24.2 DNA Supercoiling
How is cellular or viral DNA compacted into the cells or viral coats that contain it in a way that still permits access to the information in the DNA? The extreme compaction implies a high degree of structural organization. First, the many negative charges of the phosphoryl groups in the DNA backbone must be neutralized. Cations, particularly ions, and a class of molecules called polyamines provide multiple positive counterions to permit DNA compaction. Polyamines are derived from the amino acid ornithine (see Box 6-1). The second key to DNA compaction is a DNA structural alteration known as supercoiling. All cells maintain their DNA in a state that is underwound — having fewer right-handed helical turns per given length of DNA — than B-form DNA. The underwinding places structural strain on the DNA, causing it to twist upon itself. Supercoiling affects and is affected by processes such as replication and transcription. We introduce it here as a prelude to a broader discussion of DNA metabolism.
“Supercoiling” means the coiling of a coil. An old-fashioned telephone cord, for example, was typically a coiled wire. The path taken by the wire between the base of the phone and the receiver often included one or more supercoils (Fig. 24-8). DNA is coiled in the form of a double helix, with both strands of the DNA coiling around an axis. The further coiling of that axis upon itself (Fig. 24-9) produces DNA supercoiling. As detailed below, DNA supercoiling is generally a manifestation of structural strain. When there is no net bending of the DNA axis upon itself, the DNA is said to be in a relaxed state. As we shall see, the supercoiling that occurs in cells reflects underwinding of the DNA, facilitating the separation of strands required for the processes of replication and transcription (Fig. 24-10).
FIGURE 24-8 Supercoils. An old-fashioned phone cord is coiled like a DNA helix, and the coiled cord can itself coil in a supercoil. Examining phone cords helped lead Jerome Vinograd and his colleagues to the insight that many properties of small circular DNAs can be explained by supercoiling. They first detected DNA supercoiling — in small circular viral DNAs — in 1965.
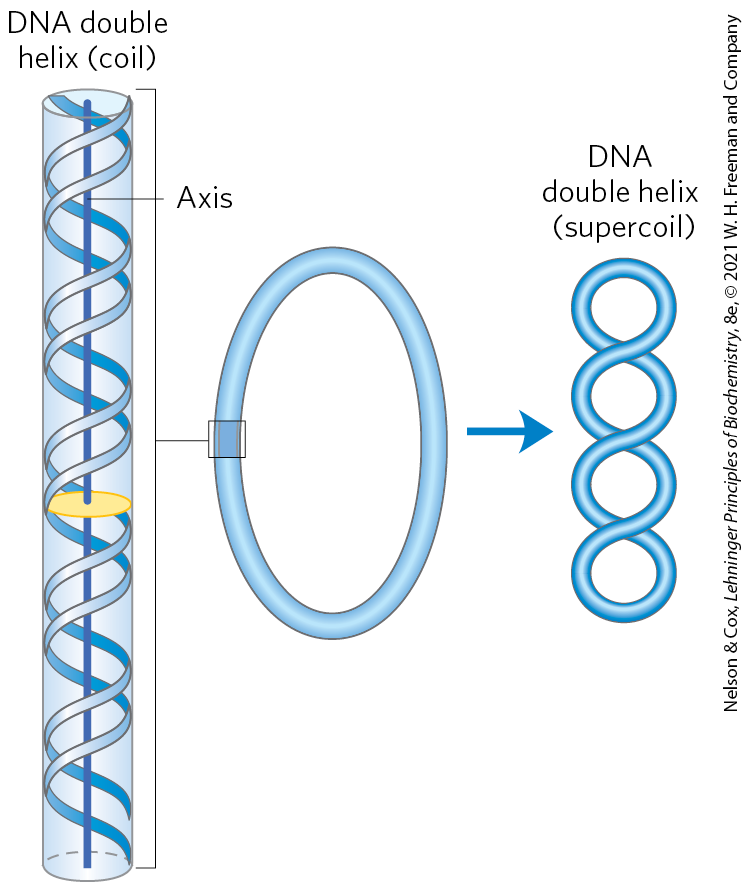
FIGURE 24-9 Supercoiling of DNA. When the axis of the DNA double helix is coiled on itself, it forms a new helix (superhelix). The DNA superhelix is usually called a supercoil.
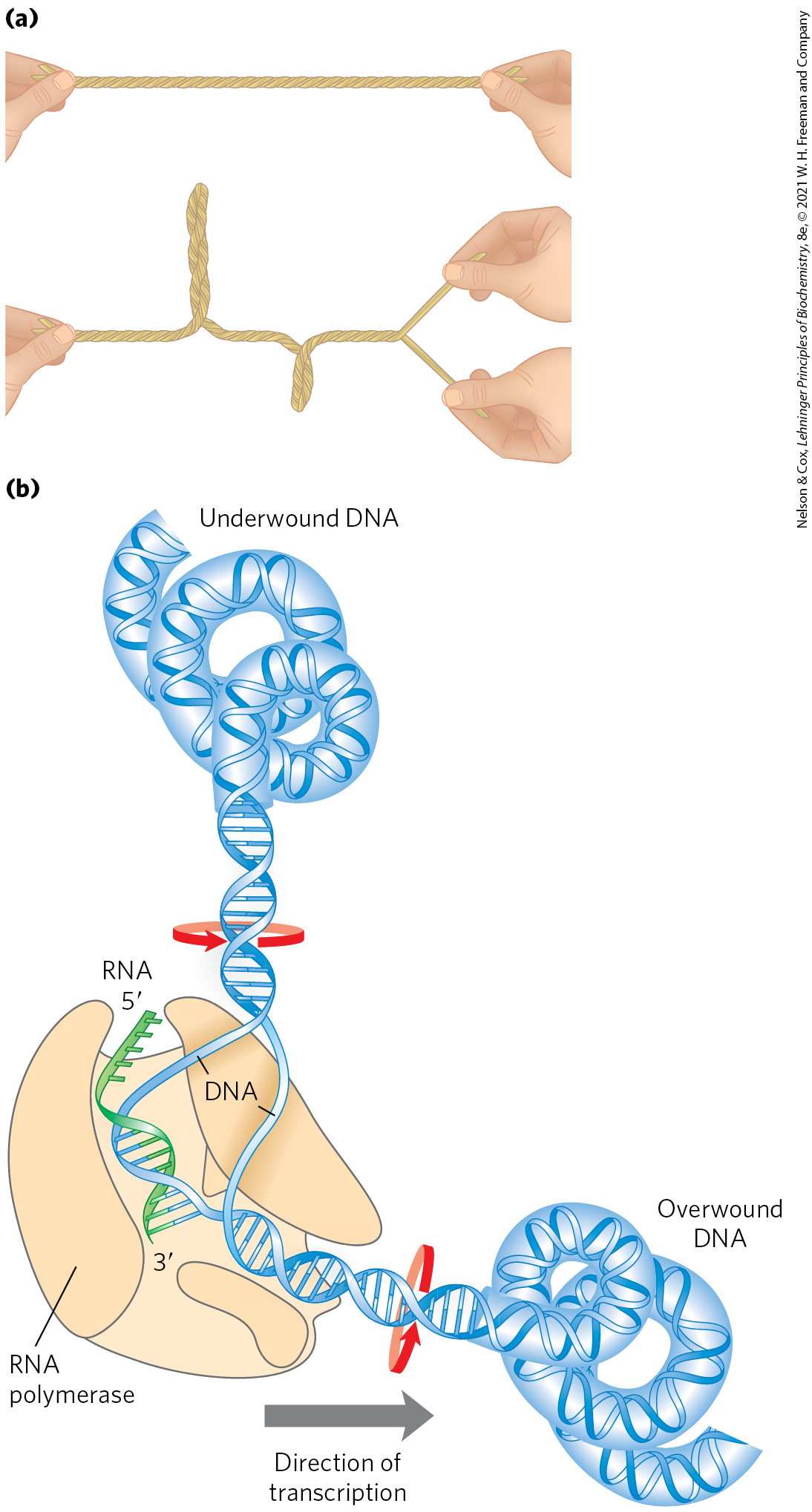
FIGURE 24-10 The effects of replication and transcription on DNA supercoiling. Because DNA is a double-helical structure, strand separation leads to added stress and supercoiling if the DNA is constrained (not free to rotate) ahead of the strand separation. (a) The general effect can be illustrated by twisting two strands of a rubber band about each other to form a double helix. If one end is constrained, separating the two strands at the other end will lead to twisting. (b) In a DNA molecule, the progress of a DNA polymerase or RNA polymerase (as shown here) along the DNA involves separation of the strands. As a result, the DNA becomes overwound ahead of the enzyme (upstream) and underwound behind it (downstream). Red arrows indicate the direction of winding.
That a DNA molecule would bend on itself and become supercoiled in tightly packaged cellular DNA would seem logical, and perhaps even trivial, were it not for one additional fact: many circular DNA molecules remain highly supercoiled even after they are extracted and purified, freed from protein and other cellular components. This indicates that supercoiling is an intrinsic property of DNA tertiary structure. It occurs in all cellular DNAs and is highly regulated by each cell.
Several measurable properties of supercoiling have been established, and the study of supercoiling has provided many insights into DNA structure and function. This work has drawn heavily on concepts derived from a branch of mathematics called topology, the study of the properties of an object that do not change under continuous deformations. For DNA, continuous deformations include conformational changes due to thermal motion or due to interaction with proteins or other molecules; discontinuous deformations involve DNA strand breakage. For circular DNA molecules, a topological property is one that is unaffected by deformations of the DNA strands as long as no breaks are introduced. Topological properties are changed only by breakage and rejoining of the backbone of one or both DNA strands.
We now examine the fundamental properties and physical basis of supercoiling.
Most Cellular DNA Is Underwound
To understand supercoiling, we must first focus on the properties of small circular DNAs such as plasmids and small viral DNAs. When these DNAs have no breaks in either strand, they are referred to as closed-circular DNAs. In aqueous solutions, DNA is most stable — that is, it is in its lowest free-energy form — in the B-form structure. If the DNA of a closed-circular molecule conforms closely to the B-form structure (Watson-Crick structure; see Fig. 8-13), with one turn of the double helix per 10.5 bp, the DNA is relaxed rather than supercoiled (Fig. 24-11). Supercoiling results when DNA is subject to some form of structural strain such that it is overwound or underwound. Purified closed-circular DNA is rarely relaxed, regardless of its biological origin. Furthermore, DNAs derived from a given cellular source have a characteristic degree of supercoiling. DNA structure is therefore strained in a manner that is regulated by the cell to induce the supercoiling.
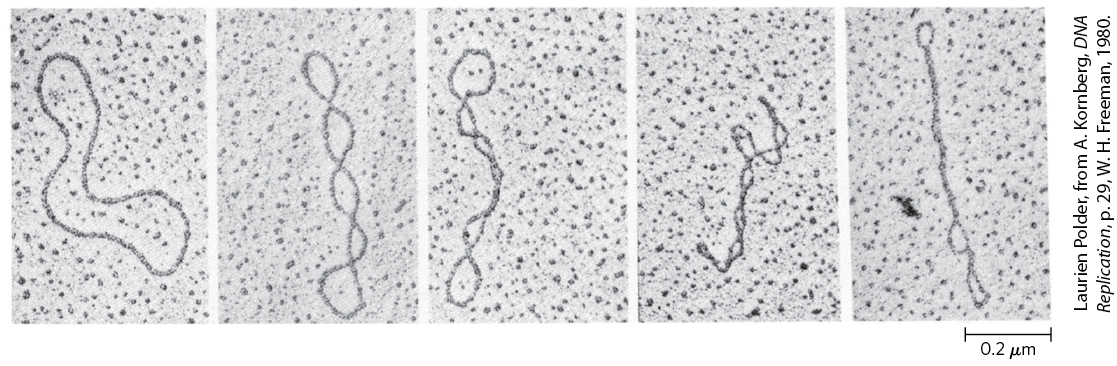
FIGURE 24-11 Relaxed and supercoiled plasmid DNAs. The molecule in the leftmost electron micrograph is relaxed; the degree of supercoiling increases from left to right.
In almost every instance, the strain is a result of underwinding of the DNA double helix in the closed circle. In other words, the DNA has fewer helical turns than would be expected for the B-form structure. The effects of underwinding are summarized in Figure 24-12. An 84 bp segment of a circular DNA in the relaxed state would contain eight double-helical turns, one for every 10.5 bp. If one of these turns were removed, there would be per turn, rather than the 10.5 found in B-DNA (Fig. 24-12b). This is a deviation from the most stable DNA form, and the molecule is thermodynamically strained as a result. Generally, much of this strain would be accommodated by coiling the axis of the DNA on itself to form a supercoil (Fig. 24-12c; some of the strain in this 84 bp segment would simply become dispersed in the untwisted structure of the larger DNA molecule). In principle, the strain could also be accommodated by separating the two DNA strands over a distance of about 10 bp (Fig. 24-12d). In isolated closed-circular DNA, strain introduced by underwinding is generally accommodated by supercoiling rather than strand separation, because coiling the axis of the DNA usually requires less energy than breaking the hydrogen bonds and disrupting the base stacking that stabilizes paired bases. Note, however, that the underwinding of DNA in vivo makes separation of the DNA strands easier, facilitating access to the information they contain.
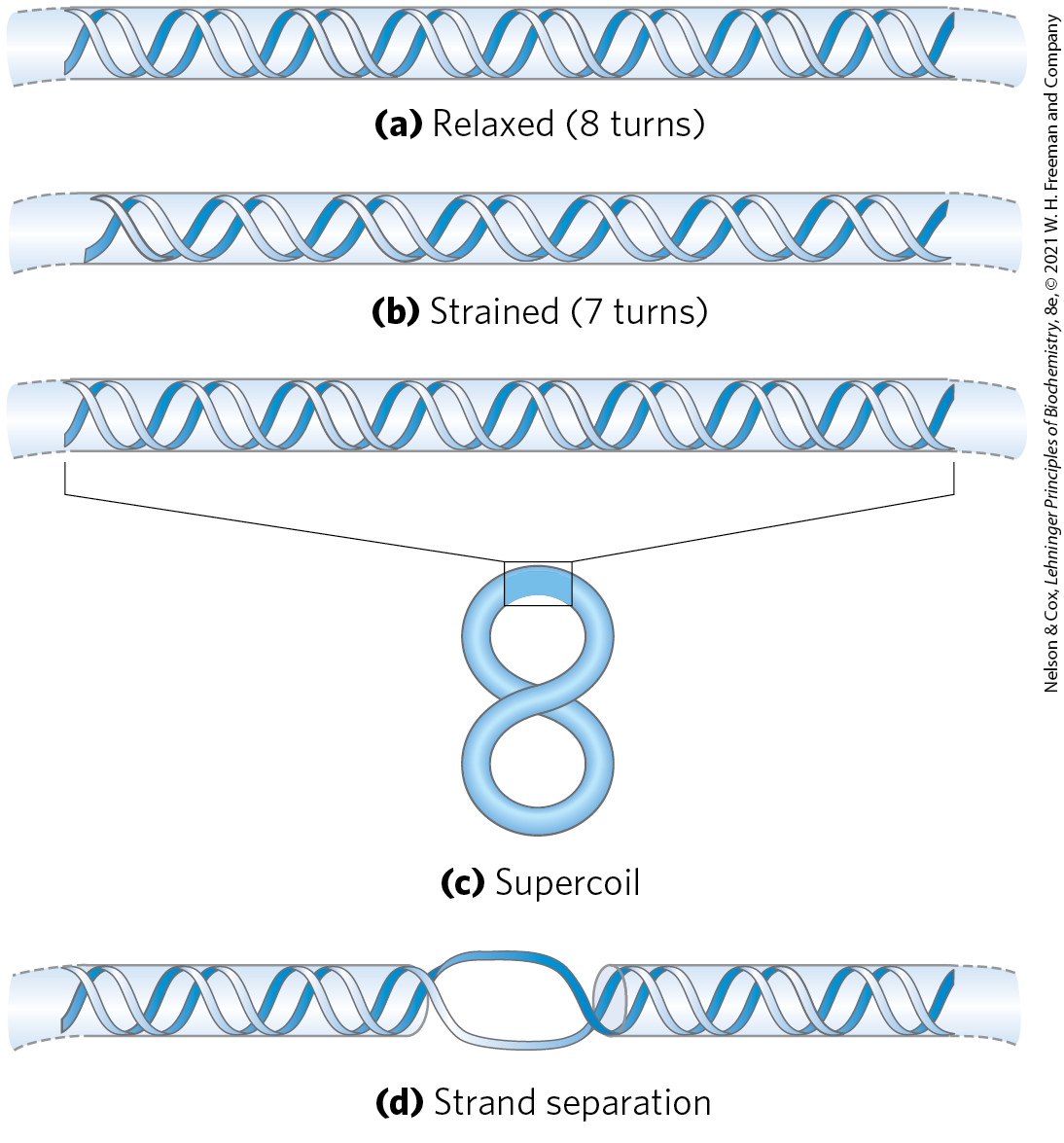
FIGURE 24-12 Effects of DNA underwinding. (a) A segment of DNA in a closed-circular molecule, 84 bp long, in its relaxed form with eight helical turns. (b) Removal of one turn induces structural strain. (c) The strain is generally accommodated by formation of a supercoil. (d) DNA underwinding also makes the separation of strands somewhat easier. In principle, each turn of underwinding should facilitate strand separation over about 10 bp, as shown here. However, the hydrogen-bonded base pairs would generally preclude strand separation over such a short distance, and the effect becomes important only for longer DNAs and higher levels of DNA underwinding.
Every cell actively underwinds its DNA with the aid of enzymatic processes (described below), and the resulting strained state represents a form of stored energy. Cells maintain DNA in an underwound state to facilitate its compaction by coiling. The underwinding of DNA is also important to enzymes of DNA metabolism that must bring about strand separation as part of their function.
The underwound state can be maintained only if the DNA is a closed circle or if it is bound and stabilized by proteins so that the strands are not free to rotate about each other. If there is a break in one strand of an isolated, protein-free circular DNA, free rotation at that point will cause the underwound DNA to revert spontaneously to the relaxed state. In a closed-circular DNA molecule, however, the number of helical turns cannot be changed without at least transiently breaking one of the DNA strands. The number of helical turns in a DNA molecule therefore provides a precise description of supercoiling.
DNA Underwinding Is Defined by Topological Linking Number
The field of topology provides some ideas that are useful to the discussion of DNA supercoiling, particularly the concept of linking number. Linking number is a topological property of double-stranded DNA, because it does not vary when the DNA is bent or deformed, as long as both DNA strands remain intact. Linking number (Lk) is illustrated in Figure 24-13. As we shall see, all cells have enzymes called topoisomerases that catalyze changes in the linking number. Because of this critical role, topoisomerases are the targets of many antibiotics and cancer chemotherapy agents.
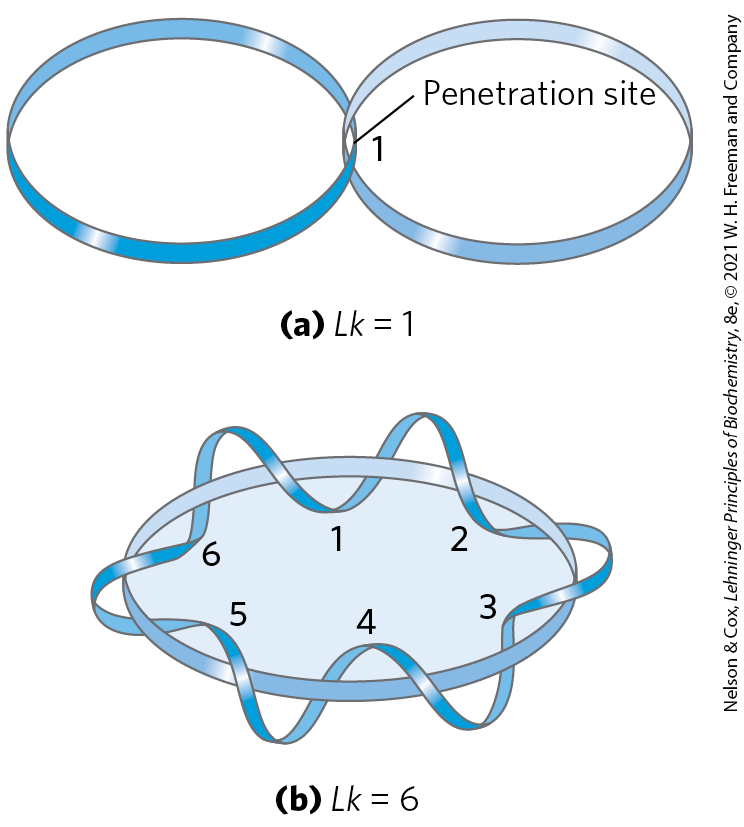
FIGURE 24-13 Linking number, Lk. Here, as usual, each blue ribbon represents one strand of a double-stranded DNA molecule. For the molecule in (a), . For the molecule in (b), . One of the strands in (b) is kept untwisted for illustrative purposes, to define the border of an imaginary surface (shaded blue). The number of times the twisting strand penetrates this surface provides a rigorous definition of linking number.
Let’s begin by visualizing the separation of the two strands of a double-stranded circular DNA. If the two strands are linked as shown in Figure 24-13a, they are effectively joined by what can be described as a topological bond. Even if all hydrogen bonds and base-stacking interactions were abolished such that the strands were not in physical contact, this topological bond would still link the two strands. Visualize one of the circular strands as the boundary of a surface (such as the soap film framed by the loop of a bubble wand before you blow a bubble). The linking number can be defined as the number of times the second strand pierces this surface. For the molecule in Figure 24-13a, ; for that in Figure 24-13b, . The linking number for a closed-circular DNA is always an integer. By convention, if the links between two DNA strands are arranged so that the strands are interwound in a right-handed helix, the linking number is defined as positive (+); for strands interwound in a left-handed helix, the linking number is negative (−). Negative linking numbers are, for all practical purposes, not encountered in DNA.
We can now extend these ideas to a closed-circular DNA with 2,100 bp (Fig. 24-14a). When the molecule is relaxed, the linking number is simply the number of base pairs divided by the number of base pairs per turn, which is close to 10.5; so in this case, . The linking number in relaxed DNA is designated . For a circular DNA molecule to have a topological property such as linking number, both strands must be intact, without a break. If there is a break in either strand, the two strands can, in principle, be unraveled and separated completely. In this case, no topological bond exists and Lk is undefined (Fig. 24-14b).
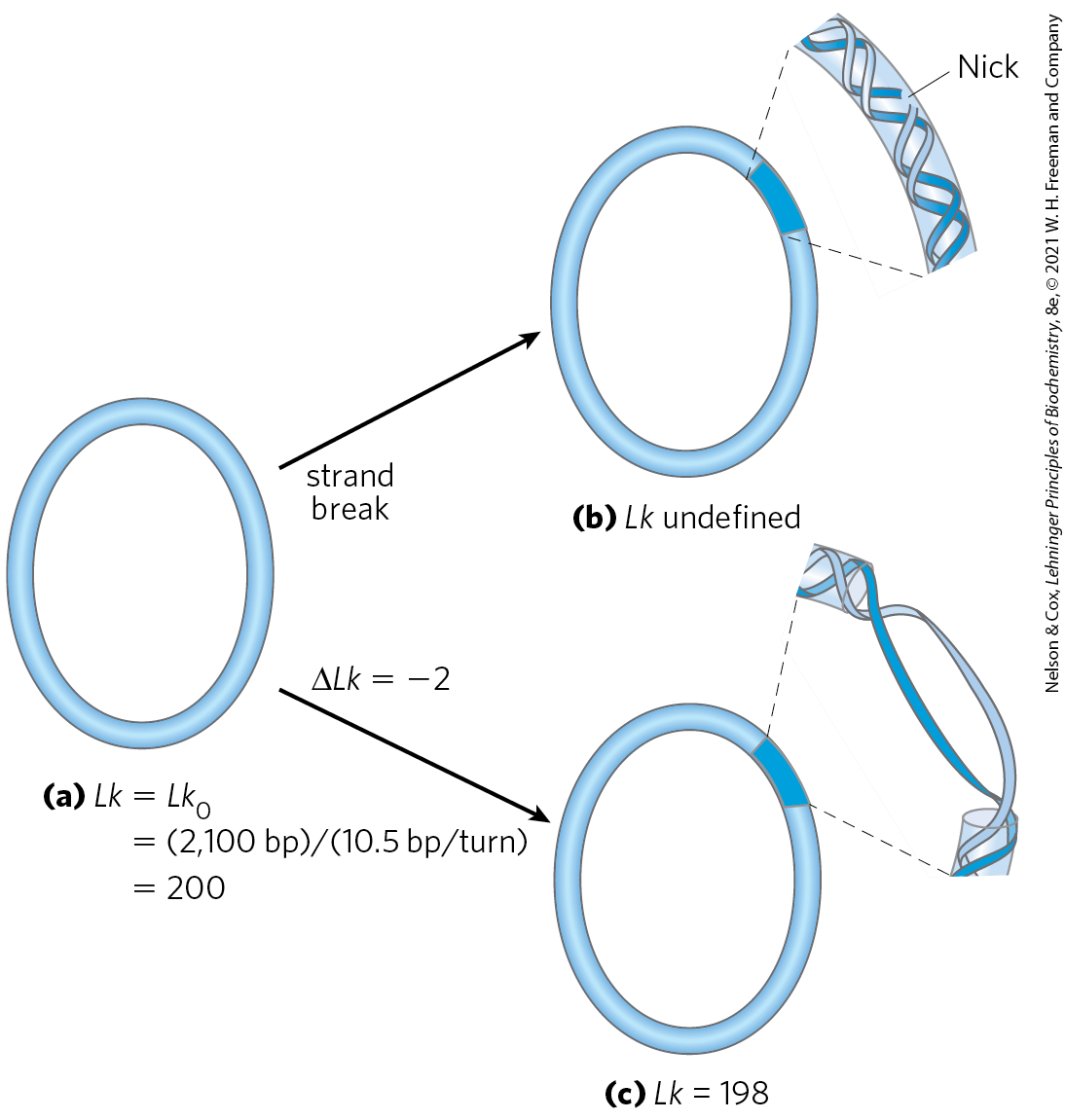
FIGURE 24-14 Linking number applied to closed-circular DNA molecules. A 2,100 bp circular DNA is shown in three forms: (a) relaxed, ; (b) relaxed with a nick (break) in one strand, Lk undefined; and (c) underwound by two turns, . The underwound molecule generally exists as a supercoiled molecule, but underwinding also facilitates the separation of DNA strands.
We can now describe DNA underwinding in terms of changes in the linking number. For the molecule shown in Figure 24-14a, ; if two turns are removed from this molecule, . The change can be described by the equation
(24-1)
It is often convenient to express the change in linking number in terms of a quantity that is independent of the length of the DNA molecule. This quantity, called the specific linking difference or superhelical density (σ), is a measure of the number of turns removed relative to the number present in relaxed DNA:
(24-2)
In the example in Figure 24-14c, , which means that 1% (2 of 200) of the helical turns present in the DNA (in its B form) have been removed. The degree of underwinding in cellular DNAs generally falls in the range of 5% to 7%; that is, to −0.07. The negative sign indicates that the change in linking number is due to underwinding of the DNA. The supercoiling induced by underwinding is therefore defined as negative supercoiling. Conversely, under some conditions DNA can be overwound, resulting in positive supercoiling. Note that the twisting path taken by the axis of the DNA helix when the DNA is underwound (negative supercoiling) is the mirror image of that taken when the DNA is overwound (positive supercoiling) (Fig. 24-15). Supercoiling is not a random process; the path of the supercoiling is largely prescribed by the torsional strain imparted to the DNA by decreasing or increasing the linking number relative to B-DNA. An increase in superhelical density brings about an increase in DNA compaction.
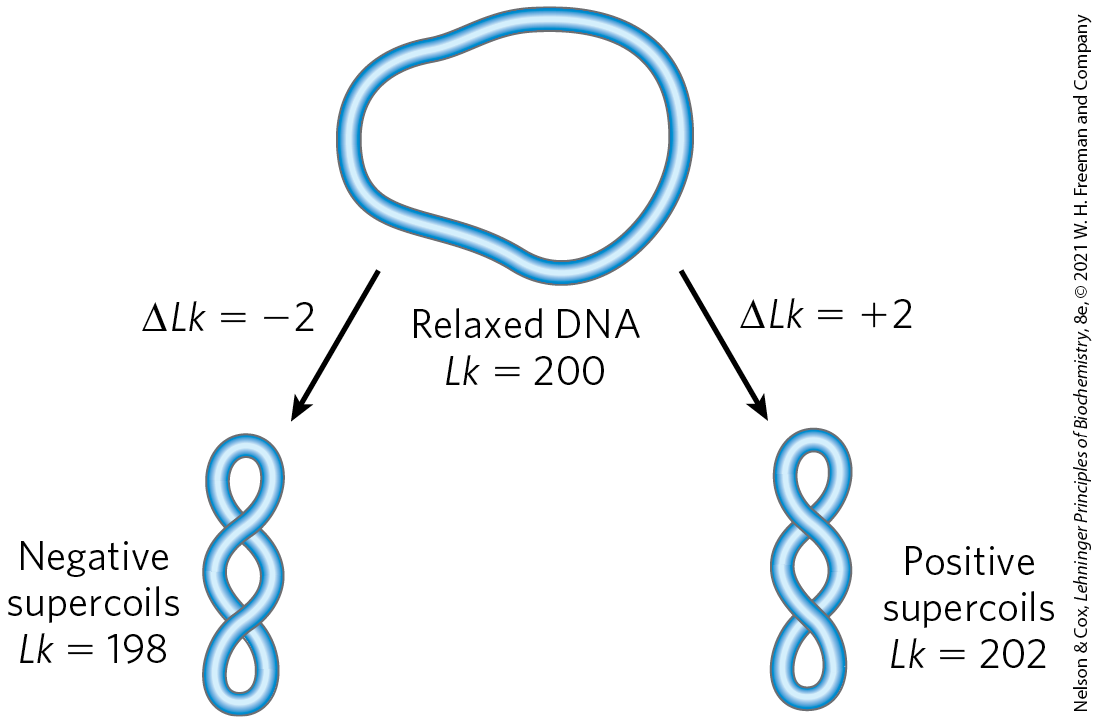
FIGURE 24-15 Negative and positive supercoils. For the relaxed DNA molecule of Figure 24-14a, underwinding or overwinding by two helical turns ( or 202) will produce negative or positive supercoiling, respectively. Notice that the DNA axis twists in opposite directions in the two cases.
Linking number can be changed by ±1 by breaking one DNA strand, rotating one of the ends 360° about the unbroken strand, and rejoining the broken ends. This change has no effect on the number of base pairs or the number of atoms in the circular DNA molecule. Two forms of a circular DNA that differ only in a topological property such as linking number are referred to as topoisomers.
WORKED EXAMPLE 24-1 Calculation of Superhelical Density
What is the superhelical density (σ) of a closed-circular DNA with a length of 4,200 bp and a linking number (Lk) of 374? What is the superhelical density of the same DNA when ? Are these molecules negatively or positively supercoiled?
SOLUTION:
First, calculate by dividing the length of the closed-circular DNA (in bp) by 10.5 bp/turn: . We can now calculate from Equation 24-1: . Substituting the values for and into Equation 24-2: . Because the superhelical density is negative, this DNA molecule is negatively supercoiled.
When the same DNA molecule has an Lk of 412, , and . The superhelical density is positive, and the molecule is positively supercoiled.
In addition to causing supercoiling and making strand separation somewhat easier, the underwinding of DNA facilitates structural changes in the molecule. These are of less physiological importance but they help illustrate the effects of underwinding. Recall that a cruciform (see Fig. 8-19) generally contains a few unpaired bases; DNA underwinding helps to maintain the required strand separation (Fig. 24-16). Underwinding of a right-handed DNA helix also enables the formation of short stretches of left-handed Z-DNA in regions where the base sequence is consistent with the Z form (see Chapter 8).
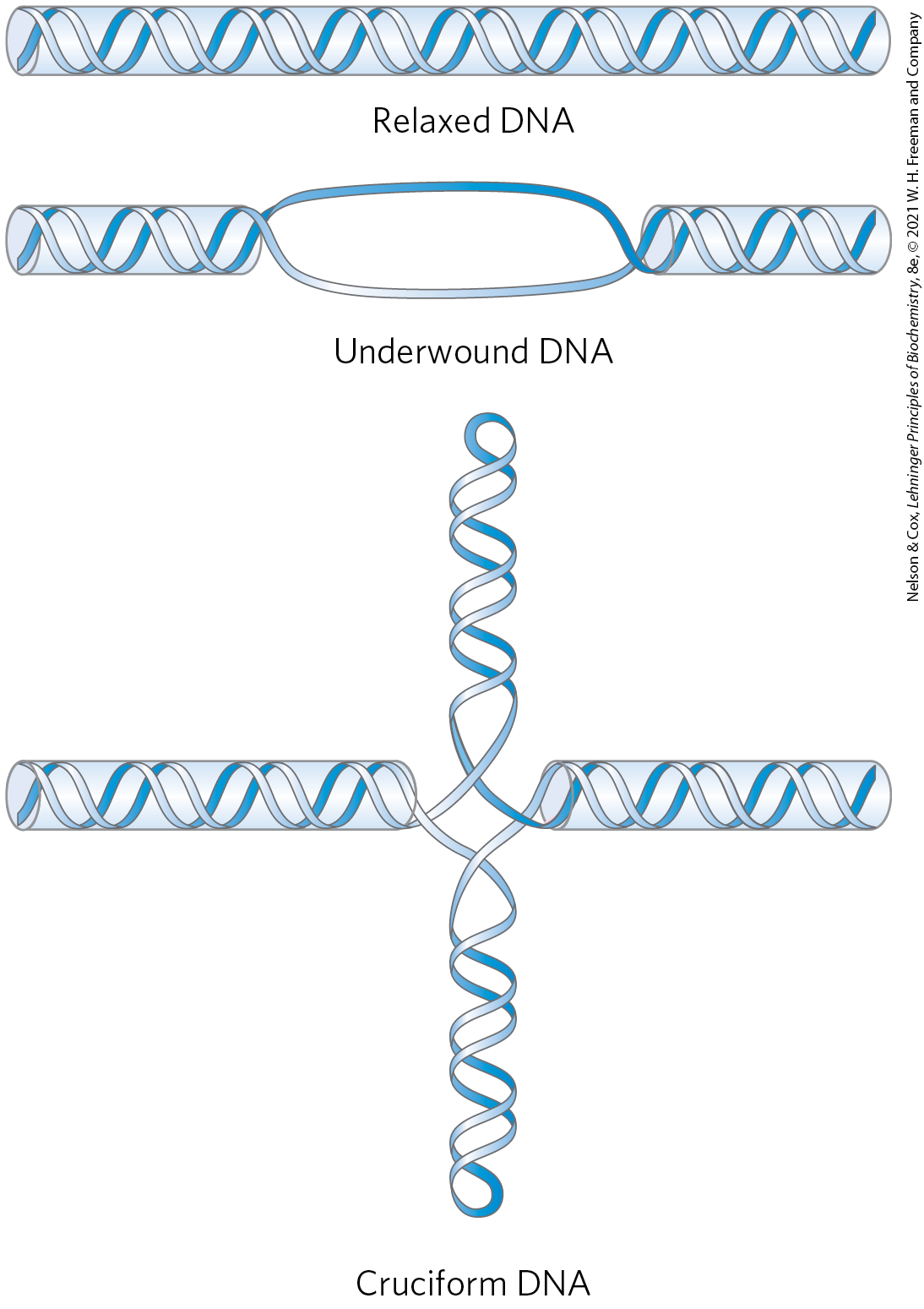
FIGURE 24-16 Promotion of cruciform structures by DNA underwinding. In principle, cruciforms can form at palindromic sequences (see Fig. 8-19), but they seldom occur in relaxed DNA because the linear DNA accommodates more paired bases than does the cruciform structure. Underwinding of the DNA facilitates the partial strand separation needed to promote cruciform formation at appropriate sequences.
Topoisomerases Catalyze Changes in the Linking Number of DNA
DNA supercoiling is a precisely regulated process that influences many aspects of DNA metabolism. Every cell has enzymes with the sole function of underwinding and/or relaxing DNA. The enzymes that increase or decrease the extent of DNA underwinding are topoisomerases; the property of DNA that they change is the linking number. These enzymes play an especially important role in processes such as replication and DNA packaging. There are two classes of topoisomerases. Type I topoisomerases act by transiently breaking one of the two DNA strands, passing the unbroken strand through the break, and rejoining the broken ends; they change Lk in increments of 1. Type II topoisomerases break both DNA strands and change Lk in increments of 2.
When a circular DNA is supercoiled, it is twisted upon itself and therefore is more compact than when it is relaxed. The supercoiled molecule will thus migrate faster in a gel matrix (Fig. 24-17). A population of identical plasmid DNAs with the same linking number migrates as a discrete band during agarose gel electrophoresis. Topoisomers with Lk values differing by as little as 1 can be separated by this method, so changes in linking number induced by topoisomerases are readily detected.
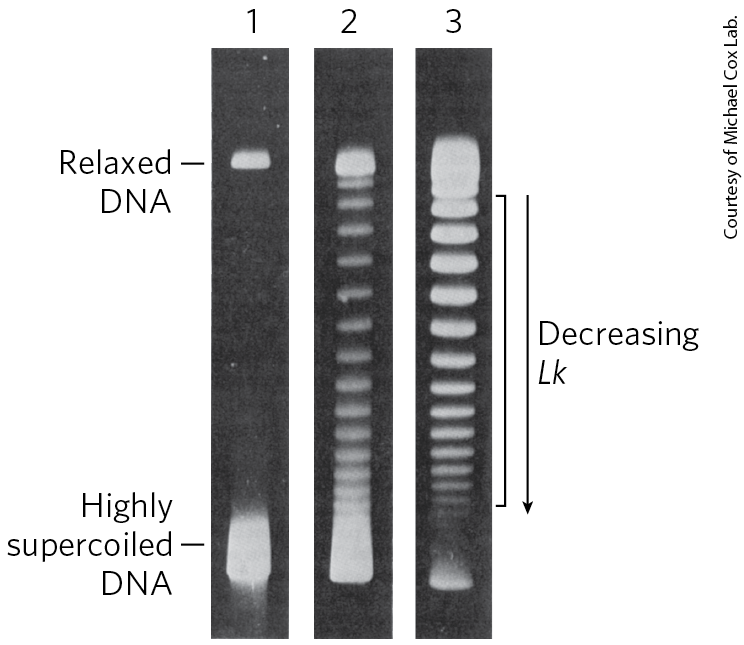
FIGURE 24-17 Visualization of topoisomers. In this experiment, all DNA molecules have the same number of base pairs but exhibit some range in the degree of supercoiling. Because supercoiled DNA molecules are more compact than relaxed molecules, they migrate more rapidly during gel electrophoresis. The gels shown here separate topoisomers (moving from top to bottom) over a limited range of superhelical density. In lane 1, highly supercoiled DNA migrates in a single band, even though different topoisomers are probably present. Lanes 2 and 3 illustrate the effect of treating the supercoiled DNA with a type I topoisomerase; the DNA in lane 3 was treated for a longer time than that in lane 2. As the superhelical density of the DNA is reduced to the point where it corresponds to the range in which the gel can resolve individual topoisomers, distinct bands appear. Individual bands in the region indicated by the bracket next to lane 3 each contain DNA circles with the same linking number; Lk changes by 1 from one band to the next.
E. coli has at least four individual topoisomerases (I through IV). Those of type I (topoisomerases I and III) generally relax DNA by removing negative supercoils (increasing Lk). The way in which bacterial type I topoisomerases change linking number is illustrated in Figure 24-18. A bacterial type II enzyme, called either topoisomerase II or DNA gyrase, can introduce negative supercoils (decrease Lk). It uses the energy of ATP to accomplish this. To alter DNA linking number, type II topoisomerases cleave both strands of a DNA molecule and pass another duplex through the break. The overall degree of supercoiling of bacterial DNA is maintained by regulation of the net activity of topoisomerases I and II. Topoisomerases III and IV have more-specialized roles in DNA metabolism.
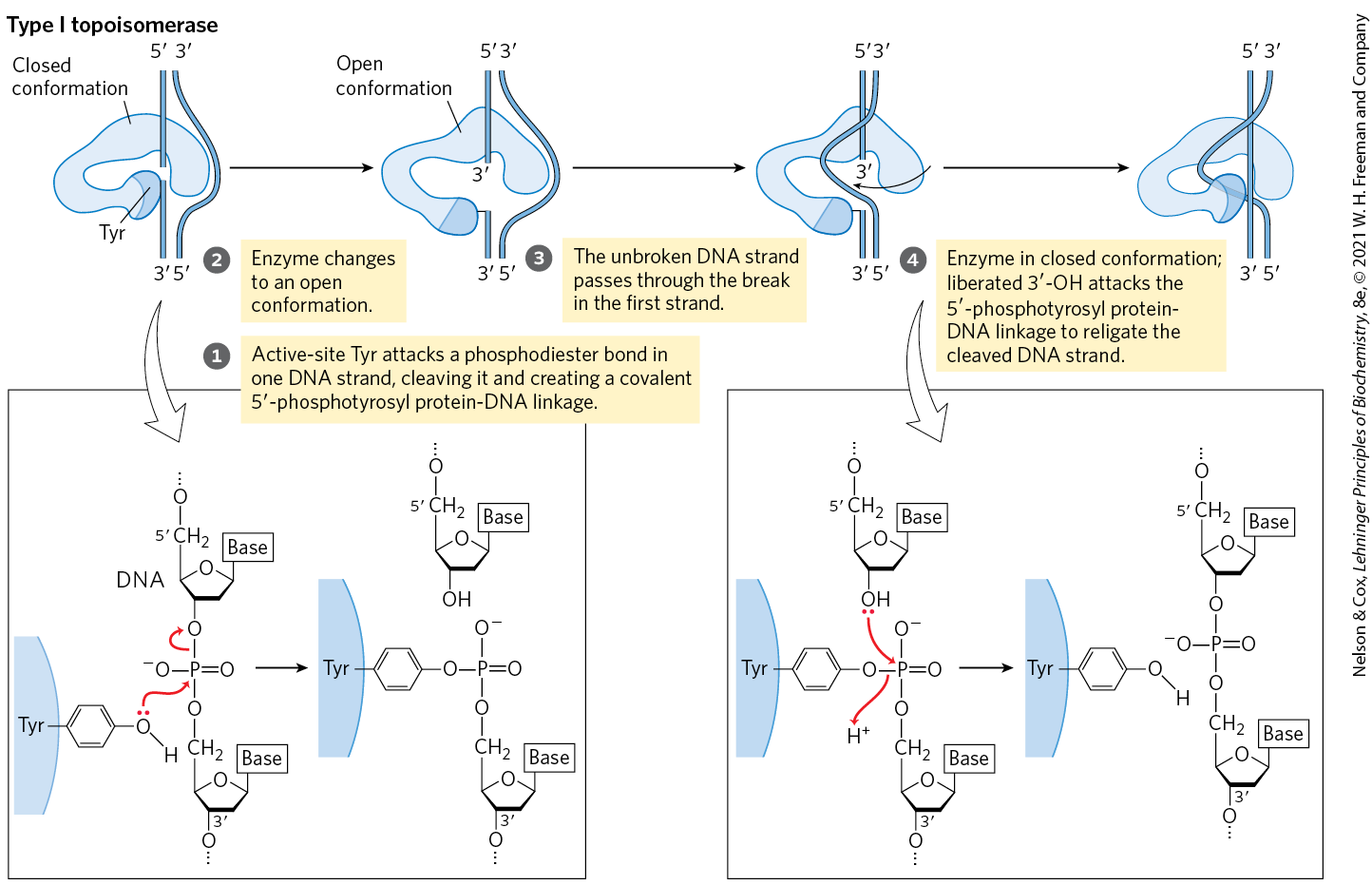
MECHANISM FIGURE 24-18 The type I topoisomerase reaction. Bacterial topoisomerase I increases Lk by breaking one DNA strand, passing the unbroken strand through the break, then resealing the break. Nucleophilic attack by the active-site Tyr residue breaks one DNA strand. The ends are ligated by a second nucleophilic attack. At each step, one high-energy bond replaces another. [Information from J. J. Champoux, Annu. Rev. Biochem. 70:369, 2001, Fig. 3.]
Eukaryotic cells also have type I and type II topoisomerases. The type I enzymes are topoisomerases I and III. The single type II enzyme has two isoforms in vertebrates, called IIα and IIβ. Most type II enzymes, including a DNA gyrase in archaea, are related and define a family called type IIA. The eukaryotic type II topoisomerases cannot underwind DNA (introduce negative supercoils), but they can relax both positive and negative supercoils (Fig. 24-19a). The capacity of type II topoisomerases to pass one duplex DNA segment through a double-strand break in another duplex allows these enzymes to untangle catenanes, DNA circles that are topologically linked (Fig. 24-19b). Some topoisomerases are specialized for decatenation functions. For example, the bacterial type II enzyme called topoisomerase IV is involved in chromosome untangling during cell division (Chapter 25).
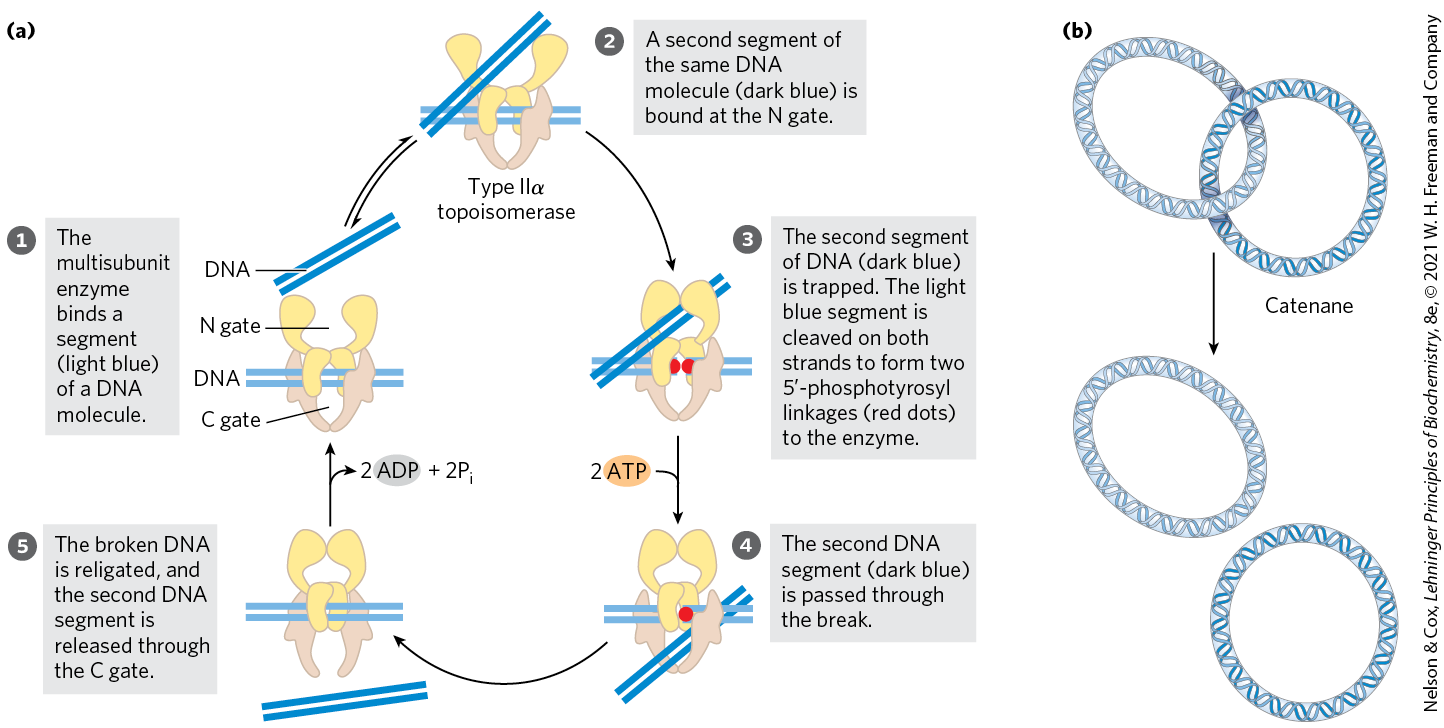
FIGURE 24-19 Alteration of the linking number by a eukaryotic type IIα topoisomerase. (a) The general mechanism features passage of one intact duplex DNA segment through a transient double-strand break in another segment. The DNA segment enters and leaves the topoisomerase through gated cavities, called the N gate and the C gate, above and below the bound DNA. Two ATPs are bound and hydrolyzed during this cycle. The enzyme structure and use of ATP are specific to this reaction. (b) When topologically linked as shown, two DNA circles are referred to as a catenane. By cleaving both strands of one circle and passing a segment of the second circle through the break, a type II topoisomerase can decatenate the circles. [(a) Information from J. J. Champoux, Annu. Rev. Biochem. 70:369, 2001, Fig. 11.]
Archaea have an unusual enzyme, topoisomerase VI, which alone defines the type IIB family. The full diversity of DNA topoisomerases is illustrated in Table 24-4. As we shall show in the next few chapters, topoisomerases play a critical role in every aspect of DNA metabolism, making them important drug targets for the treatment of bacterial infections and cancer.
| Type | Mechanism | Family (defined by structural class) | Domain(s) | Notes |
|---|---|---|---|---|
IA |
Strand passagea |
Topoisomerase I |
Bacteria, Eukarya |
Relaxes (−) |
Topoisomerase III |
Bacteria, Eukarya |
Relaxes (−) |
||
Reverse gyrase |
Archaea, Bacteria |
Uses ATP to introduce positive supercoils; thermophilic bacteria and archaea only |
||
IB |
Swivelaseb |
Topoisomerase IB |
Bacteria, Eukarya |
A few bacteria; all eukaryotes |
IC |
Swivelase |
Topoisomerase V |
Archaea |
Methanopyrus only |
IIA |
Strand passagec |
Topoisomerase II (DNA gyrase) |
Archaea, Bacteria |
Introduces negative supercoils (ATPase) |
Topoisomerase IIα |
Eukarya |
Relaxes (+ or −) |
||
Topoisomerase IIβ |
Eukarya |
Relaxes (+ or −) |
||
Topoisomerase IV |
Bacteria |
Decatenased |
||
IIB |
Strand passage |
Topoisomerase VI |
Archaea, Bacteria, Eukarya |
Among eukaryotes, plants, algae, and protists only |
aSee Figure 24-18. bA nick is made in one strand, and the other strand is allowed to rotate to relieve topological strain. cSee Figure 24-19a. dSee Figure 24-19b. |
||||
DNA Compaction Requires a Special Form of Supercoiling
Supercoiled DNA molecules are uniform in several respects. The supercoils are right-handed in a negatively supercoiled DNA molecule (Fig. 24-15), and they tend to be extended and narrow rather than compacted, often with multiple branches (Fig. 24-20). At the superhelical densities normally encountered in cells, the length of the supercoil axis, including branches, is about 40% of the length of the DNA. This type of supercoiling is referred to as plectonemic (from the Greek plektos, “twisted,” and nema, “thread”). The term can be applied to any structure with strands intertwined in some simple and regular way, and it is a good description of the general structure of supercoiled DNA in solution.
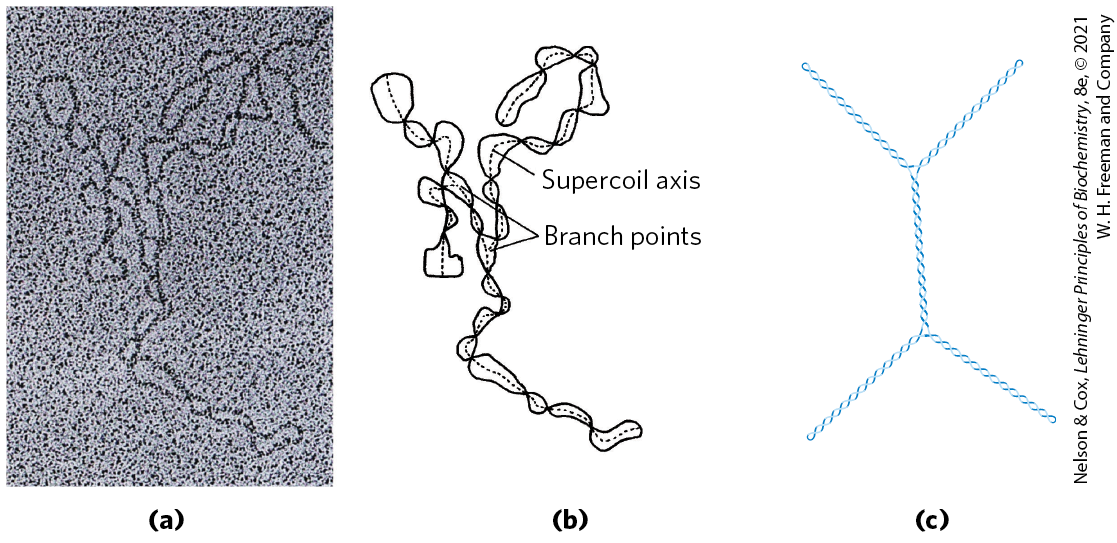
FIGURE 24-20 Plectonemic supercoiling. (a) Electron micrograph of plectonemically supercoiled plasmid DNA and (b) an interpretation of the observed structure. The dotted lines show the axis of the supercoil; notice the branching of the supercoil. (c) An idealized representation of this structure. [(a, b) Republished with permission of Elsevier, from T. C. Boles et al. (1990), “Structure of plectonemically supercoiled DNA,” J. Mol. Biol. 213:931–951, Fig. 2; permission conveyed through Copyright Clearance Center, Inc.]
Plectonemic supercoiling, the form observed in isolated DNAs in the laboratory, does not produce sufficient compaction to package DNA in the cell. A second form of supercoiling, solenoidal (Fig. 24-21), can be adopted by an underwound DNA. Instead of the extended right-handed supercoils characteristic of the plectonemic form, solenoidal supercoiling involves tight left-handed turns, similar to the shape taken up by a garden hose neatly wrapped on a reel. Although their structures are dramatically different, plectonemic and solenoidal supercoiling are two forms of negative supercoiling that can be taken up by the same segment of underwound DNA. The two forms are readily interconvertible. Although the plectonemic form is more stable in solution, the solenoidal form can be stabilized by protein binding, as it is in eukaryotic chromosomes; it provides a much greater degree of compaction. Solenoidal supercoiling is a primary mechanism by which underwinding contributes to DNA compaction in cells.
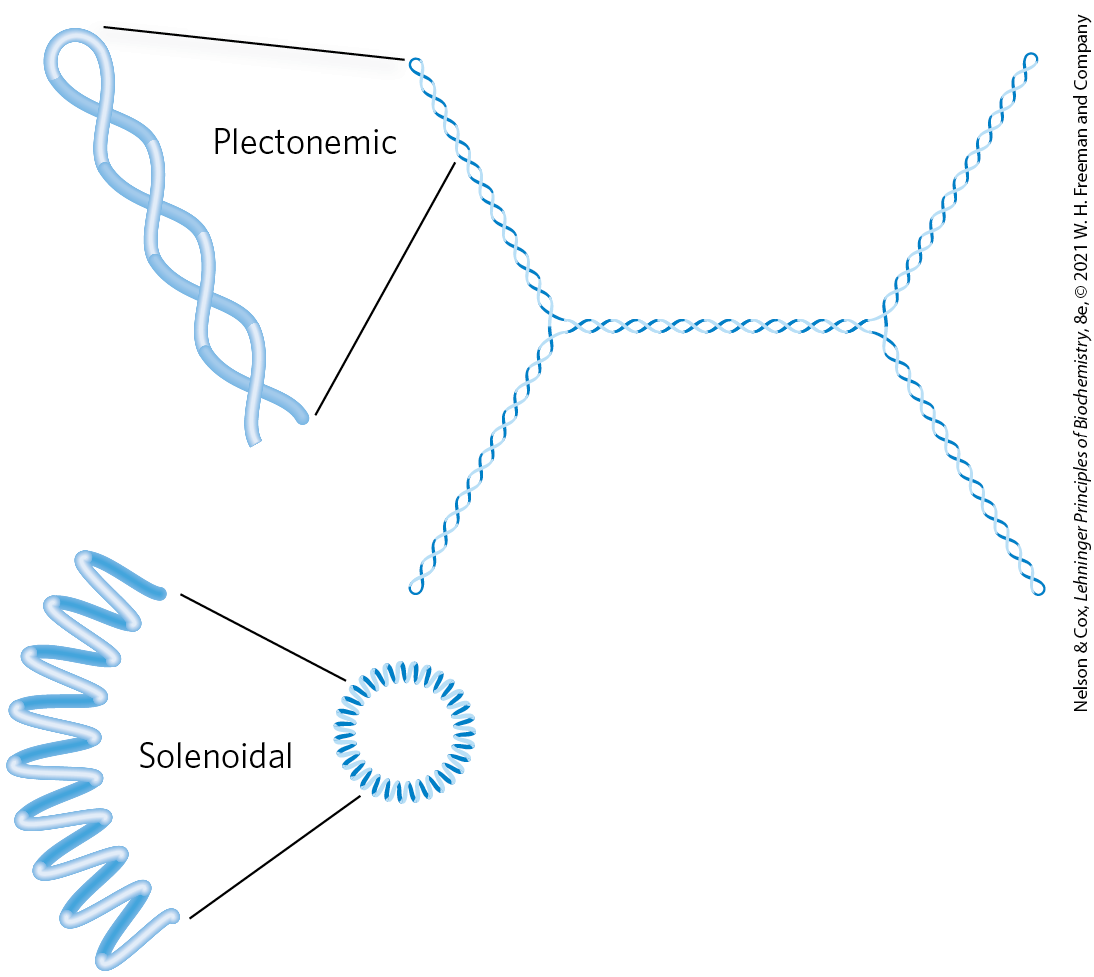
FIGURE 24-21 Plectonemic and solenoidal supercoiling of the same DNA molecule, drawn to scale. Plectonemic supercoiling takes the form of extended right-handed coils. Solenoidal negative supercoiling takes the form of tight left-handed turns about an imaginary tubelike structure. The two forms are readily interconverted, although the solenoidal form is generally not observed unless certain proteins are bound to the DNA. Solenoidal supercoiling provides a much greater degree of compaction.
SUMMARY 24.2 DNA Supercoiling
- Most cellular DNAs are supercoiled. Underwinding decreases the total number of helical turns in DNA relative to the relaxed, B form. To maintain an underwound state, DNA must be either a closed circle or bound to protein.
- Underwinding is quantified by a topological parameter called linking number, Lk. Underwinding is measured in terms of specific linking difference, or superhelical density, σ, which is . For cellular DNAs, σ is typically −0.05 to −0.07, which means that approximately 5% to 7% of the helical turns in the DNA have been removed. DNA underwinding facilitates strand separation by enzymes of DNA metabolism.
- DNAs that differ only in linking number are called topoisomers. Topoisomerases, enzymes that underwind and/or relax DNA, catalyze changes in linking number. The two classes of topoisomerases, type I and type II, change Lk in increments of 1 or 2, respectively, per catalytic event.
- Supercoiled DNA in solution, unconstrained by proteins, takes on a plectonemic supercoiling structure. When supercoiled DNA is wrapped around specialized DNA-binding proteins, it forms solenoidal supercoils.
 “Supercoiling” means the coiling of a coil. An old-fashioned telephone cord, for example, was typically a coiled wire. The path taken by the wire between the base of the phone and the receiver often included one or more supercoils (
“Supercoiling” means the coiling of a coil. An old-fashioned telephone cord, for example, was typically a coiled wire. The path taken by the wire between the base of the phone and the receiver often included one or more supercoils (
 Most cellular DNAs are supercoiled. Underwinding decreases the total number of helical turns in DNA relative to the relaxed, B form. To maintain an underwound state, DNA must be either a closed circle or bound to protein.
Most cellular DNAs are supercoiled. Underwinding decreases the total number of helical turns in DNA relative to the relaxed, B form. To maintain an underwound state, DNA must be either a closed circle or bound to protein.