22.3 Molecules Derived from Amino Acids
In addition to their role as the building blocks of proteins, amino acids are precursors of many specialized biomolecules, including hormones, coenzymes, nucleotides, alkaloids, cell wall polymers, porphyrins, antibiotics, pigments, and neurotransmitters. We describe here the pathways to a number of these amino acid derivatives.
Glycine Is a Precursor of Porphyrins
The biosynthesis of porphyrins, for which glycine is a major precursor, is our first example because of the central importance of the porphyrin nucleus in heme proteins such as hemoglobin and the cytochromes. The porphyrins are constructed from four molecules of the monopyrrole derivative porphobilinogen, which itself is derived from two molecules of δ-aminolevulinate. There are two major pathways to δ-aminolevulinate. In higher eukaryotes (Fig. 22-25a), glycine reacts with succinyl-CoA in the first step to yield α-amino-β-ketoadipate, which is then decarboxylated to δ-aminolevulinate. In plants, algae, and most bacteria, δ-aminolevulinate is formed from glutamate (Fig. 22-25b). The glutamate is first esterified to ; reduction by NADPH converts the glutamate to glutamate 1-semialdehyde, which is cleaved from the tRNA. An aminotransferase converts the glutamate 1-semialdehyde to δ-aminolevulinate.
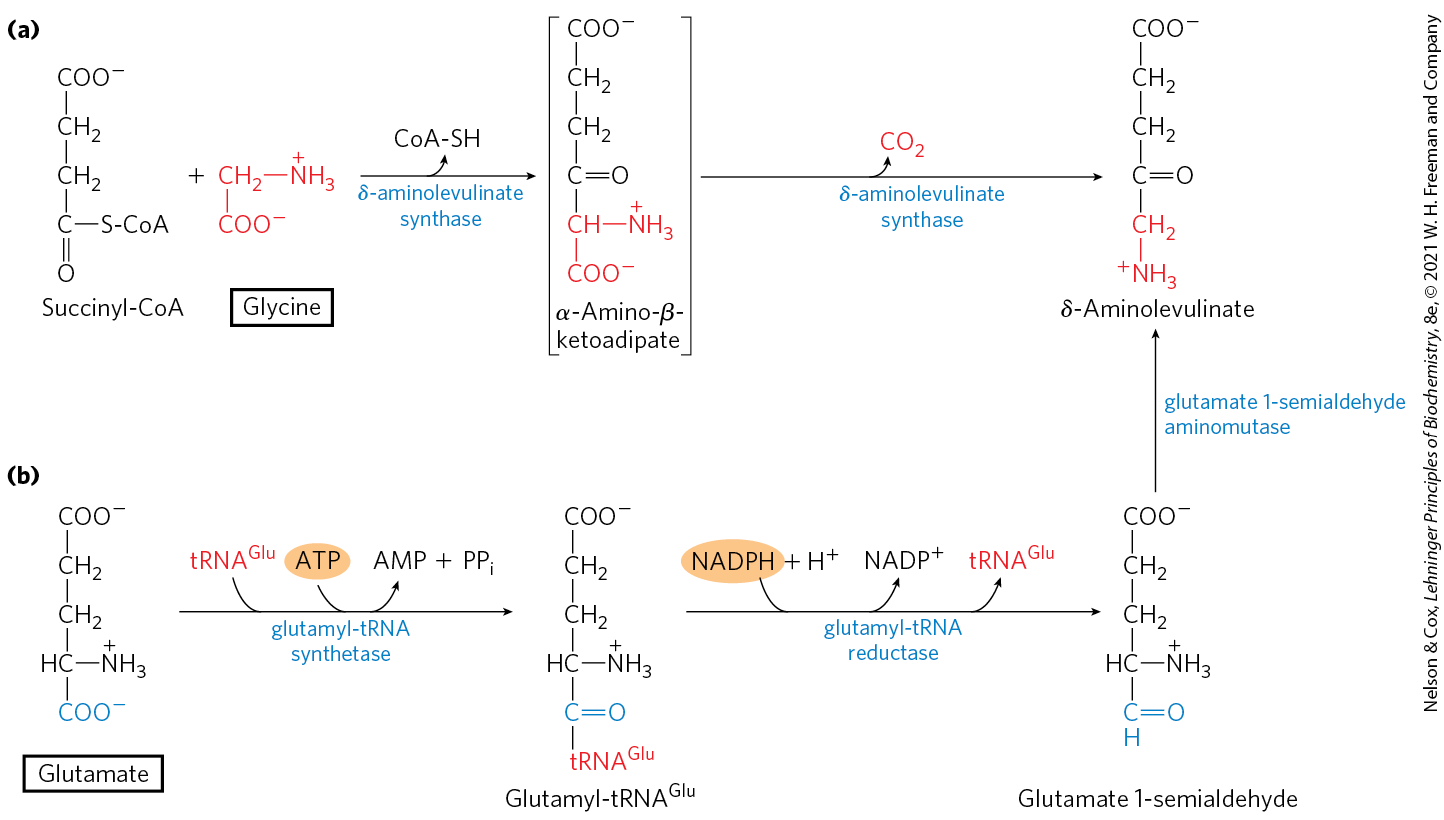
FIGURE 22-25 Biosynthesis of δ-aminolevulinate. (a) In most animals, including mammals, δ-aminolevulinate is synthesized from glycine and succinyl-CoA. The atoms furnished by glycine are shown in red. (b) In bacteria and plants, the precursor of δ-aminolevulinate is glutamate.
In all organisms, two molecules of δ-aminolevulinate condense to form porphobilinogen and, through a series of complex enzymatic reactions, four molecules of porphobilinogen come together to form protoporphyrin (Fig. 22-26). The iron atom is incorporated after the protoporphyrin has been assembled, in a step catalyzed by ferrochelatase. Porphyrin biosynthesis is regulated in higher eukaryotes by heme, which serves as a feedback inhibitor of early steps in the synthetic pathway. Genetic defects in the biosynthesis of porphyrins can lead to the accumulation of pathway intermediates, causing a variety of human diseases known collectively as porphyrias (Box 22-2).
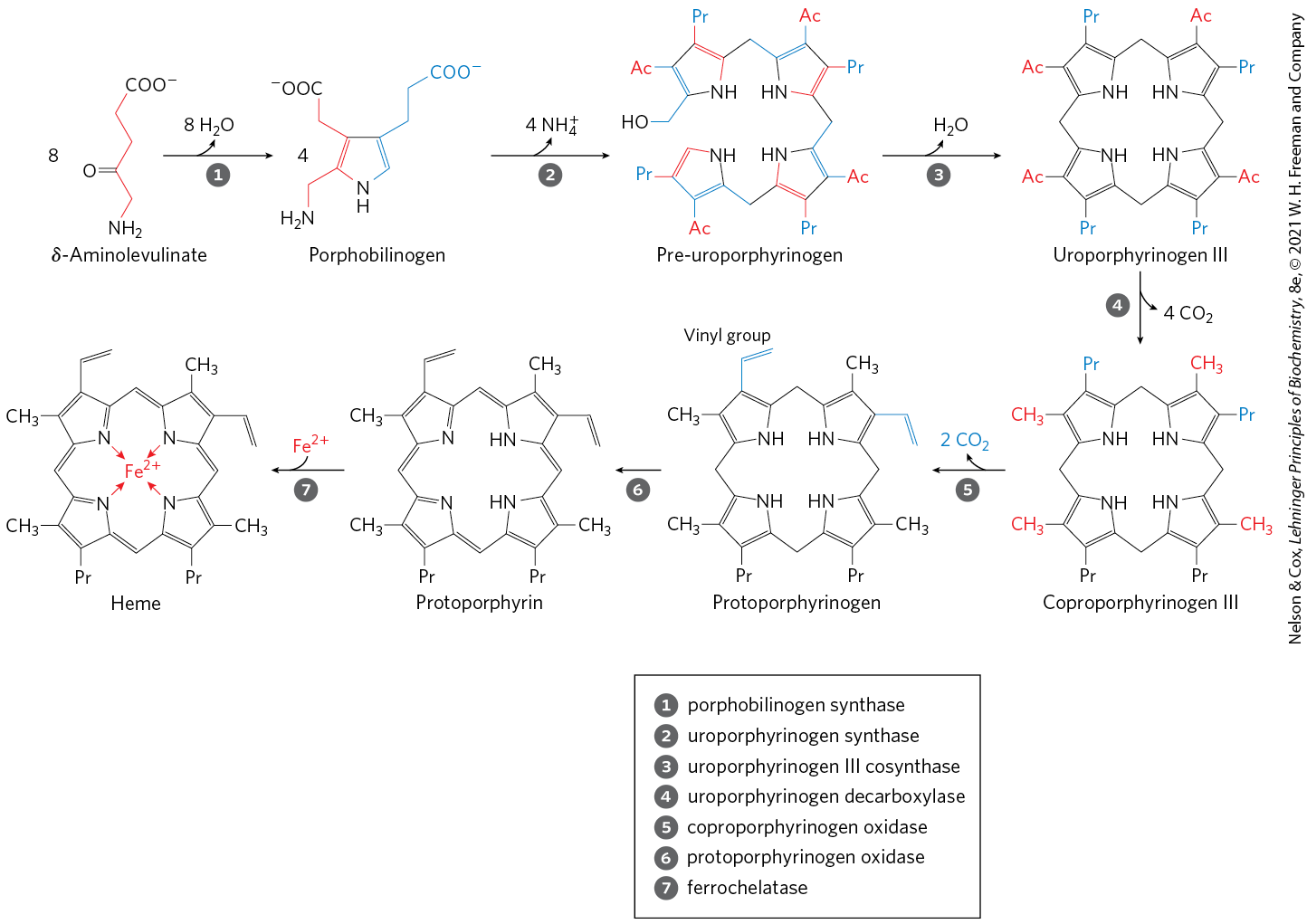
FIGURE 22-26 Biosynthesis of heme from δ-aminolevulinate. Ac represents acetyl ; Pr, propionyl .
Heme Degradation Has Multiple Functions
The iron-porphyrin (heme) group of hemoglobin, released from dying erythrocytes in the spleen, is degraded to yield free and, ultimately, bilirubin. The pathway also contributes the pigment present in mixtures of the bile salts derived from cholesterol.
The first step in the two-step pathway to bilirubin, catalyzed by heme oxygenase, converts heme to biliverdin, a linear (open) tetrapyrrole derivative (Fig. 22-27 on p. 820). The other products of the reaction are free and CO. The is quickly bound by ferritin. Carbon monoxide is a poison that binds to hemoglobin (see Box 5-1), and the production of CO by heme oxygenase ensures that, even in the absence of environmental exposure, about 1% of an individual’s heme is complexed with CO.
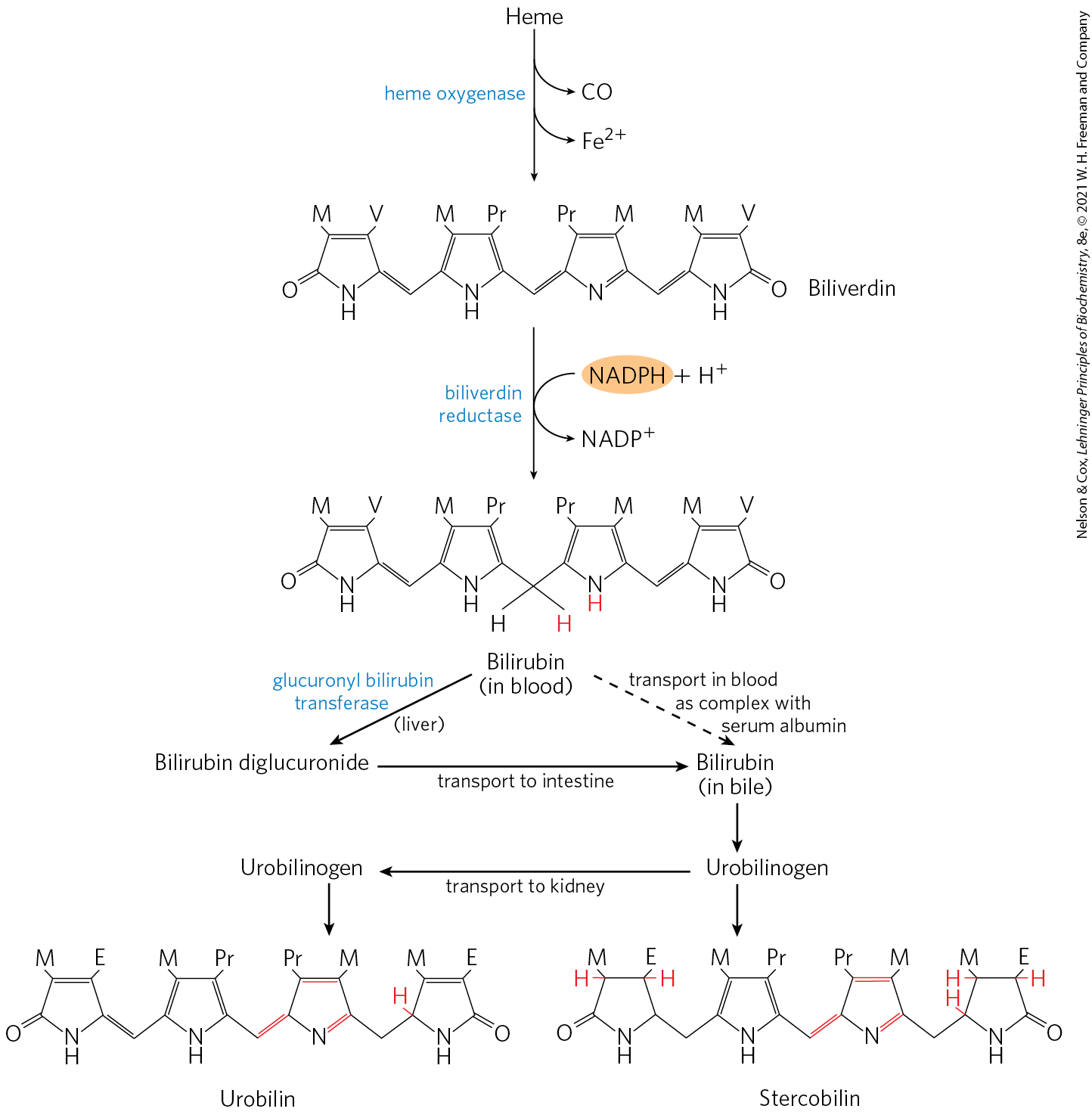
FIGURE 22-27 Bilirubin and its breakdown products. M represents methyl; V, vinyl; Pr, propionyl; E, ethyl. For ease of comparison, these structures are shown in linear form, rather than in their correct stereochemical conformations.
An arrow labeled heme oxygenase points down from heme and is accompanied by an arrow that branches to show the loss of C O above a second arrow that branches to show the loss of F e 2 plus. This yields biliverdin, which is a chain of four-five-membered rings. The left-hand ring has N at its bottom vertex substituted for C and further bonded to H, a lower left vertex double bonded to O, a double bond at its top vertex, an upper left vertex bonded to M, an upper right vertex bonded to V, and a lower right vertex with a double bond to the lower right from which a bond extends up to the lower left vertex of the second ring. In addition to the bond from its lower left vertex, the second ring has N substituted for C at its bottom vertex and bonded to H, double bonds at its upper left and right sides, an upper left vertex bonded to M, an upper right vertex bonded to P r, and a lower right vertex that extends to the lower right to meet a double bond that extends up to the lower left vertex of the third ring. In addition to the bond from its lower left vertex, the third ring has N substituted for C at its bottom vertex, double bonds at its top and lower right sides, an upper left vertex bonded to P r, an upper right vertex bonded to M, and a lower right vertex that extends to the lower right to meet a double bond that extends up to the lower left vertex of the fourth ring. In addition to the bond from its lower left vertex, the fourth ring has N substituted for C at its bottom vertex and bonded to H, a double ring at its top side, an upper left vertex bonded to M, an upper right vertex bonded to V, and a lower right vertex double bonded to O. An arrow labeled biliverdin reductase points down to bilirubin (in blood) below and is accompanied by a curved arrow showing the addition of an orange oval labeled N A D P H plus H plus and loss of N A D P plus. Bilirubin is a similar structure in which the lower right vertex of the second ring has a bonded that extends down to C to the lower left that is bonded to H to the lower left and red highlighted H to the lower right and that has a bond that extends up to the lower left vertex of the third ring. Additionally, the third ring has N substituted for C at the bottom vertex that is now bonded to a red highlighted H. Two arrows point down from bilirubin (in blood). An arrow labeled glucuronyl bilirubin transferase (liver) points to bilirubin diglucuronide at the lower left. A dashed arrow labeled transport in blood as complex with serum albumin points to bilirubin (in bile) to the lower right. An arrow labeled transport to intestine points from bilirubin diglucuronide on the left to bilirubin (in bile) on the right. An arrow points down from bilirubin (in bile) to urobilinogen below. An arrow points down from urobilinogen to stercobilin, which is also a chain of four five-membered rings. The left-hand ring has N at its bottom vertex substituted for C and further bonded to H, a lower left vertex double bonded to O, an upper left vertex connected by a red bond to H and bonded to M e, an upper right vertex connected by a red bond to H and bonded to H, and a lower right vertex with a bond to the lower right from which a bond extends up to the lower left vertex of the second ring. In addition to the bond from its lower left vertex, the second ring has N substituted for C at its bottom vertex and bonded to H, double bonds at its upper left and right sides, an upper left vertex bonded to M, an upper right vertex bonded to P r, and a lower right vertex that extends to the lower right to meet a red double bond that extends up to the lower left vertex of the third ring. In addition to the bond from its lower left vertex, the third ring has N substituted for C at its bottom vertex, red highlighted double bonds at its top and lower right sides, an upper left vertex bonded to P r, an upper right vertex bonded to M, and a lower right vertex that extends to the lower right to meet a bond that extends up to the lower left vertex of the fourth ring. In addition to this bond, the lower left vertex of the fourth ring has a red bond to H. Additionally, the fourth ring has N substituted for C at its bottom vertex and bonded to H, a double ring at its top side, an upper left vertex with a red bond to H and bonded to M, an upper right vertex with a red bond to H and bonded to E, and a lower right vertex double bonded to O. An arrow labeled transport to kidney points left from urobilinogen on the right to urobilinogen on the left, from which an arrow points down to urobilin. This is another chain of four five-membered rings. The left-hand ring has N at its bottom vertex substituted for C and further bonded to H, a lower left vertex double bonded to O, a double bond at its top vertex, an upper left vertex bonded to M, an upper right vertex bonded to E, and a lower right vertex with a double bond to the lower right from which a bond extends up to the lower left vertex of the second ring. In addition to the bond from its lower left vertex, the second ring has N substituted for C at its bottom vertex and bonded to H, double bonds at its upper left and right sides, an upper left vertex bonded to M, an upper right vertex bonded to P r, and a lower right vertex that extends to the lower right to meet a red double bond that extends up to the lower left vertex of the third ring. In addition to the bond from its lower left vertex, the third ring has N substituted for C at its bottom vertex, red double bonds at its top and lower right sides, an upper left vertex bonded to P r, an upper right vertex bonded to M, and a lower right vertex that extends to the lower right to meet a bond that extends up to the lower left vertex of the fourth ring. The lower left vertex of the fourth ring also has a red bond to red highlighted H. In addition to the bonds from its lower left vertex, the fourth ring has N substituted for C at its bottom vertex and bonded to H, a double ring at its top side, an upper left vertex bonded to M, an upper right vertex bonded to V, and a lower right vertex double bonded to O.
Biliverdin is converted to bilirubin in the second step, catalyzed by biliverdin reductase. You can monitor the reactions in the breakdown of heme (Fig. 22-27) colorimetrically in a familiar in situ experiment. When you are bruised, the black and/or purple color results from hemoglobin released from damaged erythrocytes. Over time, the color changes to the green of biliverdin, and then to the yellow of bilirubin. Bilirubin is largely insoluble, and it travels in the bloodstream as one of many metabolites, fatty acids and others, complexed with serum albumin (see Fig. 17-3). In the liver, bilirubin is transformed to the bile pigment bilirubin diglucuronide. This product is sufficiently water-soluble to be secreted with other components of bile into the small intestine, where microbial enzymes convert it to several products, predominantly urobilinogen. Some urobilinogen is reabsorbed into the blood and transported to the kidney, where it is converted to urobilin, the compound that gives urine its yellow color. Urobilinogen remaining in the intestine is converted (in another microbe-dependent reaction) to stercobilin, which imparts the red-brown color to feces.
Impaired liver function or blocked bile secretion causes bilirubin to leak from the liver into the blood, resulting in a yellowing of the skin and eyeballs, a condition called jaundice. In cases of jaundice, determination of the concentration of bilirubin in the blood may be useful in the diagnosis of underlying liver disease. Newborn infants sometimes develop jaundice because they have not yet produced enough glucuronyl bilirubin transferase to process their bilirubin. A traditional treatment to reduce excess bilirubin, exposure to a fluorescent lamp, causes a photochemical conversion of bilirubin to compounds that are more soluble and easily excreted.
These pathways of heme breakdown play significant roles in protecting cells from oxidative damage and in regulating certain cellular functions. The CO produced by heme oxygenase is toxic at high concentrations, but at the very low concentrations generated during heme degradation it seems to have some regulatory and/or signaling functions. It acts as a vasodilator, much the same as (but less potent than) nitric oxide (discussed below). Low levels of CO also have some regulatory effects on neurotransmission. Bilirubin is the most abundant antioxidant in mammalian tissues and is responsible for most of the antioxidant activity in serum. Its protective effects seem to be especially important in the developing brain of newborn infants. The cell toxicity associated with jaundice may be due to bilirubin levels in excess of the serum albumin needed to solubilize it.
Given these varied roles of heme degradation products, the degradative pathway is subject to regulation, mainly at the first step. Humans have at least three isozymes of heme oxygenase (HO). HO-1 is highly regulated; the expression of its gene is induced by a wide range of stress conditions, including shear stress, uncontrolled angiogenesis (development of blood vessels), hypoxia, hyperoxia, heat shock, exposure to ultraviolet light, hydrogen peroxide, and many other metabolic insults. HO-2 is found mainly in the brain and the testes, where it is continuously expressed. The third isozyme, HO-3, is not catalytically active, but may play a role in oxygen sensing.
Amino Acids Are Precursors of Creatine and Glutathione
Phosphocreatine, derived from creatine, is an important energy buffer in skeletal muscle (see Box 23-1). Creatine is synthesized from glycine and arginine (Fig. 22-28); methionine, in the form of S-adenosylmethionine, acts as methyl group donor.
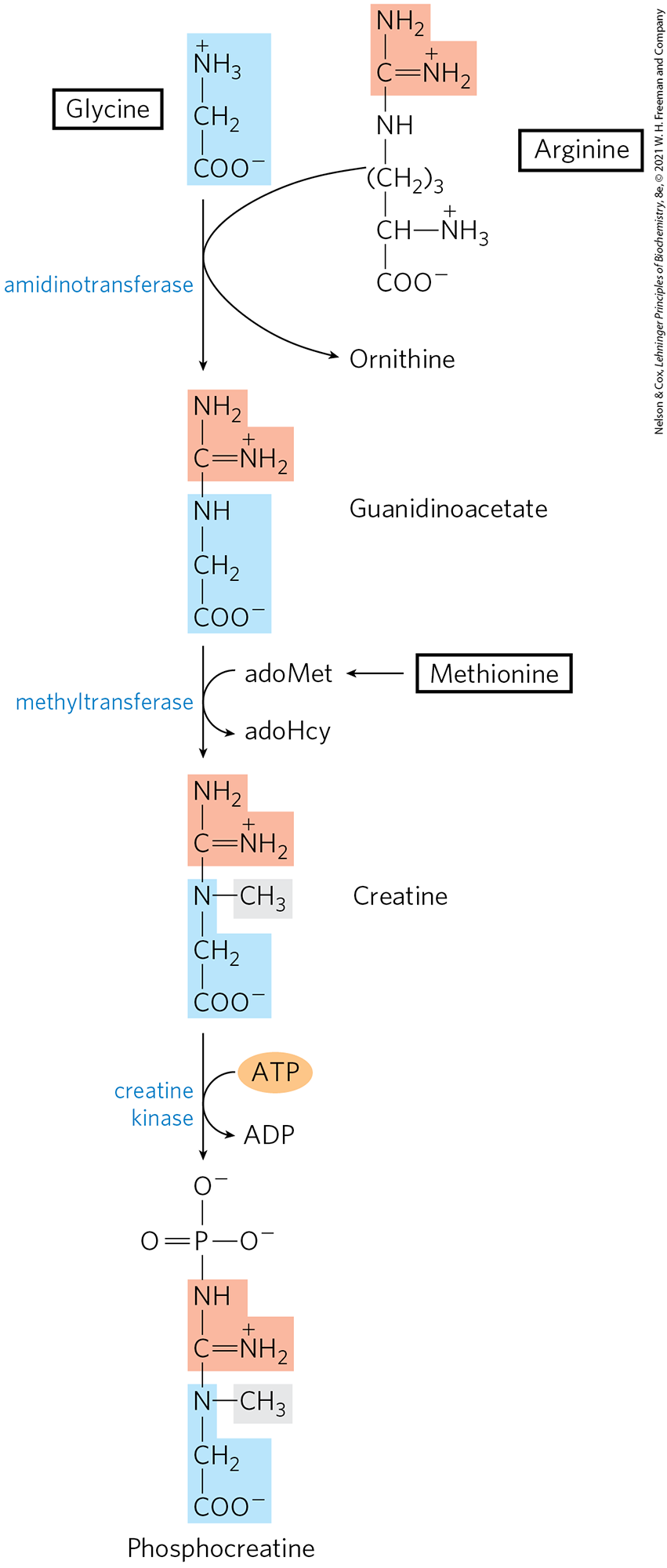
FIGURE 22-28 Biosynthesis of creatine and phosphocreatine. Creatine is made from three amino acids: glycine, arginine, and methionine. This pathway shows the versatility of amino acids as precursors of other nitrogenous biomolecules.
A molecule in a blue box is labeled by a box labeled glycine and is a chain with N H 3 at the top with a positive charge on N bonded to C H 2 below further bonded to C O O minus. An arrow labeled aminotransferase points down to guanidinoacetate accompanied by a curved arrow showing the addition of a box labeled arginine and loss of ornithine. Arginine is shown as a vertical chain with the top portion in a light red box. In the box, N H 2 is bonded to C below double bonded to N H 2 on the right with a positive charge on N and bonded to N H below, outside of the box, that is further bonded to (C H 2) 3 bonded to C H bonded to N H 3 with a positive charge on N to the right and to C O O minus below. Guanidinoacetate is a vertical chain with the top part enclosed in a light red box and the bottom part enclosed in a blue box. In the light red box, N H 2 is bonded to C below double bonded to N H 2 to the right with a positive charge on N and to N H below at the top of the blue box that is further bonded to C H 2 below further bonded to C O O minus below within the same blue box. An arrow labeled methyltransferase points down from guanidinoacetate to creatine and is accompanied by a curved arrow showing the addition of ado M e t and loss of ado H c y. An arrow points from a box labeled methionine to ado M e t involved in this reaction. Creatine is shown as a vertical chain with the top part enclosed in a light red box and the bottom part enclosed in a blue box. In the light red box, N H 2 is bonded to C below double bonded to N H 2 to the right with a positive charge on N and to N below at the top of the blue box that is bonded to C H 3 in a gray box to the right and further bonded to C H 2 below in the blue box further bonded to C O O minus below within the same blue box. An arrow labeled creatine kinase points down from creatine to phosphocreatine and is accompanied by a curved arrow showing the addition of an orange oval labeled A T P and loss of A D P. This molecule is similar to creatine except that the top N H 2 in the light red box is now N H and is bonded to nonhighlighted P above further double bonded to O on the left and bonded to O minus above and to the right.
Glutathione (GSH), present in plants, animals, and some bacteria, often at high levels, can be thought of as a redox buffer. It is derived from glutamate, cysteine, and glycine (Fig. 22-29). The γ-carboxyl group of glutamate is activated by ATP to form an acyl phosphate intermediate, which is then attacked by the α-amino group of cysteine. A second condensation reaction follows, with the α-carboxyl group of cysteine activated to an acyl phosphate to permit reaction with glycine. The oxidized form of glutathione (glutathione disulfide, or GSSG), produced in the course of its redox activities, contains two glutathione molecules linked by a disulfide bond.
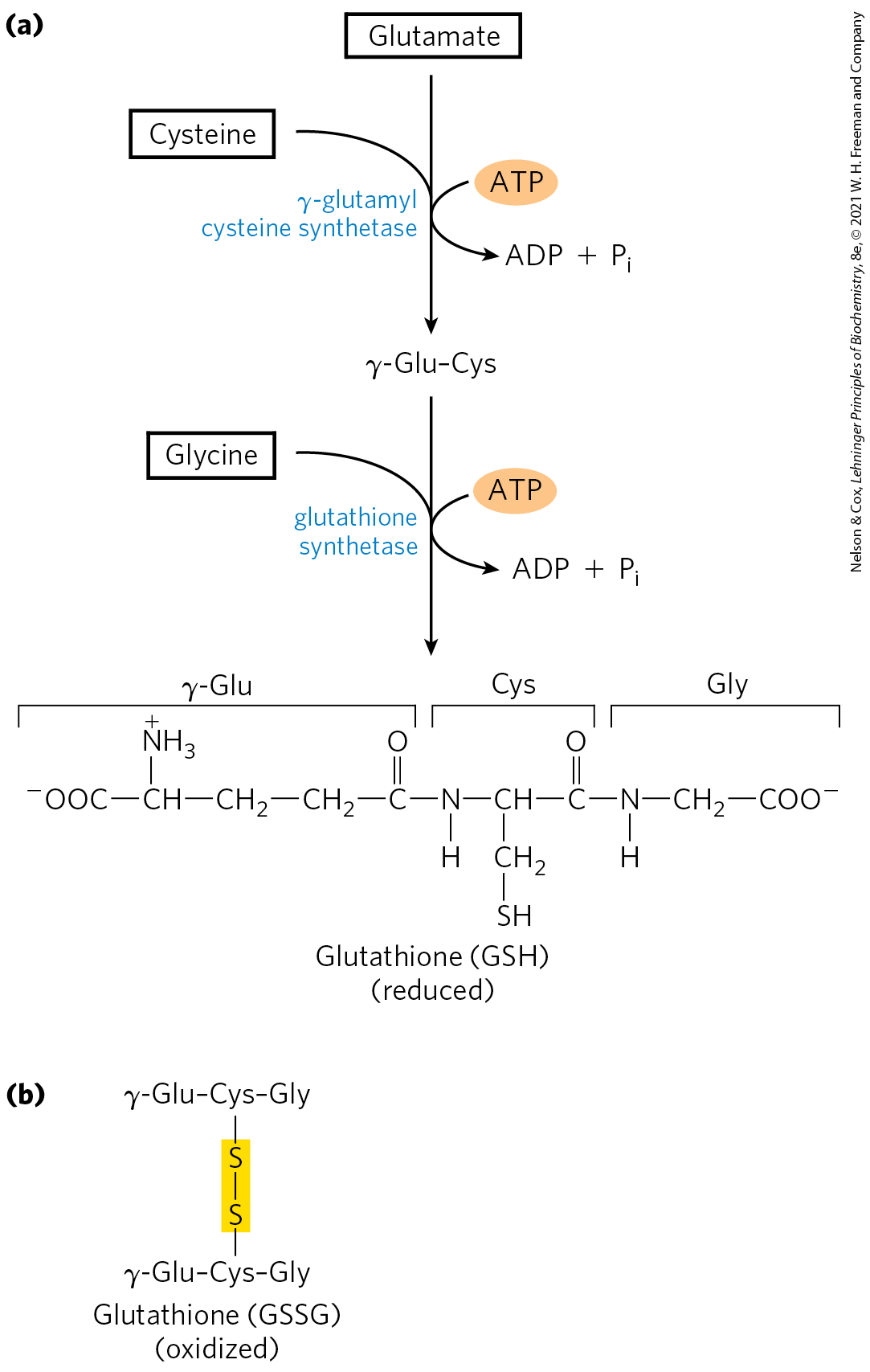
FIGURE 22-29 Glutathione metabolism. (a) Biosynthesis of glutathione. (b) Oxidized form of glutathione.
Part a shows a box labeled glutamate at the top. An arrow labeled gamma-glutamyl cysteine synthetase points down from glutamate to gamma-G l u-C y s accompanied by a curved line showing the addition of a box labeled Cysteine and a curved arrow showing the addition of an orange oval labeled A T P and loss of A D P plus P i. An arrow labeled glutathione synthetase points down from gamma-G l u-C y s to glutathione (G S H) (reduced) and is accompanied by a curved line showing the addition of a box labeled glycine and a curved arrow showing the addition of an orange oval labeled A T P and loss of A D P plus P i. This yields glutathione (G S H) (reduced), which is shown as a vertical chain divided into three parts by brackets labeled from left to right as gamma-G l u, C y s, and G l y. Beginning within the bracket labeled gamma-G l u, the molecule is C H bonded to C O O minus to the left, N H 3 above with a positive charge on N, and C H 2 to the right further bonded to C H 2 bonded to C double bonded to O above and bonded to N to the right at the start of the bracket labeled C y s. This N is bonded to H below and to C H to the right further bonded to C H 2 below further bonded to S H and bonded to C to the right double bonded to O above and bonded to N to the right at the start of the bracket labeled G l y. This N is bonded to H below and to C H 2 to the right further bonded to C O O minus. Part b shows glutathione (G S S G) (oxidized) as gamma-G l u-C y s-G l y with a bond extending down from C y s to S in a yellow box bonded to S below in the same box bonded to C y s below outside of the yellow box that is in the middle of a similar chain with gamma-G l u to the left and G l y to the right.
Glutathione helps maintain the sulfhydryl groups of proteins in the reduced state and the iron of heme in the ferrous state, and it serves as a reducing agent for glutaredoxin in deoxyribonucleotide synthesis (see Fig. 22-41). Its redox function is also used to remove toxic peroxides formed in the normal course of growth and metabolism under aerobic conditions:
This reaction is catalyzed by glutathione peroxidase, a remarkable enzyme in that it contains a covalently bound selenium (Se) atom in the form of selenocysteine (see Fig. 3-8a), which is essential for its activity.
d-Amino Acids Are Found Primarily in Bacteria
Although d-amino acids do not generally occur in proteins, they do serve some special functions in the structure of bacterial cell walls and peptide antibiotics. Bacterial peptidoglycans (see Fig. 6-32) contain both d-alanine and d-glutamate. d-Amino acids arise directly from the l isomers by the action of amino acid racemases, which have pyridoxal phosphate as cofactor (see Fig. 18-6). Amino acid racemization is uniquely important to bacterial metabolism, and enzymes such as alanine racemase are prime targets for pharmaceutical agents. One such agent, L-fluoroalanine, is being tested as an antibacterial drug. Another, cycloserine, is used to treat tuberculosis. Because these inhibitors also affect some PLP-requiring human enzymes, however, they have potentially undesirable side effects.
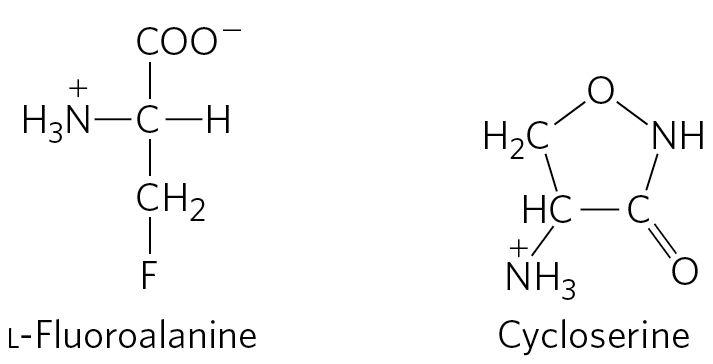
L-fluoroalanine is a vertical three-carbon chain with C 1 forming C O O minus, C 2 bonded to N H 3 to the left with a positive charge on N and to H on the right, and C 3 bonded to 2 H and to F below. Cycloserine is a five-membered ring with O at the top vertex, N substituted for C at the upper right vertex and bonded to H, C at the lower right vertex double bonded to O, C at the lower left vertex bonded to H and to N H 3 with a positive charge on N, and C at the upper left vertex bonded to 2 H.
Aromatic Amino Acids Are Precursors of Many Plant Substances
Phenylalanine, tyrosine, and tryptophan are converted to a variety of important compounds in plants. The rigid polymer lignin, derived from phenylalanine and tyrosine, is second only to cellulose in abundance in plant tissues. The structure of the lignin polymer is complex and not well understood. Tryptophan is also the precursor of the plant growth hormone indole-3-acetate, or auxin (Fig. 22-30a), which is important in the regulation of a wide range of biological processes in plants.
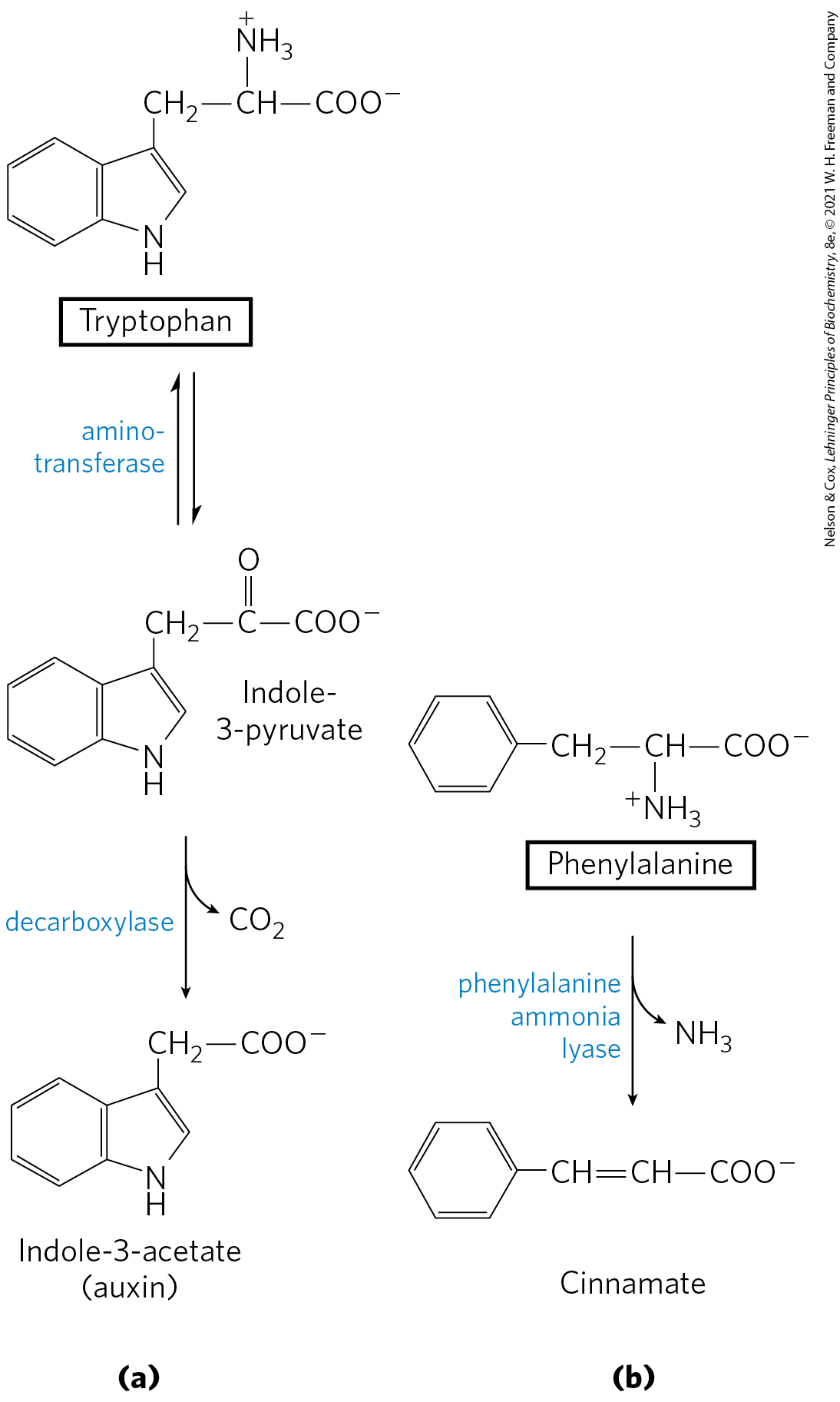
FIGURE 22-30 Biosynthesis of two plant substances from amino acids. (a) Indole-3-acetate (auxin) and (b) cinnamate (cinnamon flavor).
Part a shows a two-ring structure at the top labeled with the word tryptophan in a box. This structure has a benzene ring that shares its right side with the left side of a five-membered ring with N substituted for C at its lower right vertex and further bonded to H, a double bond at its upper right side, and an upper right vertex bonded to C H 2 further bonded to C H bonded to N H 3 above with a positive charge on N and to C O O minus to the right. Upward- and downward-pointing arrows labeled aminotransferase indicate a reversible reaction that yields indole 3-pyruvate. Indole 3-pyruvate is similar to tryptophan except that the upper right vertex of the ring is bonded to C H bonded to C double bonded to O above and bonded to C O O minus to the right. An arrow labeled decarboxylase points down from indole 3-pyruvate to indole-3-acetate (auxin) and is accompanied by an arrow that branches off to show the loss of C O 2. This yields indole-3-acetate (auxin), which has a similar structure to indole-3-pyruvate except that the upper right vertex of the ring is bonded to C H 2 bonded to C O O minus. Part b shows a benzene ring with its right vertex bonded to C H 2 bonded to C H bonded to N H 3 below with a positive charge on N and to C O O minus to the right. This is labeled with the word phenylalanine in a box. An arrow labeled phenylalanine ammonia lyase points down from phenylalanine to cinnamate and has an arrow that branches off to show the loss of N H 3. This yields cinnamate, which is a benzene ring bonded to C H double bonded to C H bonded to C O O minus.
Phenylalanine and tyrosine also give rise to many commercially significant natural products, including the tannins that inhibit oxidation in wines; alkaloids such as morphine, which have potent physiological effects; and the flavoring of cinnamon oil (Fig. 22-30b), nutmeg, cloves, vanilla, cayenne pepper, and other products.
Biological Amines Are Products of Amino Acid Decarboxylation
Many important neurotransmitters are primary or secondary amines, derived from amino acids in simple pathways. In addition, some polyamines that form complexes with DNA are derived from the amino acid ornithine, a component of the urea cycle. A common denominator of many of these pathways is amino acid decarboxylation, another PLP-requiring reaction (see Fig. 18-6).
The synthesis of some neurotransmitters is illustrated in Figure 22-31. Tyrosine gives rise to a family of catecholamines that includes dopamine, norepinephrine, and epinephrine. Levels of catecholamines are correlated with, among other things, changes in blood pressure. The neurological disorder Parkinson disease is associated with an underproduction of dopamine, and it has traditionally been treated by administering l-dopa. Overproduction of dopamine in the brain may be linked to psychological disorders such as schizophrenia.
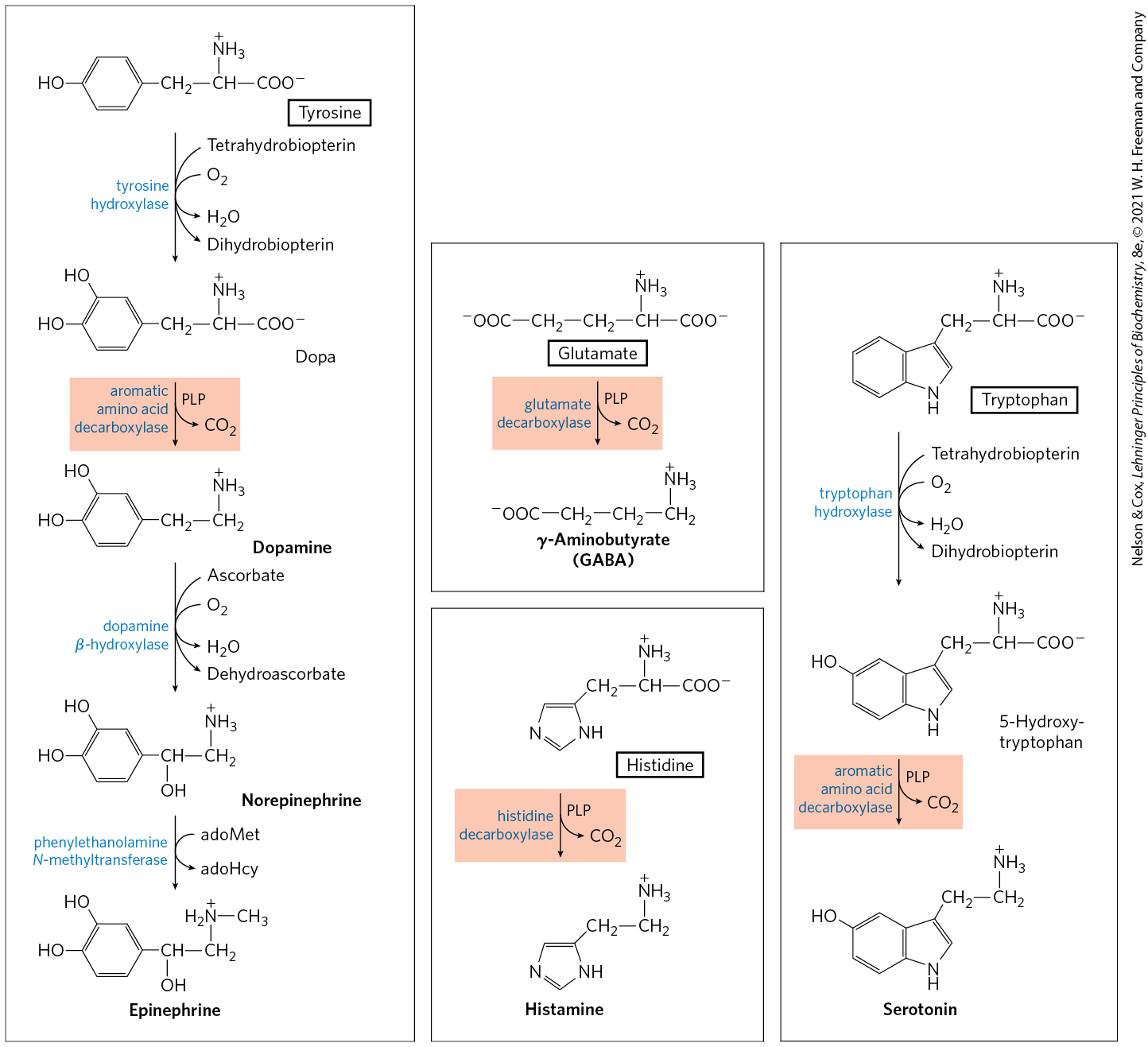
FIGURE 22-31 Biosynthesis of some neurotransmitters from amino acids. The key step is the same in each case: a PLP-dependent decarboxylation (shaded light red).
Four boxes are shown that contain separate reaction pathways. The left-hand box shows the synthesis of dopamine, norepinephrine, and epinephrine from tyrosine. Tyrosine is a benzene ring with its left side vertex bonded to O H and its right side vertex bonded to C H 2 bonded to C H bonded to N H 3 above with a positive charge on N and to C O O minus on the right. An arrow labeled tyrosine hydroxylase points down from tyrosine to dopa accompanied by two curved arrows. The outer curved arrow shows the addition of tetrahydrobiopterin and loss of dihydrobiopterin. The inner curved arrow shows the addition of O 2 and loss of H 2 O. This yields dopa, which is similar to tyrosine except that both the left side and upper left vertices of the benzene ring is bonded to O H. An arrow labeled aromatic amino acid decarboxylase is enclosed in a brown rectangle and points down from dopa to dopamine accompanied by the word P L P at the top and an arrow below that branches to show the loss of C O 2. The word dopamine is bolded. Dopamine is similar to dopa except that the right side vertex of the benzene ring is bonded to C H 2 bonded to C H 2 that is bonded to N H 3 above with a positive charge on N. An arrow labeled dopamine beta-hydroxylase points down from dopamine to norepinephrine, which is also bolded. This arrows is accompanied by two curved arrows. The outer curved arrow shows the addition of ascorbate and loss of dehydroascorbate. The inner curved arrow shows the addition of O 2 and loss of H 2 O. This yields norepinephrine, which is similar to dopamine except that the right side vertex of benzene is bonded to C H bonded to O H below and to C H to the right further bonded to N H 3 above with a positive charge on N. An arrow labeled phenylethanolamine italicized N end italics methyltransferase points down from norepinephrine to epinephrine, which is also bolded. The arrow is accompanied by a curved arrow showing the addition of ado M e t and loss of ado H c y. This yields epinephrine, which is similar except that N H 3 with a positive charge on N has been replaced by N H 2 with a positive charge on N further bonded to C H 3. The upper middle box shows the synthesis of gamma-aminobutyrate (G A B A) from glutamate. A box labeled glutamate is shown as C H 2 bonded to C O O minus to the left and to C H 2 to the right further bonded to C H bonded to N H 3 above with a positive charge on N and to C O O minus to the right. An arrow labeled glutamate decarboxylate points down from glutamate to gamma-aminobutyrate (G A B A), which is bolded. The arrow is enclosed in a brown rectangle and has P L P at the top before a curved arrow showing the loss of C O 2. This yields gamma-aminobutyrate, shown as C H 2 bonded to C O O minus to the left and C H 2 to the right further bonded to C H 2 bonded to N H 3 above with a positive charge on N. The lower middle box shows the synthesis of histamine from histidine. A five-membered ring has N substituted for C at the lower left vertex, N substituted for C at the lower right vertex and bonded to H, a double bond at the lower left side, a double bond at the top side, and an upper right vertex bonded to C H 2 bonded to C H bonded to N H 3 above with a positive charge on N and to C O O minus to the right. This is labeled with the word histidine in a box. An arrow labeled histidine decarboxylase points down from histidine to histamine, which is bolded. The arrow is enclosed in a brown rectangle and has P L P at the top before a curved arrow showing the loss of C O 2. This yields histamine, which is similar except that the upper right vertex of the ring is bonded to C H 2 bonded to C H 2 bonded to N H 3 above with a positive charge on N. The right-hand box shows the synthesis of serotonin from tryptophan. A benzene ring shares its right side with the left side of a five-membered ring that has N substituted for C at its bottom vertex and bonded to H, a double bond at its upper right side, and an upper right vertex bonded to C H 2 bonded to C H bonded to N H 3 above with a positive charge on N and to C O O minus to the right. This is labeled with the word tryptophan in a box. An arrow labeled tryptophan hydroxylase points down from tryptophan to 5-hydroxytryptophan. The arrow is accompanied by two curved arrows. The outer curved arrow shows the addition of tetrahydrobiopterin and loss of dihydrobiopterin. The inner curved arrow shows the addition of O 2 and loss of H 2 O. 5-hydroxytryptophan has a similar structure to tryptophan except that the benzene ring has an upper left vertex bonded to O H. An arrow labeled aromatic amino acid decarboxylase points down from 5-hydroxytryptophan to serotonin, which is bolded. The arrow is enclosed in a brown rectangle and has P L P at the top before a curved arrow showing the loss of C O 2. This yields serotonin, which is similar except that the upper right vertex of the five-membered ring is bonded to C H 2 bonded to C H bonded to N H 3 above with a positive charge on N.
Glutamate decarboxylation gives rise to γ-aminobutyrate (GABA), an inhibitory neurotransmitter. Its underproduction is associated with epileptic seizures. GABA analogs are used in the treatment of epilepsy and hypertension. Levels of GABA can also be increased by administering inhibitors of the GABA-degrading enzyme GABA aminotransferase. Another important neurotransmitter, serotonin, is derived from tryptophan in a two-step pathway.
Histidine undergoes decarboxylation to histamine, a powerful vasodilator in animal tissues. Histamine is released in large amounts as part of the allergic response, and it also stimulates acid secretion in the stomach. A growing array of pharmaceutical agents are being designed to interfere with either the synthesis or the action of histamine. A prominent example is the histamine receptor antagonist cimetidine (Tagamet), a structural analog of histamine:
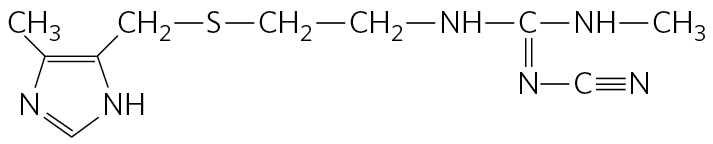
The molecule has a five-membered ring with N substituted for C at its lower left vertex, N substituted for C at its lower right vertex and bonded to H, a double bond at its lower left vertex, a double bond at its top vertex, an upper left vertex bonded to C H 3, and an upper right vertex bonded to C H 2 bonded to S bonded to C H 2 bonded to C H 2 bonded to N H bonded to C that is double bonded to N below further bonded to C triple bonded to N and that is also bonded to N H to the right further bonded to C H 3.
It promotes the healing of duodenal ulcers by inhibiting secretion of gastric acid.
Polyamines such as spermine and spermidine, involved in DNA packaging, are derived from methionine and ornithine by the pathway shown in Figure 22-32. The first step is decarboxylation of ornithine, a precursor of arginine (Fig. 22-12). Ornithine decarboxylase, a PLP-requiring enzyme, is the target of several powerful inhibitors used as pharmaceutical agents (see Box 6-1).
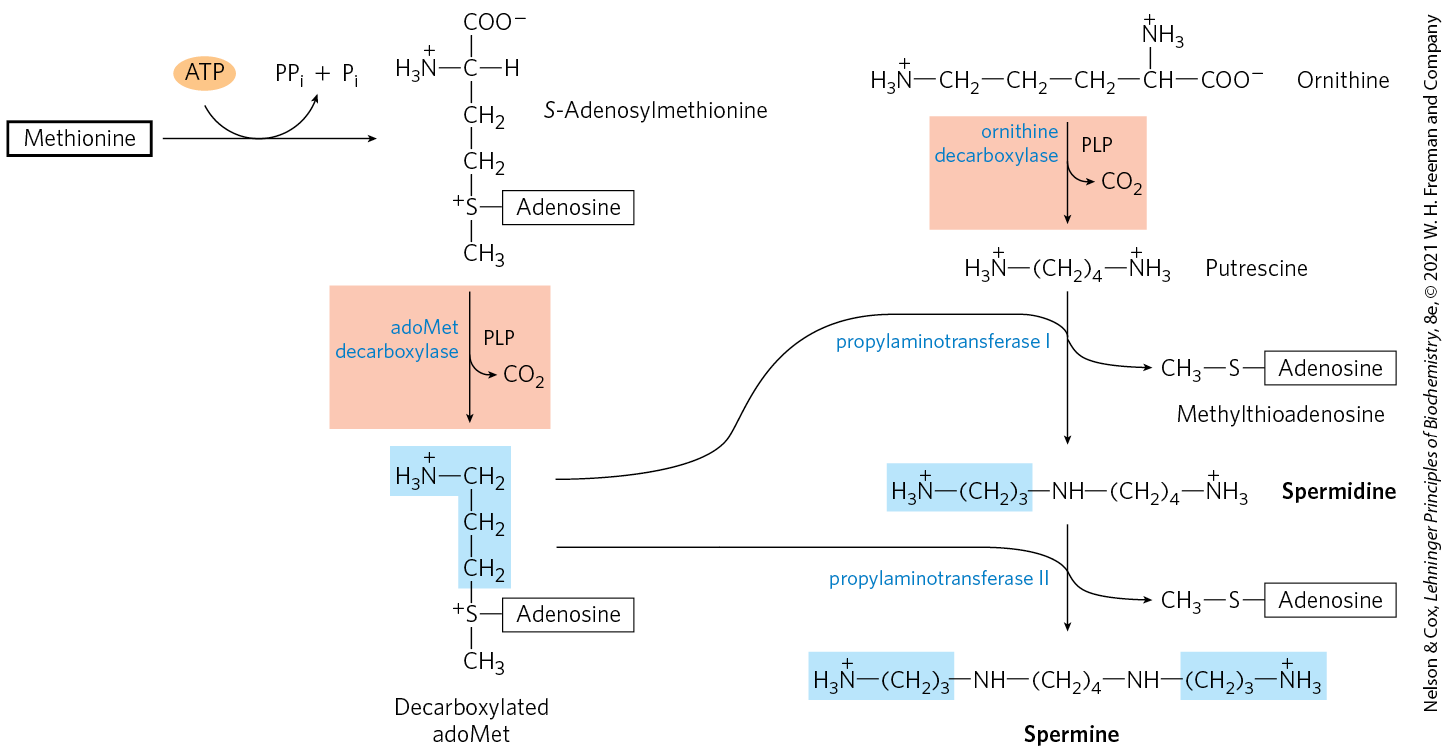
FIGURE 22-32 Biosynthesis of spermidine and spermine. The PLP-dependent decarboxylation steps are shaded light red. In these reactions, S-adenosylmethionine (in its decarboxylated form) acts as a source of propylamino groups (shaded blue).
An arrow points right from a box labeled methionine to italicized S end italics-adenosylmethionine and is accompanied by a curved arrow showing the addition of an orange oval labeled A T P and loss of P P subscript i end subscript plus P subscript i end subscript. Italicized S end italics-adenosylmethionine is a vertical six-membered chain with C 1 forming C O O minus, C 2 bonded to N H 3 on the left with a positive charge on N and to H on the right, C 3 and C 4 each bonded to 2 H, and S plus in position 5 bonded to a box labeled adenosine to the right and to C H 3 below. An arrow labeled ado M e t decarboxylase points down from italicized S end italics-adenosylmethionine to decarboxylated ado M e t. The arrow is enclosed in a brown rectangle and has P L P at the top before a curved arrow showing the loss of C O 2. Decarboxylated ado M e t has a blue highlighted top part with C H 2 bonded to N H 3 on the left with a positive charge on N and to C H 2 below further bonded to C H 2 bonded to S plus outside of the blue region that is further bonded to a box labeled adenosine to the right and to C H 3 below. Two arrows point right from decarboxylated ado M e t to intersect reactions to the right. This series of reactions to the right begins with ornithine. Ornithine is N H 3 with a positive charge on N bonded to C H 2 bonded to C H 2 bonded to C H 2 bonded to C H bonded to N H 3 above with a positive charge on N and bonded to C O O minus to the right. An arrow labeled ornithine decarboxylase points down from ornithine to putrescene. The arrow is enclosed in a brown rectangle and has P L P at the top before a curved arrow showing the loss of C O 2. This yields putrescine, which is N H 3 with a positive charge on N bonded to (C H 2) 4 bonded to N H 3 with a positive charge on N. A vertical arrow pointing down from putrescine to spermidine below is intersected by a mostly horizontal arrow from decarboxylated ado M e t to methylthioadeonosine. The point of intersection is labeled propylaminotransferase Roman numeral 1. Methylthioadenosine is C H 3 bonded to S bonded to a box labeled adenosine. Spermidine, which is bolded, has a blue highlighted portion on the left. From left to right, it is N H 3 with a positive charge on N in the blue box bonded to (C H 2) 3 in the same blue box bonded to N H outside of the blue box bonded to (C H 2) 4 bonded to N H 3 with a positive charge on N. A vertical arrow points down fro spermidine to spermine and is intersected by a mostly horizontal arrow from decarboxylated ado M e t to methylthioaenosine. The point of intersection is labeled propylaminotransferase Roman numeral 2. Spermine, which is bolded, is similar to spermidine except that the right-hand N H 3 plus has been replaced by N H that is bonded to (C H 2) 3 in a blue box further bonded to N H 3 to the right with a positive charge on N that is within the same blue box.
Arginine Is the Precursor for Biological Synthesis of Nitric Oxide
A surprise finding in the mid-1980s was the role of nitric oxide (NO) — previously known mainly as a component of smog — as an important biological messenger. This simple gaseous substance diffuses readily through membranes, although its high reactivity limits its range of diffusion to about a 1 mm radius from the site of synthesis. In humans NO plays a role in a range of physiological processes, including neurotransmission, blood clotting, and the control of blood pressure. Its mode of action is described in Box 12-2.
Nitric oxide is synthesized from arginine in an NADPH-dependent reaction catalyzed by nitric oxide synthase (Fig. 22-33), a dimeric enzyme structurally related to NADPH cytochrome P-450 reductase (see Box 21-1). The reaction is a five-electron oxidation. Each subunit of the enzyme contains one bound molecule of each of four different cofactors: FMN, FAD, tetrahydrobiopterin, and heme. NO is an unstable molecule and cannot be stored. Its synthesis is stimulated by interaction of nitric oxide synthase with -calmodulin (see Fig. 12-17).
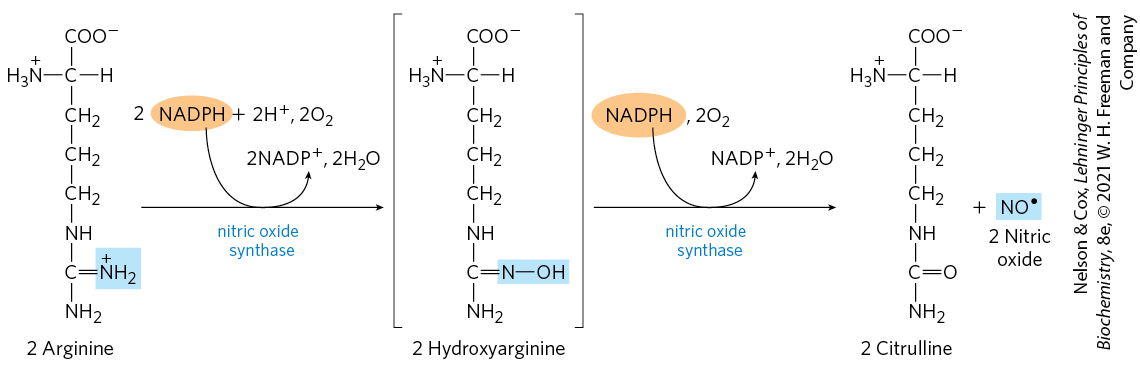
FIGURE 22-33 Biosynthesis of nitric oxide. The nitrogen of the NO is derived from the guanidinium group of arginine.
The reaction begins with 2 arginine. Arginine is shown as a vertical eight-member chain with C 1 forming C O O minus; C 2 bonded to N H 3 plus to the left with a positive charge on N and bonded to H on the right; C 3 and C 4 bonded to 2 H; and C 5 bonded to 2 H and to N H below further bonded to C double bonded to N H 2 with a positive charge on N in a blue box to the right and to N H 2 below. An arrow labeled nitric oxide synthase points right from 2 arginine to 2 hydroxyarginine and is accompanied by a curved arrow showing the addition of 2 of an orange oval labeled N A D P H plus 2 H plus, 2 O 2 and the loss of 2 N A D P plus, 2 H 2 O. Hydroxyarginine is shown in brackets and is similar to arginine except that the blue highlighted N H 2 with a positive charge on N is now N bonded to O H. An arrow labeled nitric oxide synthase points from hydroxyarginine to 2 citrulline plus 2 nitric oxide and is accompanied by a curved arrow showing the addition of an orange oval labeled N A D P H, 2 O 2 and loss of N A D P plus, 2 H 2 O. 2 citrulline resembles hydroxyarginine except that C that had been bonded to the blue box containing N bonded to O H is now double bonded to O. N O with a dot in a blue box is labeled 2 nitric oxide.
SUMMARY 22.3 Molecules Derived from Amino Acids
- Many important biomolecules are derived from amino acids. Glycine is a precursor of porphyrins.
- Degradation of iron-porphyrin (heme) generates bilirubin, which is converted to bile pigments with several physiological functions.
- Glycine and arginine give rise to creatine and phosphocreatine, an energy buffer. Glutathione, formed from three amino acids, is an important cellular reducing agent.
- Bacteria synthesize d-amino acids from l-amino acids in racemization reactions requiring pyridoxal phosphate. d-Amino acids are commonly found in certain bacterial walls and certain antibiotics.
- Plants make many substances from aromatic amino acids.
- The PLP-dependent decarboxylation of some amino acids yields important biological amines, including neurotransmitters and polyamines.
- Arginine is the precursor of nitric oxide, a biological messenger.
 reduction by NADPH converts the glutamate to glutamate 1-semialdehyde, which is cleaved from the tRNA. An aminotransferase converts the glutamate 1-semialdehyde to δ-aminolevulinate.
reduction by NADPH converts the glutamate to glutamate 1-semialdehyde, which is cleaved from the tRNA. An aminotransferase converts the glutamate 1-semialdehyde to δ-aminolevulinate. The iron-porphyrin (heme) group of hemoglobin, released from dying erythrocytes in the spleen, is degraded to yield free and, ultimately, bilirubin. The pathway also contributes the pigment present in mixtures of the bile salts derived from cholesterol.
The iron-porphyrin (heme) group of hemoglobin, released from dying erythrocytes in the spleen, is degraded to yield free and, ultimately, bilirubin. The pathway also contributes the pigment present in mixtures of the bile salts derived from cholesterol. Given these varied roles of heme degradation products, the degradative pathway is subject to regulation, mainly at the first step. Humans have at least three isozymes of heme oxygenase (HO). HO-1 is highly regulated; the expression of its gene is induced by a wide range of stress conditions, including shear stress, uncontrolled angiogenesis (development of blood vessels), hypoxia, hyperoxia, heat shock, exposure to ultraviolet light, hydrogen peroxide, and many other metabolic insults. HO-2 is found mainly in the brain and the testes, where it is continuously expressed. The third isozyme, HO-3, is not catalytically active, but may play a role in oxygen sensing.
Given these varied roles of heme degradation products, the degradative pathway is subject to regulation, mainly at the first step. Humans have at least three isozymes of heme oxygenase (HO). HO-1 is highly regulated; the expression of its gene is induced by a wide range of stress conditions, including shear stress, uncontrolled angiogenesis (development of blood vessels), hypoxia, hyperoxia, heat shock, exposure to ultraviolet light, hydrogen peroxide, and many other metabolic insults. HO-2 is found mainly in the brain and the testes, where it is continuously expressed. The third isozyme, HO-3, is not catalytically active, but may play a role in oxygen sensing. 
 Many important biomolecules are derived from amino acids. Glycine is a precursor of porphyrins.
Many important biomolecules are derived from amino acids. Glycine is a precursor of porphyrins.