20.3 Evolution of a Universal Mechanism for ATP Synthesis
The combined activities of the two plant photosystems move electrons from water to , conserving some of the energy of absorbed light as NADPH (Fig. 20-12). Simultaneously, protons are pumped across the thylakoid membrane and energy is conserved as an electrochemical potential. We turn now to the process by which this proton gradient drives the synthesis of ATP, the other energy-conserving product of the light-dependent reactions.
A Proton Gradient Couples Electron Flow and Phosphorylation
Although the energy source and electron carriers in photophosphorylation in chloroplasts differ from those of oxidative phosphorylation in mitochondria, they use essentially the same mechanism to capture the energy of the proton gradient. Electron-transferring molecules in the chain of carriers connecting PSII and PSI are oriented asymmetrically in the thylakoid membrane, so photoinduced electron flow results in the net movement of protons across the membrane, from the stromal side to the thylakoid lumen (Fig. 20-21).
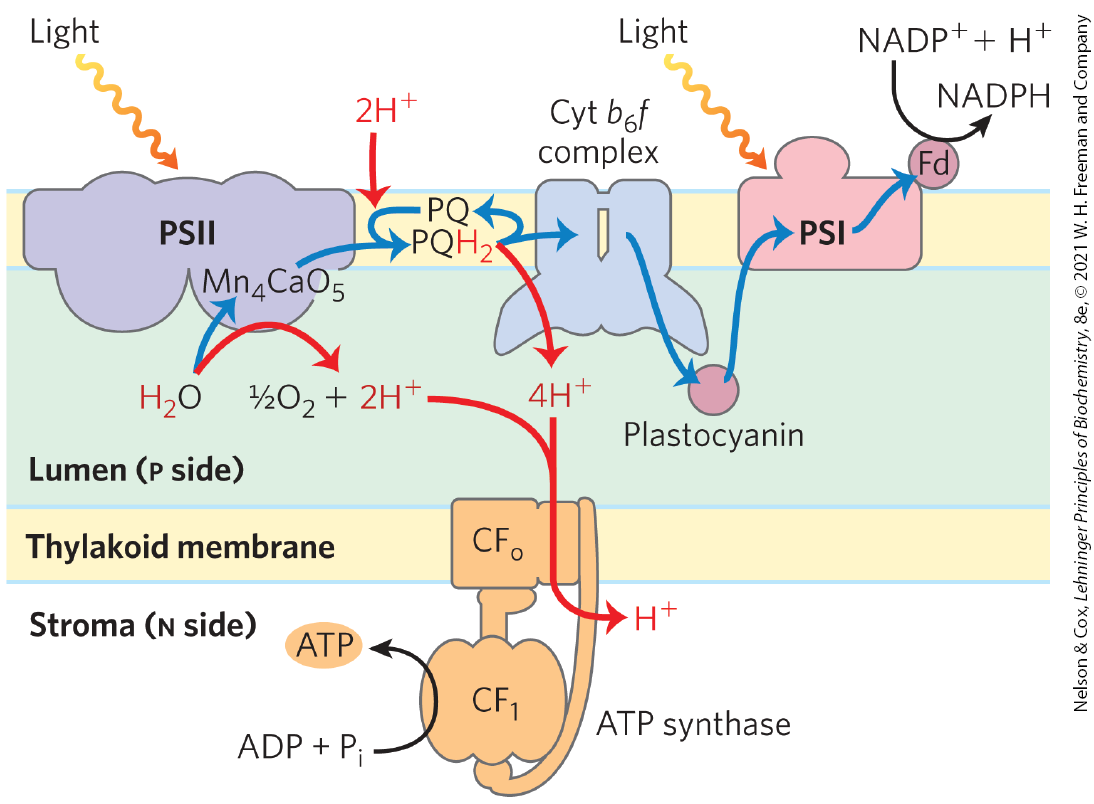
FIGURE 20-21 Proton and electron circuits during photophosphorylation. In the linear electron pathway (blue arrows), electrons move from through PSII, through the intermediate chain of carriers of the cytochrome complex, through PSI, and finally to . In the cyclic pathway, electrons move from PSI back to plastoquinone and cytochrome . Protons (red arrows) are pumped into the thylakoid lumen by the flow of electrons through cytochrome , and they reenter the stroma through proton channels formed by of ATP synthase. The subunit catalyzes synthesis of ATP.
The Approximate Stoichiometry of Photophosphorylation Has Been Established
As electrons move from water to in chloroplasts, about 12 protons move from the stroma into the thylakoid lumen per 4 electrons passed (that is, per formed). Of these protons, 4 are moved by the oxygen-evolving center, and up to 8 are moved by the cytochrome complex. The measurable result is a 1,000-fold difference in concentration across the thylakoid membrane . Recall that the energy stored in a proton gradient (the electrochemical potential) has two components: a proton concentration difference and an electrical potential due to charge separation. In chloroplasts, is the dominant component; counterion movement apparently dissipates most of the electrical potential. In illuminated chloroplasts, the energy stored in the proton gradient per mole of protons is
so the movement of 12 mol of protons across the thylakoid membrane represents conservation of about 200 kJ of energy — enough energy to drive the synthesis of several moles of ATP . Experimental measurements yield values of about 3 ATP per produced.
At least 8 photons must be absorbed to drive 4 electrons from to 2 NADPH (one photon per electron at each reaction center). The energy in 8 photons of visible light is more than enough for the synthesis of three molecules of ATP.
ATP synthesis is not the only energy-conserving reaction of photosynthesis in plants; the NADPH formed in the final electron transfer is also energetically rich. The overall equation for this linear photophosphorylation is
(20-6)
The ATP Synthase Structure and Mechanism Are Nearly Universal
The enzyme responsible for ATP synthesis in chloroplasts is a large complex with two functional components, and (C denoting its location in chloroplasts). is a transmembrane proton pore composed of several integral membrane proteins and is homologous to mitochondrial . is a peripheral membrane protein complex very similar in subunit composition, structure, and function to mitochondrial .
Electron microscopy of sectioned chloroplasts shows ATP synthase complexes as projections on the outside (stromal, or n) surface of thylakoid membranes; these complexes correspond to the ATP synthase complexes that project on the inside (matrix, or n) surface of the inner mitochondrial membrane. Thus, the relationship between the orientation of the ATP synthase and the direction of proton pumping is the same in chloroplasts and mitochondria. In both cases, the portion of ATP synthase is located on the more alkaline (n) side of the membrane through which protons flow down their concentration gradient; the direction of proton flow relative to is the same in both cases: p to n (Fig. 20-22).
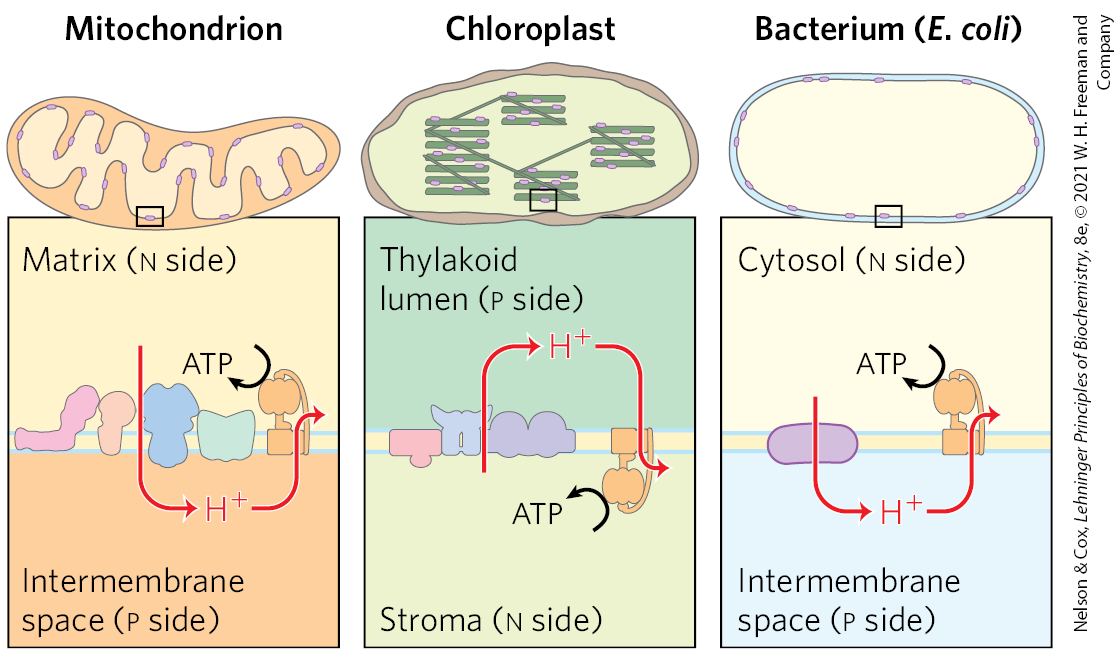
FIGURE 20-22 Orientation of ATP synthase is fixed relative to the proton gradient. Superficially, the direction of proton pumping in chloroplasts may seem to be opposite to that in mitochondria and bacteria. In mitochondria and bacteria, protons are pumped out of the organelle or cell, and is on the inside of the membrane; in chloroplasts, protons are pumped into the thylakoid lumen, and is on the outside of the thylakoid membrane. However, exactly the same mechanism of energy conversion (from proton gradient to ATP) occurs in all three cases. ATP is synthesized in the matrix of mitochondria, the stroma of chloroplasts, and the cytosol of bacteria.
The mechanism of chloroplast ATP synthase is essentially identical to that of its mitochondrial analog; ADP and readily condense to form ATP on the enzyme surface, and the release of this enzyme-bound ATP requires a proton-motive force. Rotational catalysis sequentially engages each of the three β subunits of the ATP synthase in ATP synthesis, ATP release, and binding (see Figs. 19-26 and 19-27).
The appearance of oxygenic photosynthesis on Earth about 2.5 billion years ago was a crucial event in the evolution of the biosphere. Before that, Earth’s atmosphere was composed of methane, , and . The planet was essentially devoid of molecular oxygen and lacked the ozone layer that protects organisms from solar UV radiation. Oxygenic photosynthesis made available a nearly limitless supply of reducing agent to drive the production of organic compounds by reductive biosynthetic reactions. And mechanisms evolved that allowed organisms to use as a terminal electron acceptor in highly energetic electron transfers from organic substrates, employing the energy of oxidation to support metabolism. The complex photosynthetic apparatus of a modern vascular plant is the culmination of a series of evolutionary events, the most recent of which was the acquisition by eukaryotic cells of a cyanobacterial endosymbiont.
The chloroplasts of modern organisms share several properties with mitochondria and originated by the same mechanism that gave rise to mitochondria: endosymbiosis. Like mitochondria, chloroplasts contain their own DNA and protein-synthesizing machinery. Some of the polypeptides of chloroplast proteins are encoded by chloroplast genes and synthesized in the chloroplast; others are encoded by nuclear genes, synthesized outside the chloroplast, and imported (Chapter 27). When plant cells grow and divide, chloroplasts give rise to new chloroplasts by division, during which their DNA is replicated and divided between daughter chloroplasts. The machinery and mechanisms for light capture, electron flow, and ATP synthesis in modern cyanobacteria are similar in many respects to those in plant chloroplasts. These observations led to the now widely accepted hypothesis that the evolutionary progenitors of modern plant cells were primitive eukaryotes that engulfed photosynthetic cyanobacteria and established stable endosymbiotic relationships with them (see Fig. 1-37).
At least half of the photosynthetic activity on Earth now occurs in microorganisms — algae, other photosynthetic eukaryotes, and photosynthetic bacteria. Cyanobacteria have PSII and PSI in tandem, and the PSII has an associated oxygen-evolving activity resembling that of plants. However, the other groups of photosynthetic bacteria have single reaction centers and do not split or produce . Many are obligate anaerobes and cannot tolerate ; they must use some compound other than as an electron donor. Some photosynthetic bacteria use inorganic compounds as electron (and hydrogen) donors. For example, green sulfur bacteria use hydrogen sulfide:
These bacteria, instead of producing molecular , form elemental sulfur as the oxidation product of . (They further oxidize the S to .) Other photosynthetic bacteria use organic compounds such as lactate as electron donors:
The fundamental similarity of photosynthesis in plants and bacteria, despite the differences in the electron donors they employ, becomes more obvious when the equation of photosynthesis is written in the more general form
in which is an electron (and hydrogen) donor and D is its oxidized form. may be water, hydrogen sulfide, lactate, or some other organic compound, depending on the species. Most likely, the bacteria that first developed photosynthetic ability used as their electron source.
Modern cyanobacteria can synthesize ATP by oxidative phosphorylation or by photophosphorylation, although they have neither mitochondria nor chloroplasts. The enzymatic machinery for both processes is in a highly convoluted plasma membrane (Fig. 20-23). Three protein components function in both processes, giving evidence that the processes have a common evolutionary origin (Fig. 20-24). First, the proton-pumping cytochrome complex carries electrons from plastoquinone to cytochrome in photosynthesis, and also carries electrons from ubiquinone to cytochrome in oxidative phosphorylation — the role played by cytochrome in mitochondria. Second, cytochrome , homologous to mitochondrial cytochrome c, carries electrons from Complex III to Complex IV in cyanobacteria; it can also carry electrons from the cytochrome complex to PSI — a role performed in plants by plastocyanin. We therefore see the functional homology between the cyanobacterial cytochrome complex and the mitochondrial cytochrome complex, and between cyanobacterial cytochrome and plant plastocyanin. The third conserved component is the ATP synthase, which functions in oxidative phosphorylation and photophosphorylation in cyanobacteria, and in the mitochondria and chloroplasts of photosynthetic eukaryotes. The structure and remarkable mechanism of this enzyme have been strongly conserved throughout evolution.
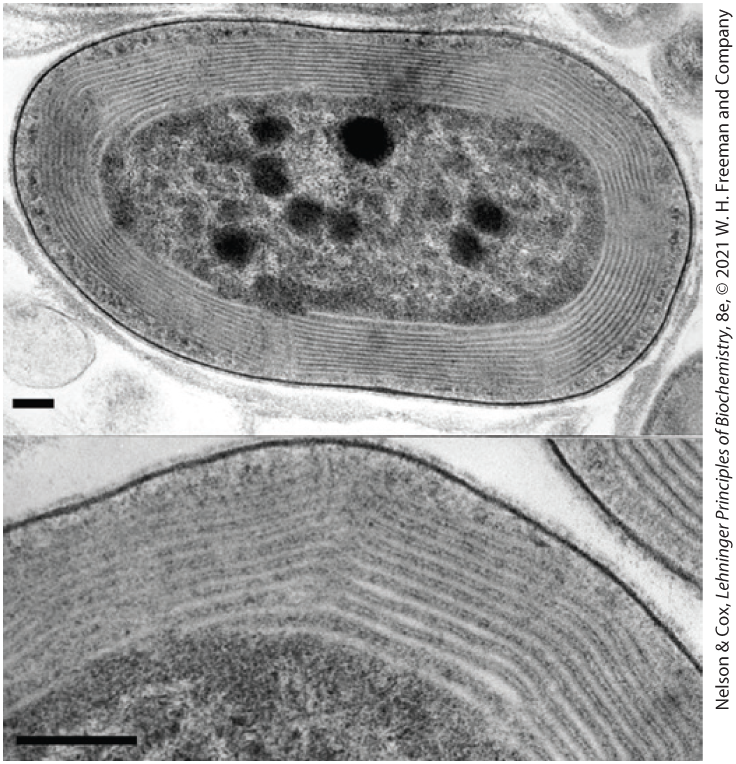
FIGURE 20-23 The photosynthetic membranes of a cyanobacterium. In these thin sections of a cyanobacterium, viewed with a transmission electron microscope, the multiple layers of the internal membranes are seen to fill half the total volume of the cell. The extensive membrane system serves the same role as the thylakoid membranes of vascular plants, providing a large surface area containing all of the photosynthetic machinery. [S. R. Miller et al. Discovery of a free-living chlorophyll d-producing cyanobacterium with a hybrid proteobacterial/cyanobacterial small-subunit rRNA gene. Proc. Natl. Acad. Sci. USA 102:850, 2005, Fig. 2. © 2005 National Academy of Sciences.]
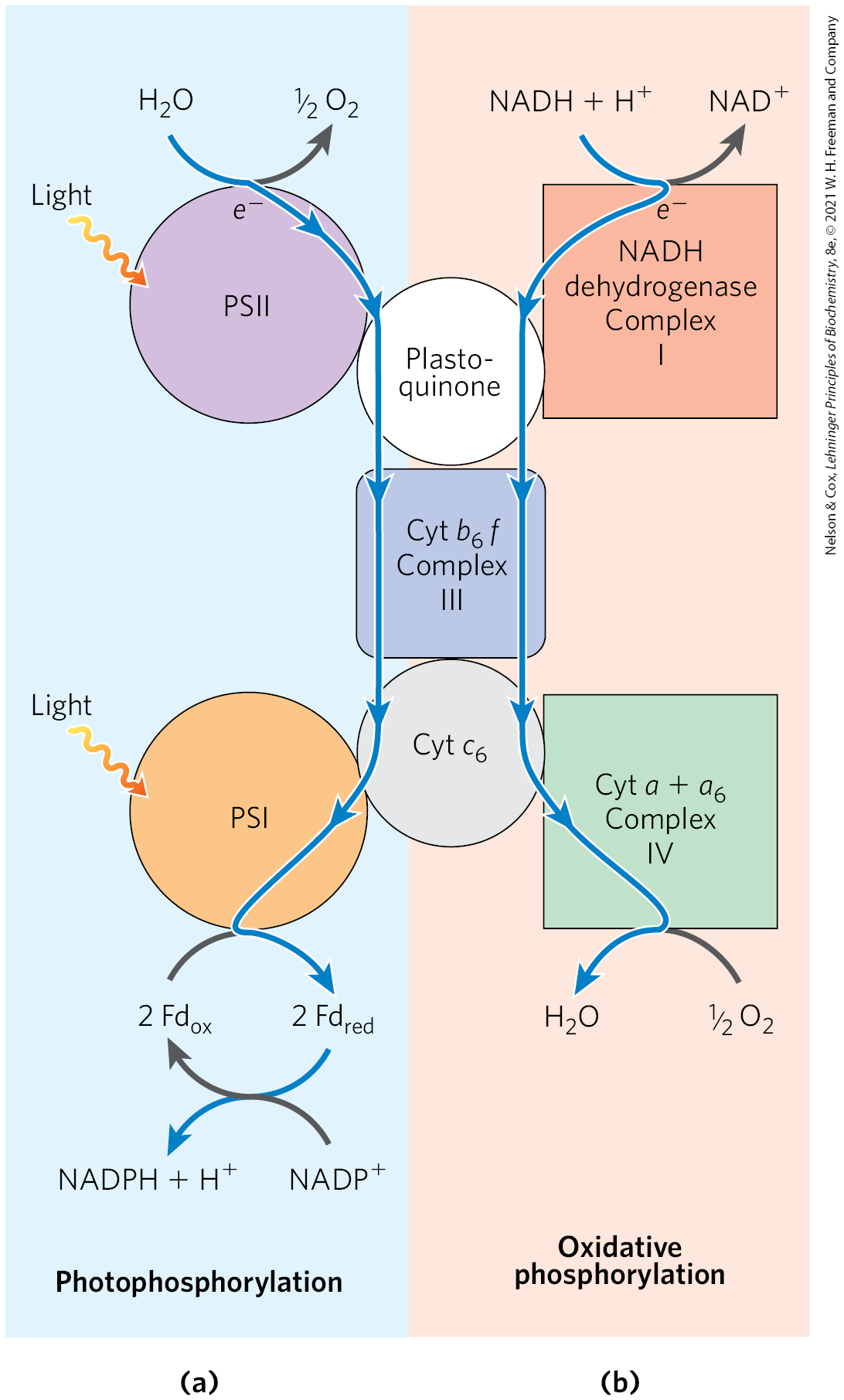
FIGURE 20-24 Dual roles of cytochrome and cytochrome in cyanobacteria reflect evolutionary origins. Cyanobacteria use cytochrome , cytochrome , and plastoquinone for both oxidative phosphorylation and photophosphorylation. (a) In photophosphorylation, electrons flow (blue arrows) from water to . (b) In oxidative phosphorylation, electrons flow from NADH to . Both processes are accompanied by proton movement across the membrane, accomplished by a Q cycle.
SUMMARY 20.3 Evolution of a Universal Mechanism for ATP Synthesis
- In plants, both the water-splitting reaction and electron flow through the cytochrome complex are accompanied by proton pumping across the thylakoid membrane. The proton-motive force thus created drives ATP synthesis by a complex similar to the mitochondrial complex in both structure and catalytic mechanism.
- Direct measurements show that eight photons drive the production of one from oxidation of two , making three ATP molecules.
- About 2.5 billion years ago, cyanobacteria appeared on Earth. They had acquired two photosystems — one of the type now found in purple bacteria, the other of the type found in green sulfur bacteria — that operated in tandem, and a water-splitting activity that released oxygen into the atmosphere.
- Many photosynthetic microorganisms obtain electrons for photosynthesis not from water but from donors such as , forming an oxidized product such as elemental sulfur (not oxygen).
- Chloroplasts, like mitochondria, evolved from bacteria living as endosymbionts in early eukaryotic cells. The ATP synthases of bacteria, cyanobacteria, mitochondria, and chloroplasts share a common evolutionary precursor and a common enzymatic mechanism.
 Although the energy source and electron carriers in photophosphorylation in chloroplasts differ from those of oxidative phosphorylation in mitochondria, they use essentially the same mechanism to capture the energy of the proton gradient. Electron-transferring molecules in the chain of carriers connecting PSII and PSI are oriented asymmetrically in the thylakoid membrane, so photoinduced electron flow results in the net movement of protons across the membrane, from the stromal side to the thylakoid lumen (
Although the energy source and electron carriers in photophosphorylation in chloroplasts differ from those of oxidative phosphorylation in mitochondria, they use essentially the same mechanism to capture the energy of the proton gradient. Electron-transferring molecules in the chain of carriers connecting PSII and PSI are oriented asymmetrically in the thylakoid membrane, so photoinduced electron flow results in the net movement of protons across the membrane, from the stromal side to the thylakoid lumen ( In plants, both the water-splitting reaction and electron flow through the cytochrome complex are accompanied by proton pumping across the thylakoid membrane. The proton-motive force thus created drives ATP synthesis by a complex similar to the mitochondrial complex in both structure and catalytic mechanism.
In plants, both the water-splitting reaction and electron flow through the cytochrome complex are accompanied by proton pumping across the thylakoid membrane. The proton-motive force thus created drives ATP synthesis by a complex similar to the mitochondrial complex in both structure and catalytic mechanism.