Lecture 9
Readings
http://umdberg.pbworks.com/w/page/63473552/Boltzmann%20distribution%20%282013%29
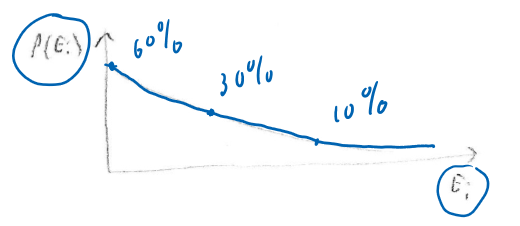
Clarification:
- This is the probability for a small part of a large system.
- Small is typically 1 molecule.
- Large is around
- So this probability distribution doesn't work for say 2 large objects in contact with each other
Change with temperature:
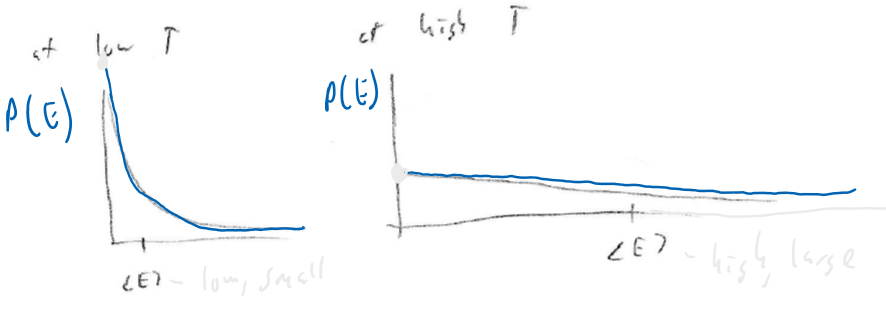
Average energy goes up as temperature goes up
Still,
Spread of possible energies increases greatly at high
Thus, finding a molecule at
The
But there is also a correction term for entropy
- If some
- If some
Gibb's Free Energy exactly accounts for this
So the best form for biology is:
Where
Note, not
- This is absolute
- This is absolute
But mostly, just think of