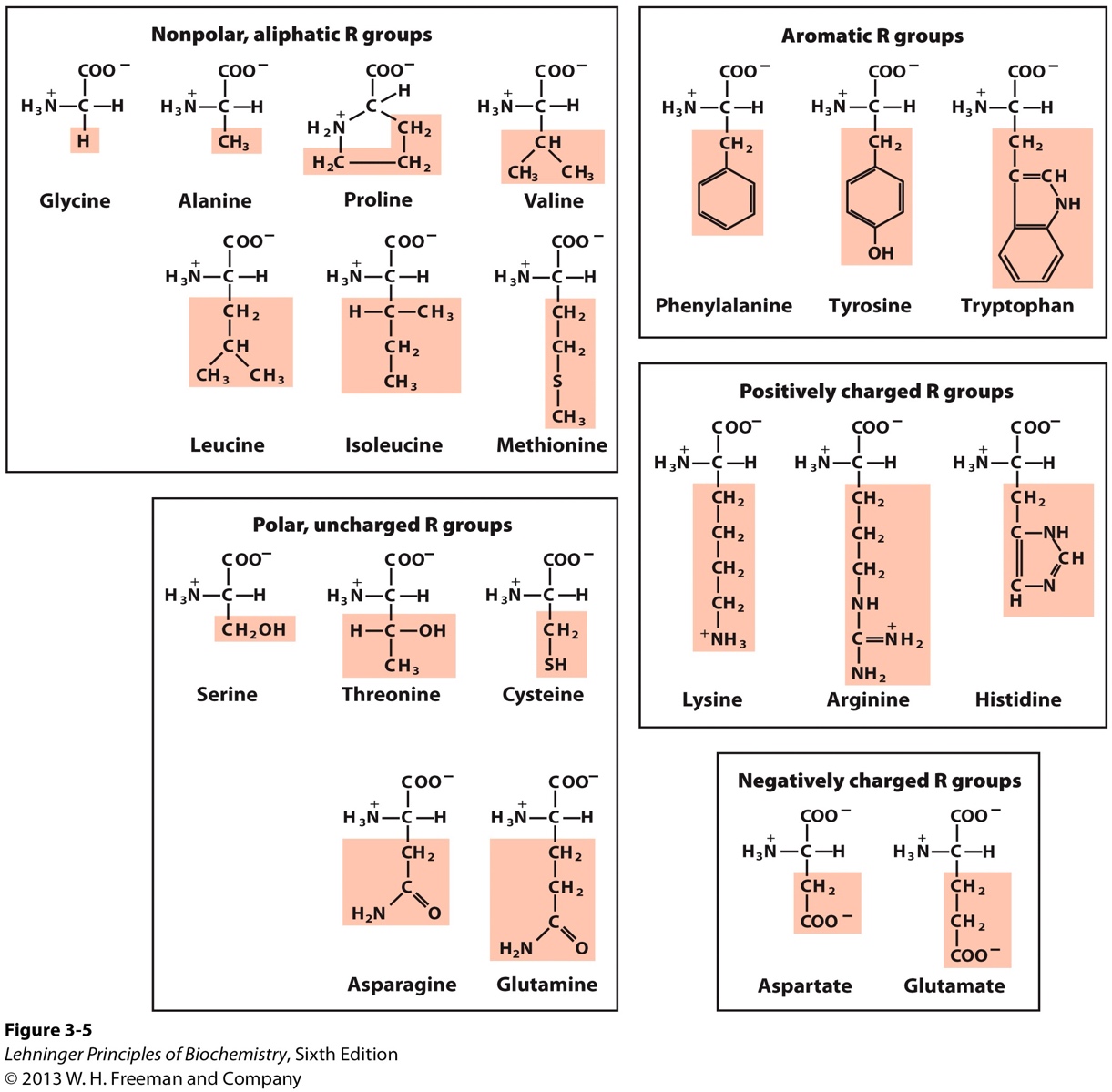
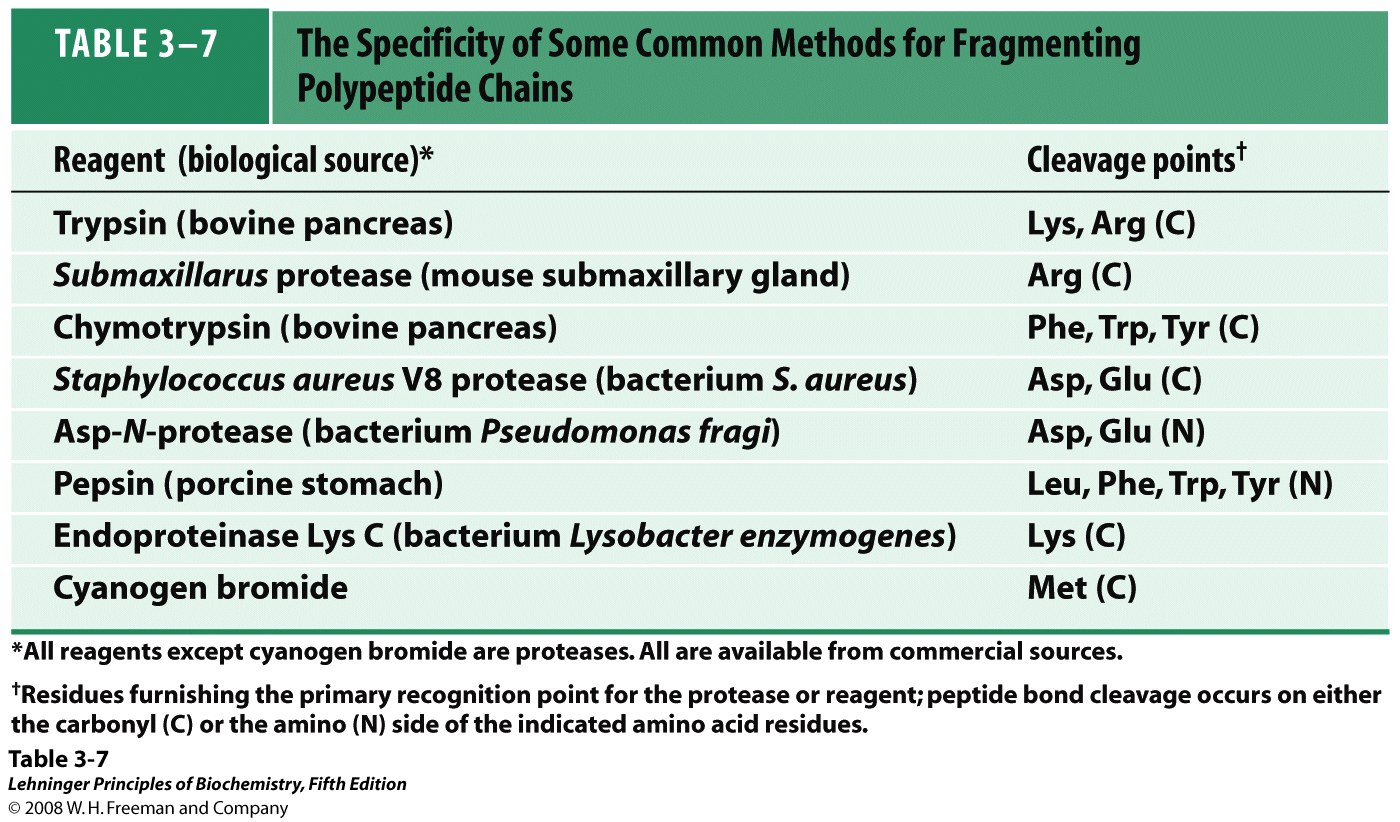
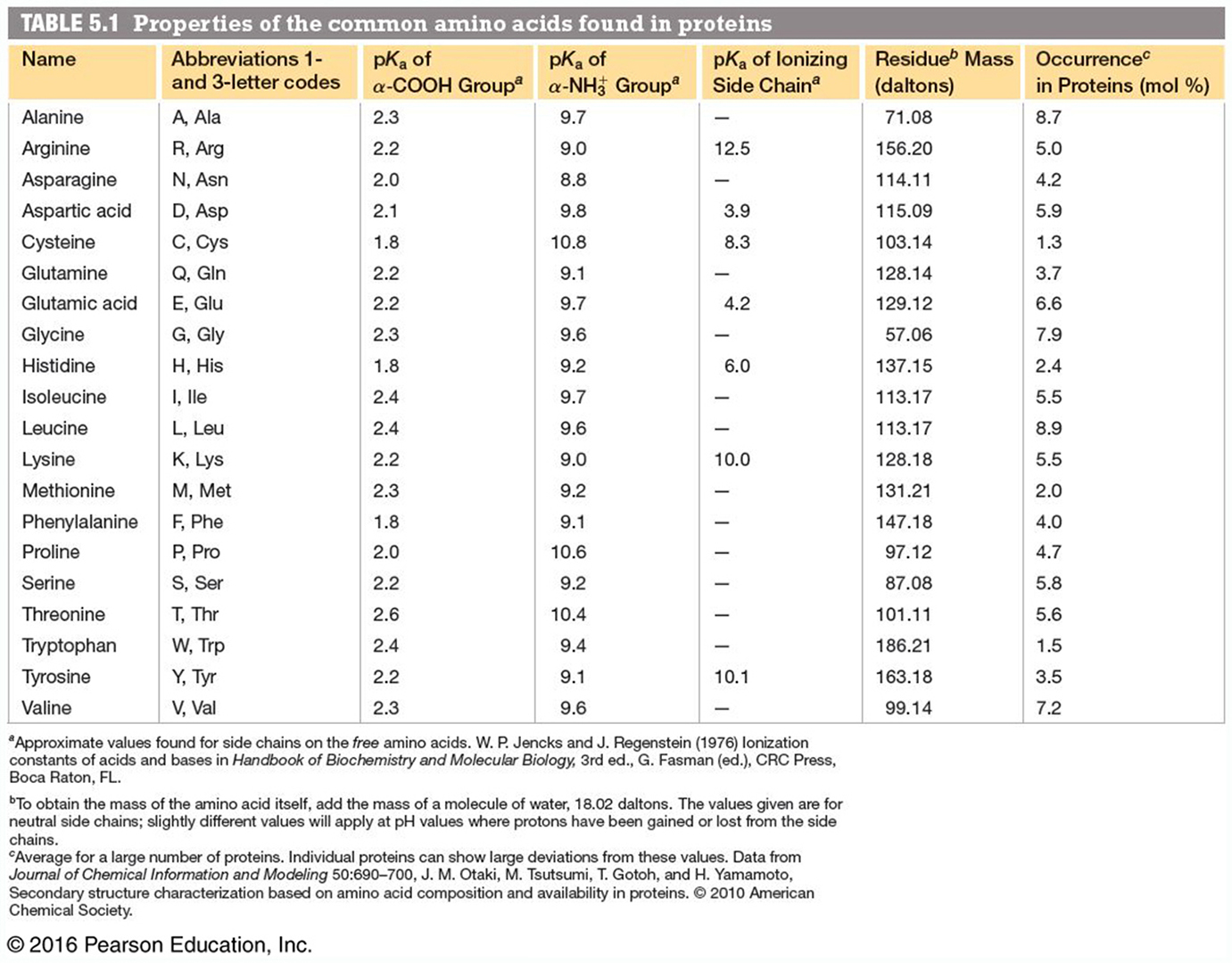
Reaction Velocity
Rate Equations
Half Life Equations
Misc
Definitions
- indicates by how many kJ/mol the transition state is stabilized in the catalyzed reaction
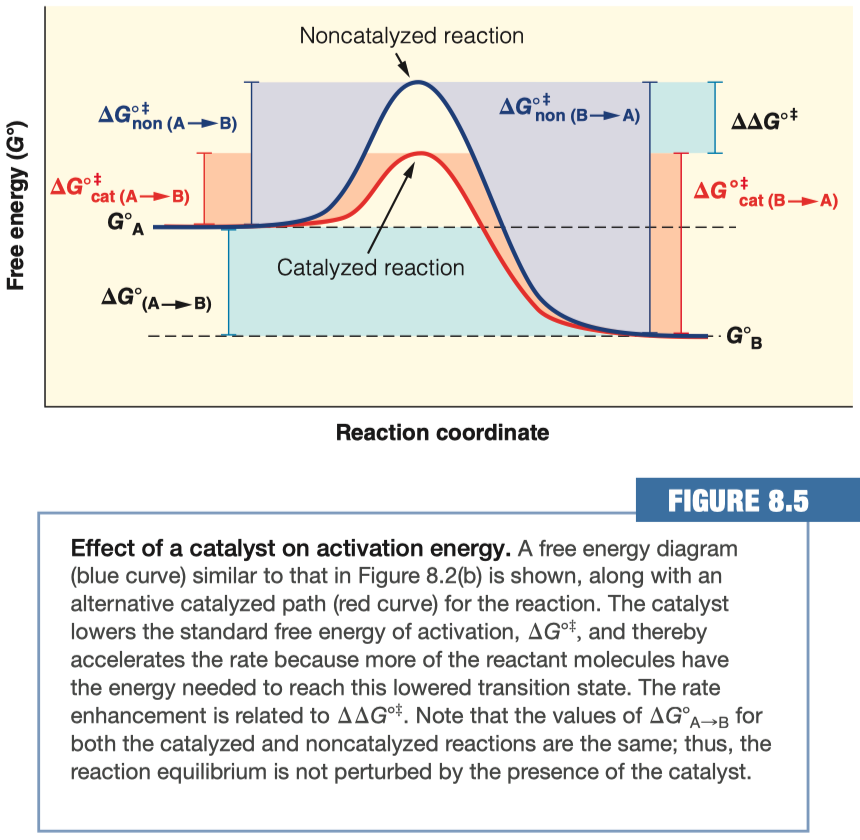
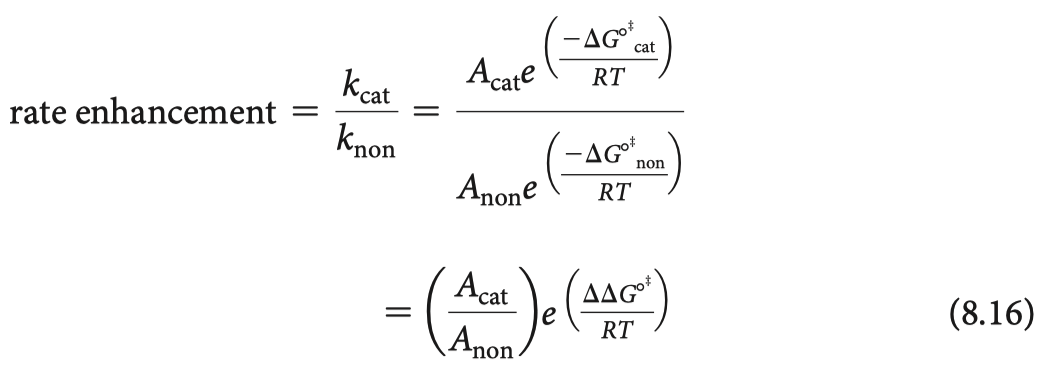
Competitive Inhibition :
- Apparent
- Apparent
- Lines = intersect on y-axis
- Apparent
Uncompetitive Inhibition :
- Apparent
- Apparent
- Lines = Parallel
- Apparent
Mixed Inhibition :
- Apparent
- Apparent
- Lines = Intersect near the x-intercept
- Apparent