Lecture 32
Readings
http://umdberg.pbworks.com/w/page/52122743/Three%20models%20of%20light
http://umdberg.pbworks.com/w/page/65464521/The%20photon%20model%20%282013%29
http://umdberg.pbworks.com/w/page/125510822/Workout%3A%20The%20photon%20model
http://umdberg.pbworks.com/w/page/53301740/Basic%20principles%20of%20the%20photon%20model
3 Models of Light
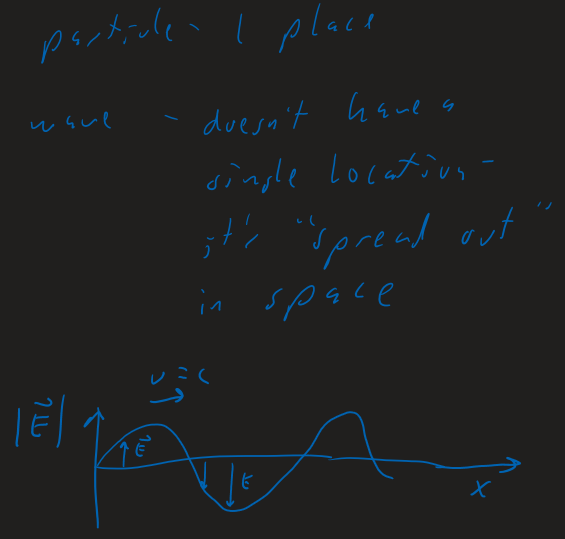
Waves
Rays
Particles
"Light" ( = Electromagnetic (EM) waves ) are really just 1 thing
- Which "picture" or "model" we use depends on the application
EM waves go by all/any of the following names:
- Gamma Rays
- X-rays
- Ultraviolet
- Visible light
- Infrared
- EHF , UHF , VHF
- Microwaves
- Radio Waves
- Gamma Rays
Does not include:
- Sound , ocean waves
- Ultrasound
- Gravity waves
Photon Model
Photo = "particle" ( but will have "wave" properties )
A fixed amount of energy ( for a particular frequency / wavelength )
For each wavelength
- If
- Or if
- Remember
- For EM waves in vacuum ,
- If
Photon activity
3 Possible Results
- Vibration of Molecule (infrared)
- Rotation of Molecule (microwave)
- Breaking of Molecule (ultraviolet)
- Vibration of Molecule (infrared)
Photoelectric Effect
Hit matter with light (photon)
Electrons only emitted (ejected from molecule) when light has a high enough frequency
Remember, photons are light ( as in light waves)
- They have no mass
- They are not "matter"
Electrons do have mass
- They are matter
Remember , each photon has a specific energy, determined by its freqency ,
This phenomenon proves that light comes in discrete packets
But notice the contradiction:
- Frequency is only makes sense when dealing with waves
- Particles aren't waves
You must "suspend your logic" when thinking about light in these different ways