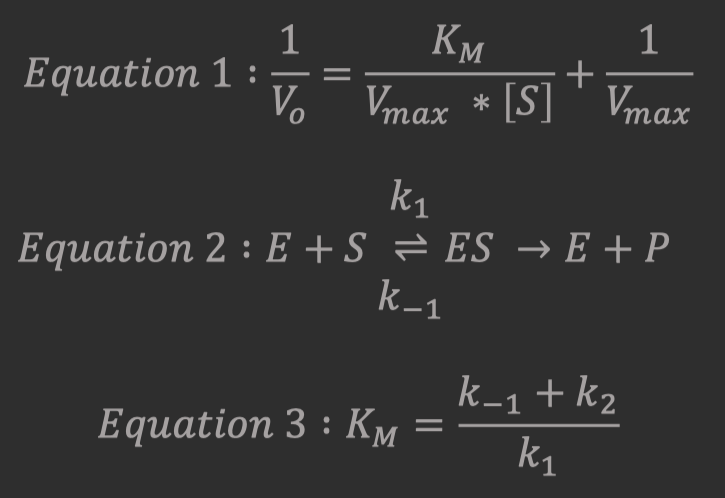Michaelis-Menton Equation
- The Michaelis-Menten equation describes the rate of an enzymatic reaction as a function of substrate concentration.
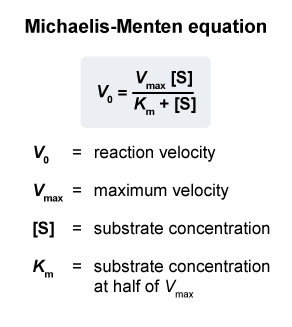
- It is written in the form :
where :
= substrate concentration
= observed reaction rate at a given
= maximum possible reaction rate
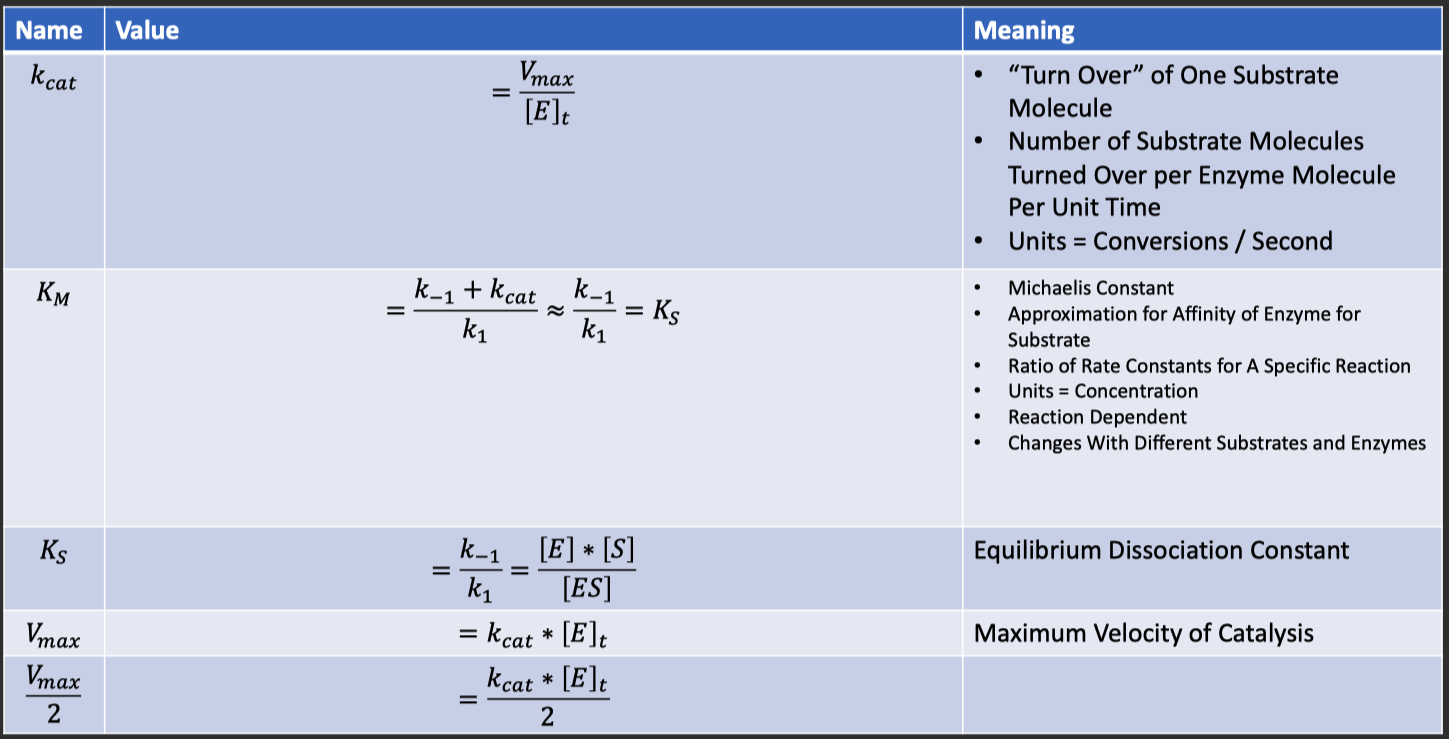
:
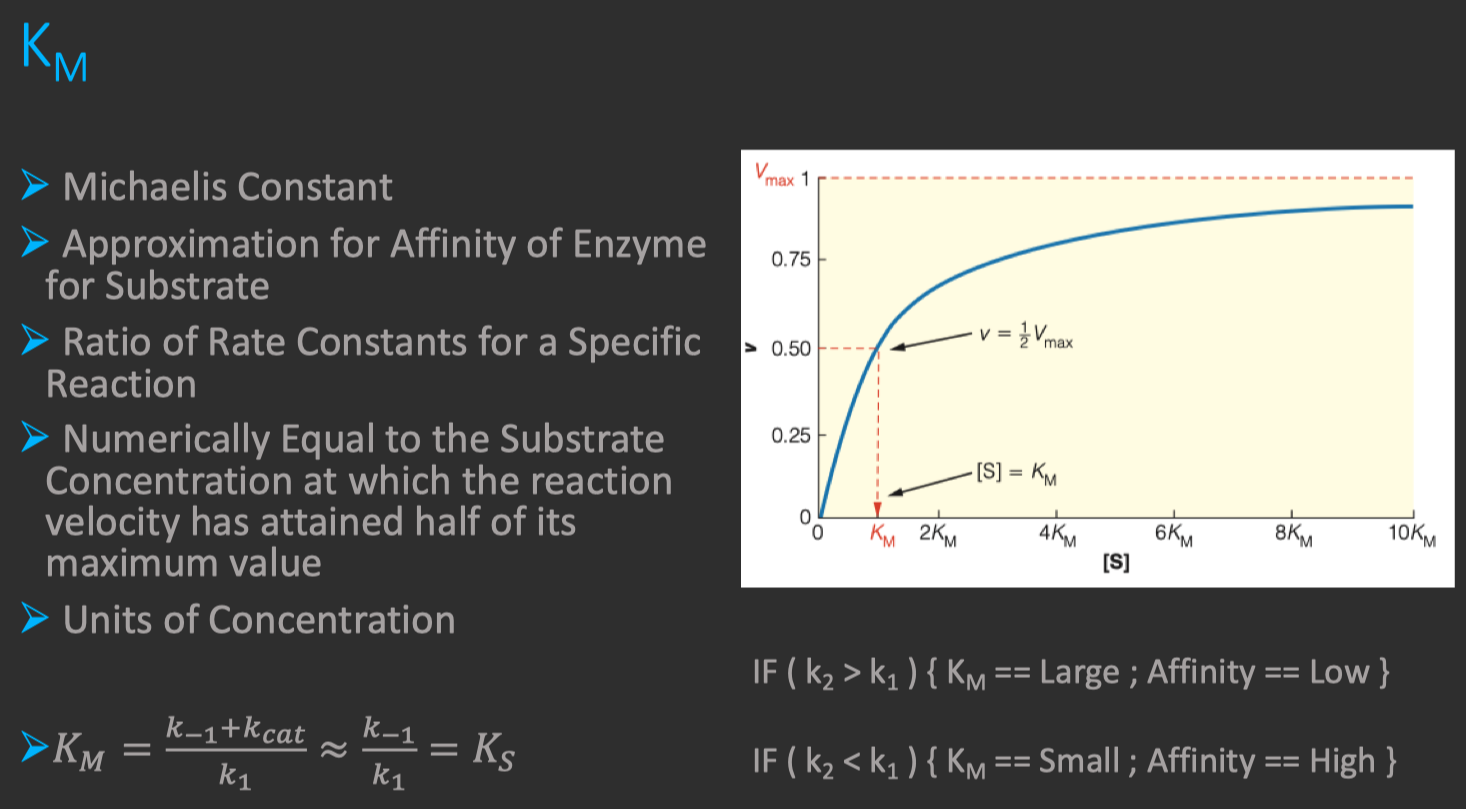
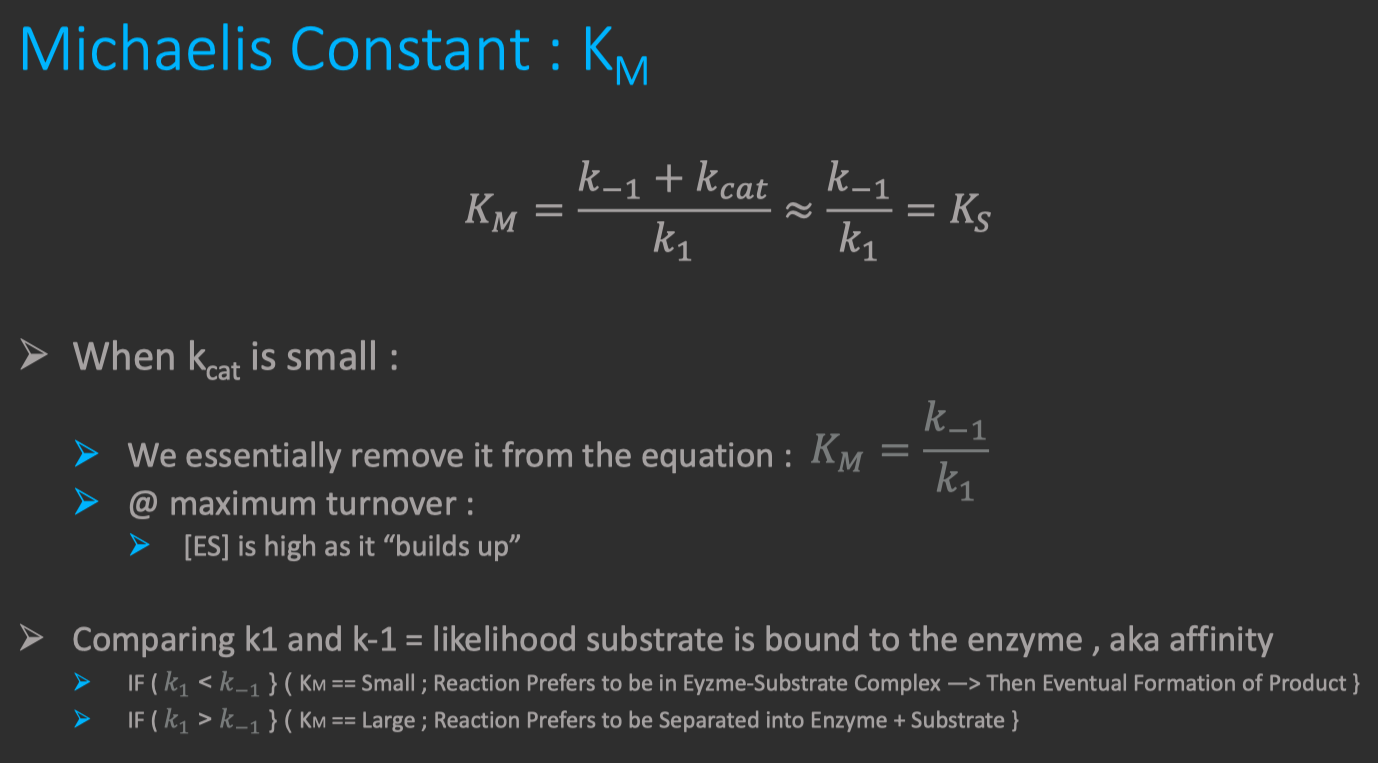
Michaelis constant
substrate concentration at which
is achieved also a "measure" of the affinity of Enzyme for Substrate
Binding Tightness of ES complex
- smaller values of
mean the Enzyme is more tightly bound to the Substrate
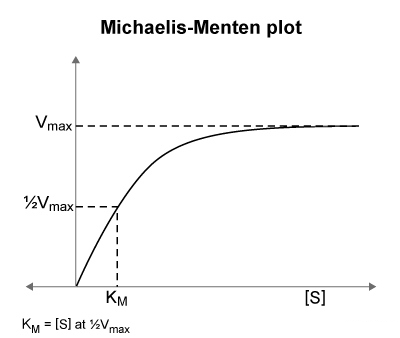
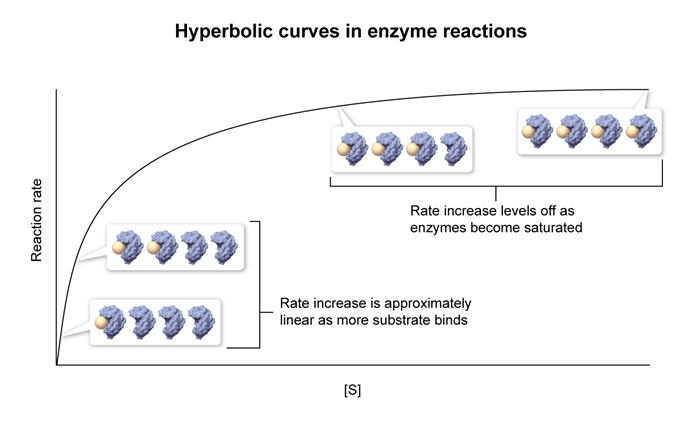
- As
increases , increases hyperbolically , approaching a plateau at - When
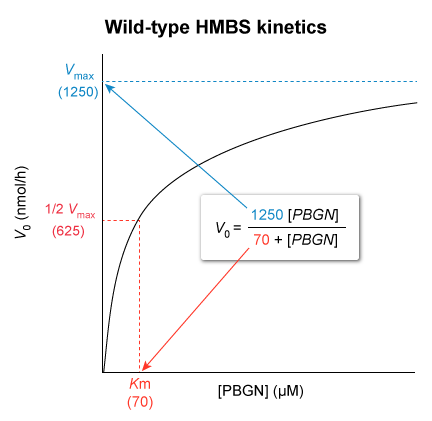
= , the Michaelis-Menten equation can be rewritten as :
- Therefore , when
is equal to , the reaction proceeds at
- The Michaelis-Menten equation describes the rate of an enzymatic reaction as a function of substrate concentration.
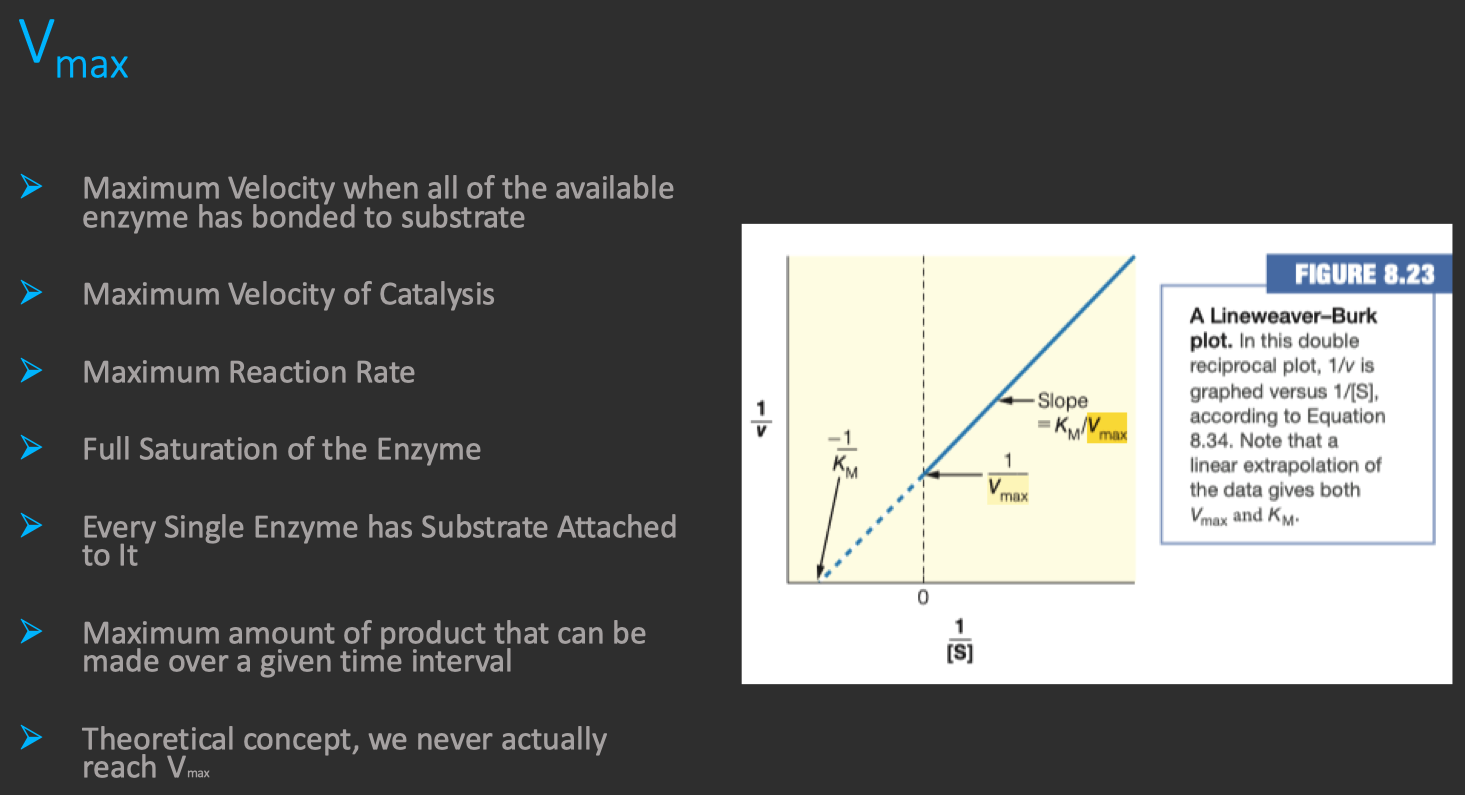
- The maximum reaction velocity
, found in the numerator of the equation , indicates the height of the plateau that the graph approaches. - The
, found in the denominator , is the substrate concentration at which is achieved.
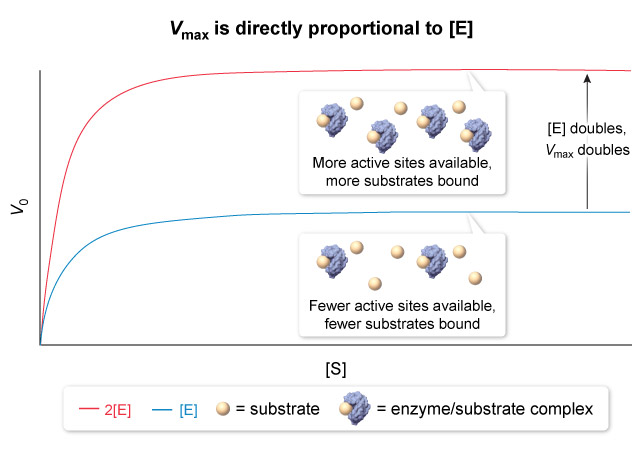
is achieved when is high enough that all enzyme active sites are bound by substrate , a condition known as saturation. is directly proportional to the concentration of enzyme in solution , expressed mathematically as :
- Where
is the total enzyme concentration and is the turnover number , or the number of reactions catalyzed per second of enzyme at saturation. - At saturation , increasing
will not increase the reaction rate because has already been achieved. - Increasing the enzyme concentration , on the other hand , increases the number of active sites present in solution , and therefore increases
- The rate of an enymatic reaction is directly proportional to the concentration of enzyme present in the reaction.
- When the active sites on an enzyme are saturated , an increase in substrate concentration will NOT change the reaction rate but an increase in enzyme concentration will.
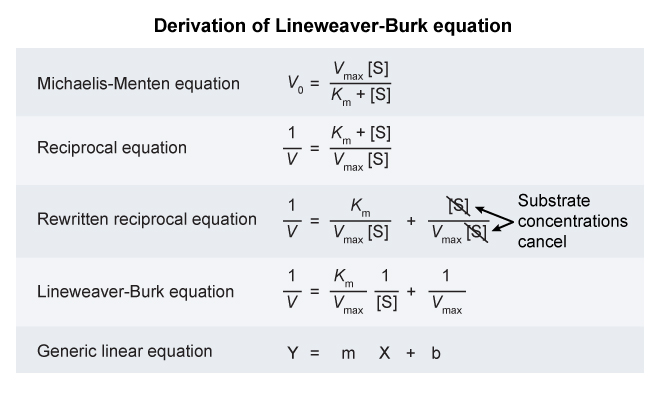
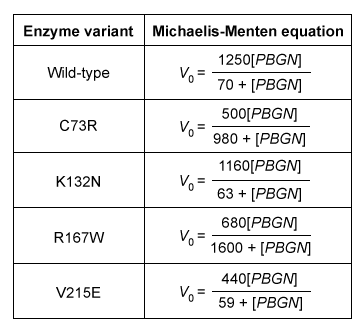
Experimentally Determining
and
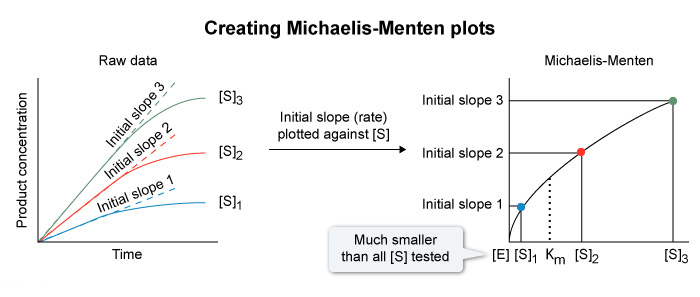
Michaelis-Menten kinetics are determined by measuring the rate of product formation in the presence of varying concentrations of substrate.
The reaction rate ( initial slope ) at each substrate concentration is than plotted as a single point , with concentration on the x-axis and rate on the y-axis , yielding a hyperbolic curve ( Michaelis-Menten plot ) described by the Michaelis-Menten equation
The Michaelis-Menten equation relies on three assumptions :
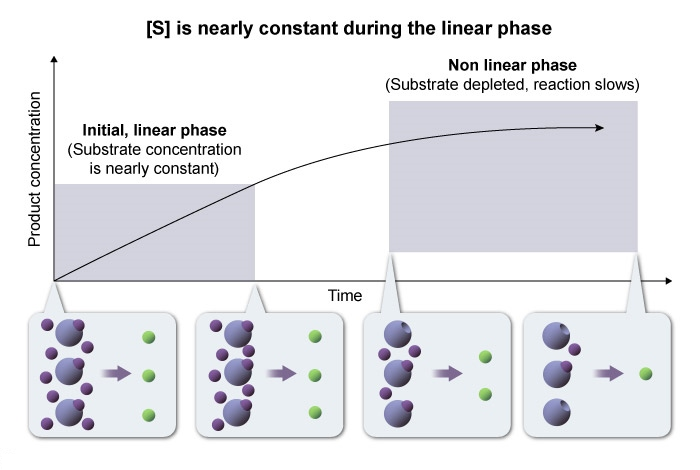
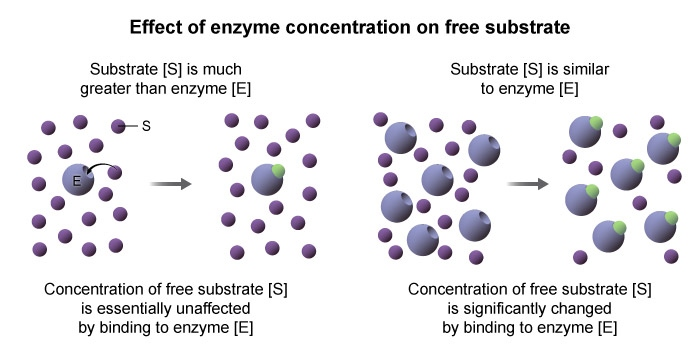
- The free ligand approximation states that substrate concentration
is constant during the reaction. - This approximation is only true during the initial phase of the reaction , before a significant amount of substrate is converted to product.
- Substrate can also be depleted when it binds the enzyme to form the enzyme-substrate complex ( ES ).
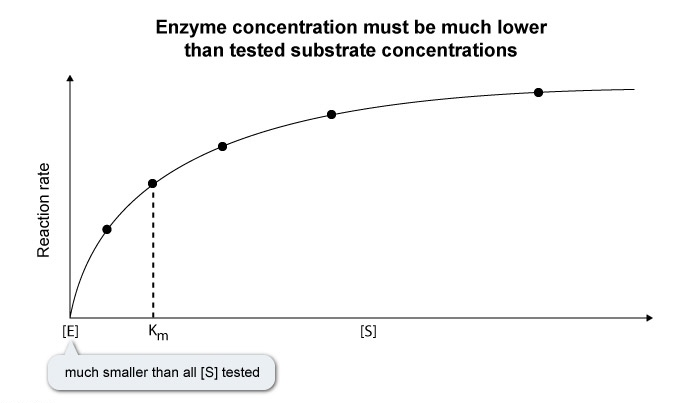
- To ensure that ES formation does not significantly impact
, the total concentration of enzyme in solution should be much smaller than any substrate concentration tested The steady state assumption states that the concentration of ES remains constant over the course of the reaction , allowing the rate of product formation to remain constant.
- Once
becomes significantly depleted , ES levels decrease and the reaction slows. The irreversibility assumption states that the reaction proceeds only in the forward direction , and product does not get converted back to substrate.
- Once enough product accumulates , the reverse reaction occurs at non-negligible levels and further slows the net rate of product formation.
Each of these assumptions holds true only during the initial phase of the reaction , before substrate is depleted or product accumulates.
Therefore , for each substrate concentration measured , only the initial reaction rate should be reported.
is the substrate concentration at which occurs. Because enzyme concentration must be substantially smaller than all substrate concentrations tested , enzyme concentration must be much smaller than
, not larger.
- The Michaelis-Menten equation models the initial rates of a reaction at various substrate concentrations.
- Rates are measured as the slope of the initial , linear phase of the reaction before substrate is depleted and product accumulates.
- Enzyme concentration should be much lower than all substrate concentrations tested.
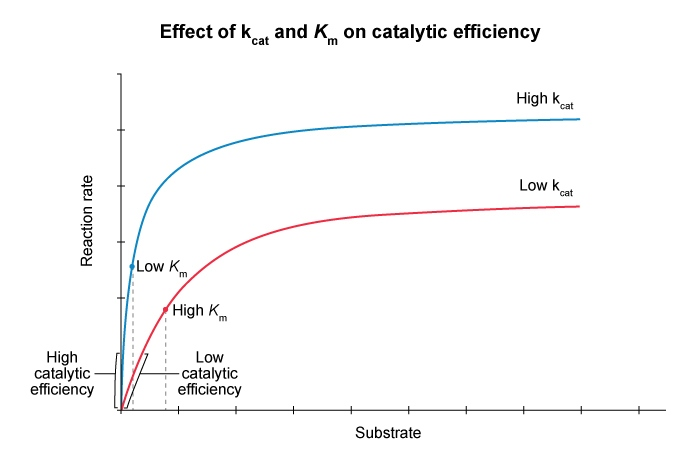
Catalytic Efficiency
Catalytic efficiency is a measure of how well an enzyme facilitates reactions at low substrate concnetrations.
When enzyme concentration is held constant , catalytic efficiency is proportional to the initial slope of a Michaelis-Menten curve
- when
This slope is governed by both the catlytic turnover
and the Michaelis constant Mathematically , catalytic efficiency is calculated as the ratio of
to An increase in
increases the catalytic efficiency , and a decrease in also increases the efficiency. Therefore , an allosteric activator that both increases
can decreases will increase the catalytic efficiency of an enzyme. Conceptually , a large ( steep ) slope indicates an efficient enzyme because a small amout of substrate yields a high reaction rate.
Catalytic turnover (
) measures the number of substrate molecules converted to product per enzyme per second when the enzyme is saturated
Therefore , a high
corresponds to a high maximum rate ( )
- and , by extension a larger slope at lower substrate concentrations
The Michaelis constant (
) is an indicator of binding affinity. A small
indicates high affinity , allowing the reaction to approach more rapidly ( higher slope ) with smaller amounts of substrate.
- Therefore , a small
contributes to high catalytic efficiency.
#concept , #equation , #kinetic , #rate-law