Lecture 17
Readings
http://umdberg.pbworks.com/w/page/73084895/Nernst%20potential%20%282013%29
Nernst Potential
- Situation:
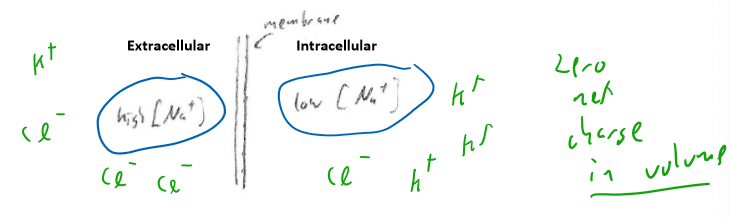
Not worrying at the momement about how this situation with [Na] occured
- It just exists
But still, each side has equal positive and negative ions due to other ions not shown here.
Na+ wants to move from outside to inside (extracellular to intracellular) due to random diffusion/entropy effect:

- Now a few extra positive ions are inside:
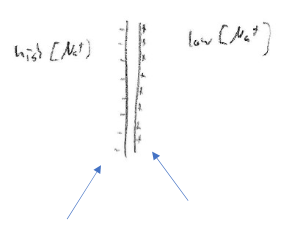
Another Na+ wants to go inside, but what does it see?
- A wall of positive charges on the inside membrane
So it's tougher to move inside
Eventually, the random motion/entropy effect (Na+ wanting to move inside) is balanced by the opposing electric field:
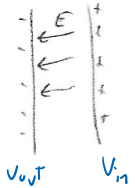
Which resists + charges moving inside
Now there's a potential difference between inside and outside, with the inside potential being higher than outside.
- This is not the "normal" case for biological cells.
- This was what would happen if only Na+ were involved.
This potential difference is called the Nernst potential for sodium
For resting/normal membranes, it is potassium, K+ , that primarily determines the membrane potential:
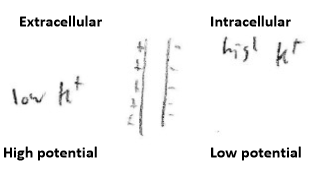
The intracellular space is at
Membranes normally are much more permeable to
Notice two concepts: "Membrane potential" and "Nernst Potential"
Each ion has a Nernst potential
So there are many Nernst potentials for a single membrane, one for each ion
The membrane potential is a weighted average of each Nernst potential
The ions that can move through membrane most easily ( highest permeability ) get the most weight
The membrane is much more permeable to
- Therefore, the membrane potential is close to the
- Therefore, the membrane potential is close to the
Typical Numbers:
- Nernst:
- Nernst:
- Membrane Potential
- Nernst:
Value of the Nernst potential is determined by a Boltzmann distribution:
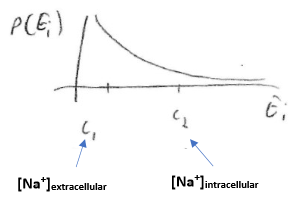
- The "
- Rearranging:
- OR:
This says that the Nernst potential across a membrane for an ion is determined by the concentrations of that ion on each side of the membrane.
The Nernst potential reflects the electrical energy difference that opposes the thermal/entropy tendency to even out the concentration
Reminder: something else caused the concentration of
We aren't dealing with why concentration of
- It just is