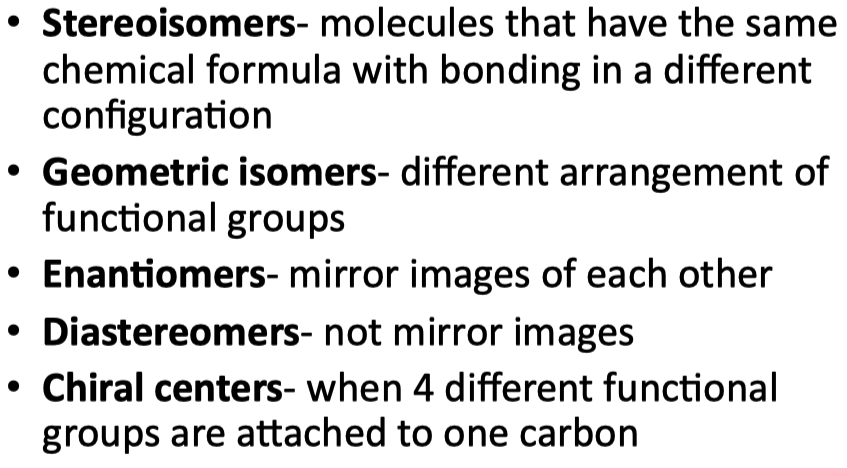
Describe the differences between the types of stereoisomers found in biomolecules
Calculate standard free energy change of a reaction
Enthalpy ( H ) is the heat exchanged between the system and the surroundings at constant pressure
Problem :
Given:
What is ΔG° for :
Describe the importance of hydrogen bonds in water and biomolecules such as DNA and proteins
- important for stabilization , protein folding , clathrate cage formation
Describe the factors that determine hydrogen bond strength
Geometry of the bond
- linear = stronger
- bent = weaker
Describe the function of a buffer in biochemical systems
resists changes to pH
- helps keep things in equilibrium
- provides protection in acidic environments
Define and calculate pH and
pH = available hydronium ion in solution
small
large
Problem :
- 1 liter of a phosphate buffer (
- How much would the pH of the buffer change if
- 1 liter of a phosphate buffer (
Problem :
Does a strong acid have a greater or lesser tendency to lose its proton than a week acid?
- greater
Does the strong acid have a higher or lower
- higher
Does the strong acid have a higher or lower
- lower
Describe the general structure of amino acids
Peptide bond = condensation reaction
Planar = because of thermodynamics ( steric hindrance )
Rigid = double bonds ( pi bonding )
- Because of Resonance structure , 40% of it is in a double bond
Because peptides are planar and rigid , it gives us our “torsional angles” aka
Both can rotate freely from -180 to +180
- In reality they don’t get this full range because of steric hindrance
Calculate pI for an amino acids and small peptides
organize all
- 1 =
- 2 = C-terminal carboxylic group
- 3 ... = Any ionizable side chains on any of the amino acids
- 1 =
sort all of the
make an educated guess for where it "might" be neutral
- default , start at pH = 7
- try , just above or just below each of your
write out and solve each
- Protonated Amine = +1
- Deprotonated Amine = 0
- Protonated Carboxylic Acid = 0
- Deprotonated Carboxylic Acid = -1
xxxxxxxxxxif ph < pKa:protonatedif pH > pKa:deprotonatedadjust your "test" pH value up or down depending on the last net charge you calculated
once you find where the peptide is neutral ,
take the first
take the first
take their average
Describe the condensation reaction that forms peptides
- hydroxyl on C-terminus carboxylic acid combines with hydrogen on N-terminus amine to release a water molecule
Polar , uncharged R side chains can form hydrogen bonds
- Serine , Threonine , Asparagine , Glutamine
Cysteine and Methionine forms disulfide bonds
Problem :
- Calculate the pI of the peptide : Glu-His-Trp-Ser-Gly-Leu-Arg-Pro-Gly
What is the charge of the molecule at pH 3 , 8 , and 11?
pH = 3 :
- 2.0
pH = 8 :
- 0.0
pH = 11 :
- -1
Describe how proteins are separated and quantified by chromatography
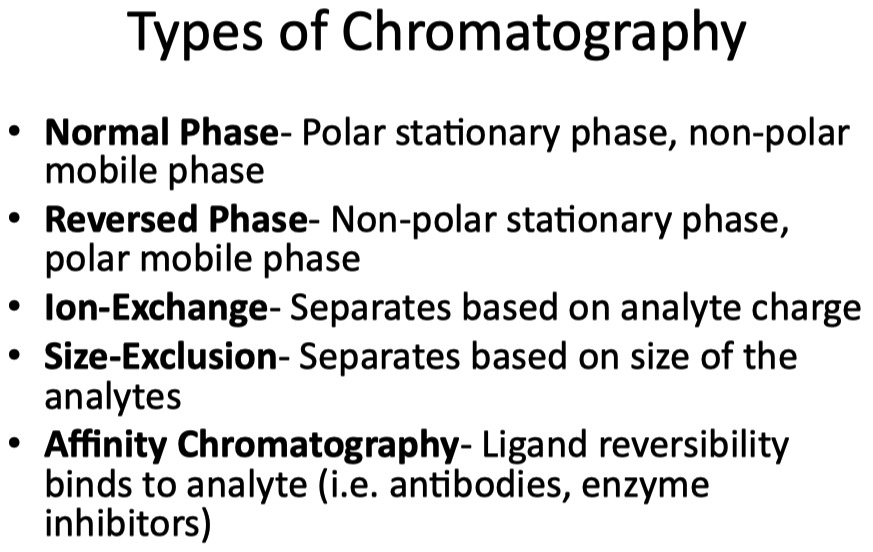
Evaluate the best type of protein separation for a given research scenario
Problem :
A researcher has produced a protein of interest using a peptide synthesizer. The product material is a mixture of the desired protein and smaller fragments ( incomplete synthesis ). For the experiment the researcher needs a large quantity of the protein with the smaller fragments removed.
- size-exclusion chromatography with beads with small pores
Identify the importance of determining amino acid sequences of proteins
- drug design , general understanding
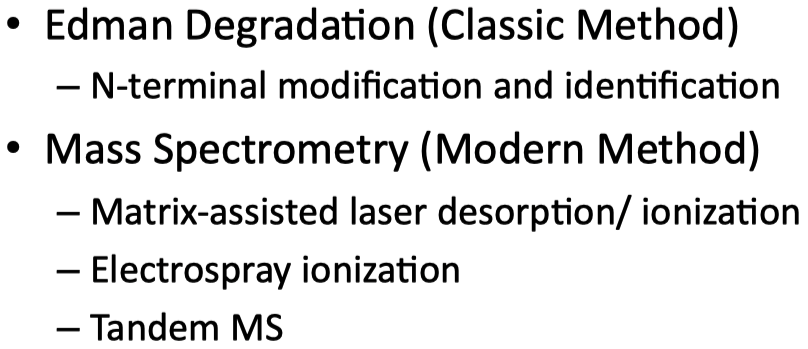
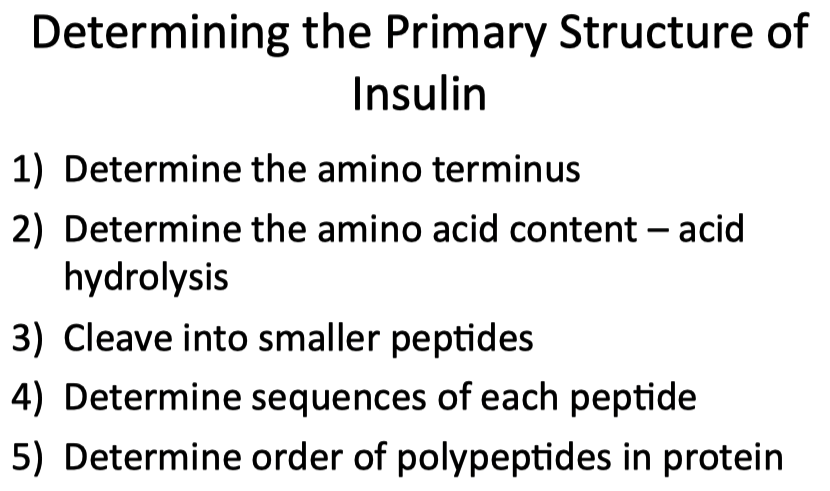
Identify the different methods of determining the primary structure of proteins
Determine the amino acid sequence of a small polypeptide
Problem :
Sequencing of an unknown polypeptide has yielded the following information. What is the sequence of the polypeptide?
Gly, Leu, Phe, and Tyr are in a 2:1:1:1 molar ratio
Treatment with 1-fluoro-2,4-dinitrobenzene ( FDNB ) yielded complete hydrolysis and 2,4-dinitrophenyl tyrosine and no free tyrosine
Digestion with chymotrypsin yielded free tyrosine and leucine , and a tripeptide of Phe and Gly
- Chymotrypsin = Phe , Trp , Tyr ( C )
First bullet point = tells us molar ratio only
- Gly , Gly , Leu , Phe , Tyr
2nd bullet point = tells us peptide was labeled on N-terminus , shot it apart with an acid , tells us that the N-terminus was tyrosine
3rd bullet point = the only way chymotrypsin could be applied and it cleaves a peptide length of 3 , is for phenylalanine to be on the end
- Gly-Gly-Phe
We know leucine and tyrosine are “free”
Write down all combinations for chymotrypsin cleavage
Via trial and error , we know where chymotrypsin cleaved
- Tyr || Gly-Gly-Phe || Leu
Structure must be :
1 2 3 4 5 Tyr Gly Gly Phe Leu
Diagram secondary structure features of proteins
Predict the type of secondary structure of a protein segment
Problem :
Which of the following peptides would be more likely to form an
LKAENDEAARAMSEA
- there are no glycine or prolines here
CRAGGFPWDQPGTSN
How many turns would the helix have?
Describe the structural differences between fiberous and globular proteins
Fiberous = scaffolding , and give structure
- Collagen , keratin
- Make up the majority of the “volume”
- woven together polymers of either helixes or beta sheets
Globular = cytosolic protein
- large proteins with domains / motifs
- Enzymes
- We have more globular proteins , majority of the “population”
Evaluate the structural features of a protein
different domains / motifs
- combinations / orientations of them
Problem :
- characterize 3-D quaternary structure
Describe how factors such as heat contribute to protein denaturation
- breaks hydrogen bonds , eventually breaks peptide bonds
Diagram how chaperones / chaperonins contribute to protein folding
- they can provide a scaffold for where different functional groups can undergo chemical changes
Problem :
Why does the Gro EL/ES system only function in one direction ?
the inside cavity provides a hydrophobic environment
the ATP hydrolysis used to power , creates an energy barrier almost impossible to reverse
- Once you hydrolyze ATP , the Delta G to reattach phosphate group is almost impossible
Describe oxygen binding to myoglobin using both graphical and mathematical approaches
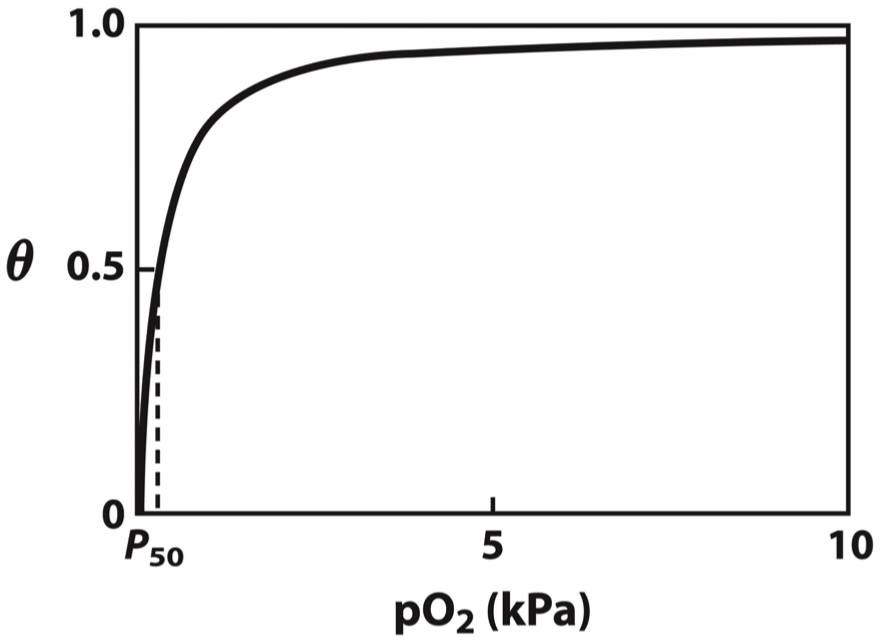
Describe cooperative ligand binding
shifting the partial pressure to the right is not enough
- We need some conformational change
It starts in the low affinity state
Once it reaches a certain partial pressure of oxygen , it changes from low affinity state to high affinity
- Becomes saturated more quickly now ( cooperativity ) ( positive homotrophic allosteric effector )
When first oxygen binds , it moves heme to be in plane with the iron
Normally , there is an amino acid that causes the Tense / Strain state
That causes the proximal histidine to move with the iron
The proximal histidine is attached to the F-helix
This moves the F-helix
- causes 15-degree rotation at interface between alpha and beta subunits
Deoxystate = 4 oxygens are closed off
Once 15 degree rotation happens , it moves to high affinity state
- Oxygen binding sites are more open and accessible
Proximal Histidine = works with
Distal Histidine = further stabilizes oxygen binding
Problem :
Could myoglobin transport
- No , myoglobin has to high of a binding affinity to oxygen for it to work as a transport protein
Problem :
A protein binds a ligand with associtation rate of
Describe the following and how they affect
- Bohr effect
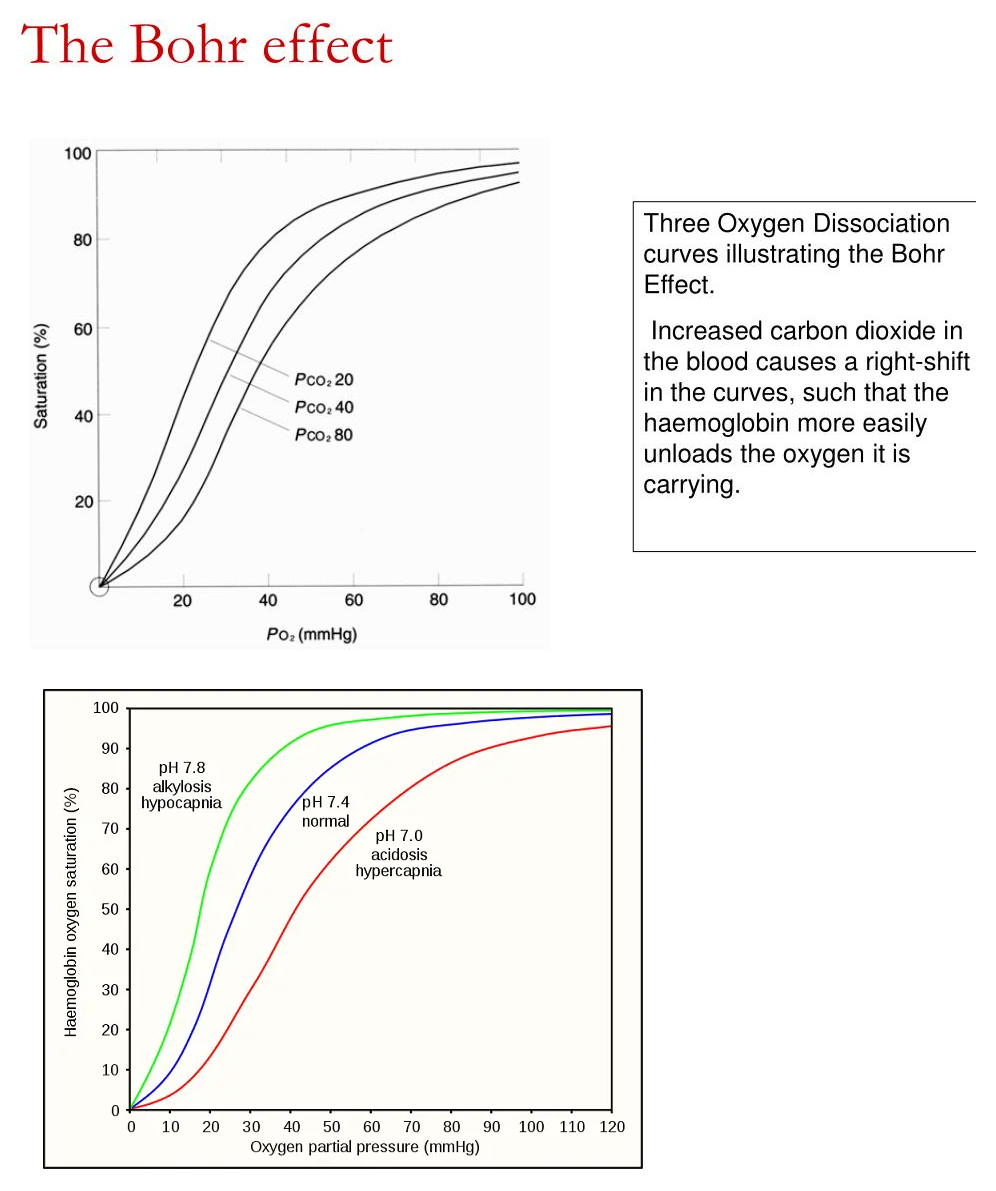
2,3-BPG
In the R-state , the positive charges can no longer interact with 2,3-BPG , causing 2,3-BPG to leave
2,3-BPG = negative allosteric effector , causes right shift , increases
Adult hemoglobin has positively charged histidine
- Binds 2,3-BPG
- Oxygen = positive homotrophic effector
- PH = negative heterotrophic
- 2,3-BPG = negative heterotrophic
Fetal hemoglobin
Fetal hemoglobin has the histidine swapped out for serine
Does not bind 2,3-BPG
- Left Shift
Sickle cell anemia
- When hemoglobin is in R state , the glutamate is not exposed
Conformational change from the T state to the R state involves breaking ion pairs between the
Acidic environment stabilizes the
- leads to the release of
- leads to the release of
Basic environment stabilizes the
Problem :
Hemoglobin S homozygotes who are severely anemic often have elevated levels of BPG in their red blood cells. Is this good or bad?
Bad , because even though the 2,3-BPG promotes more oxygen release in the tissues ,
- it promotes an even bigger problem of polymerization of the erythrocytes