Lecture 8
Readings
http://umdberg.pbworks.com/w/page/68375276/Powers%20and%20exponents%20%282013%29
http://umdberg.pbworks.com/w/page/104491534/How%20energy%20is%20distributed%3A%20Fluctuations
Fluctuations in Energy Distributions
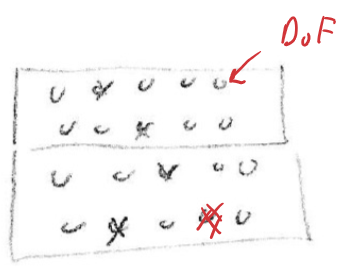
At thermal equilibrium, the average energy per degree of freedom is the same
- 2 energy packets, 10 degrees of freedom
- Average energy per degree of freedom = <E> = 0.2 energy packets
- Same for top and bottom = thermal equilibrium
The energy in a particular DoF will fluctuate
- 80% of the time, a DoF will have 0 energy, 20% of the time, a DoF will have 1 energy packet
But.... you can also put 2 energy packets into a single DoF!
- It can happen randomly
- This makes the probability calculations harder
- There is no upper limit on the amount of energy in a single DoF
Actual odds are as follows
| Probability | Number of Energy Packets |
|---|---|
| 79% | 0 |
| 19% | 1 |
| 1.9% | 2 |
| 0.08% | 3 |
| 0.02% | 4 |
- This is the P(E) that the reading referred to, the probability of each amount of energy
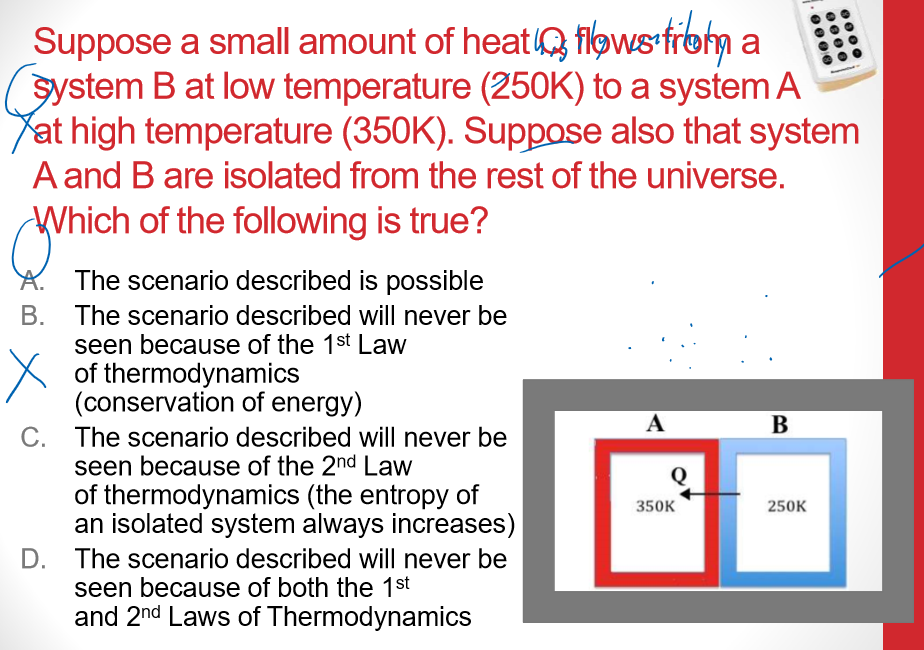
Multiple Choice
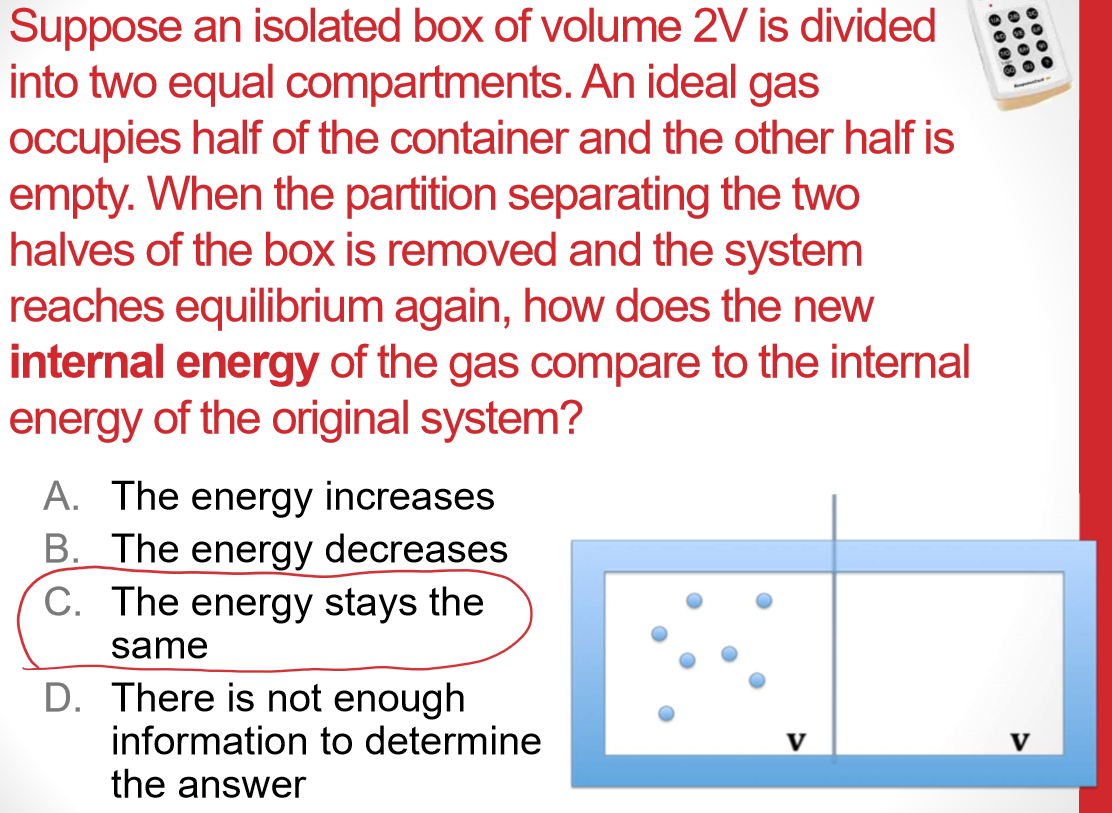
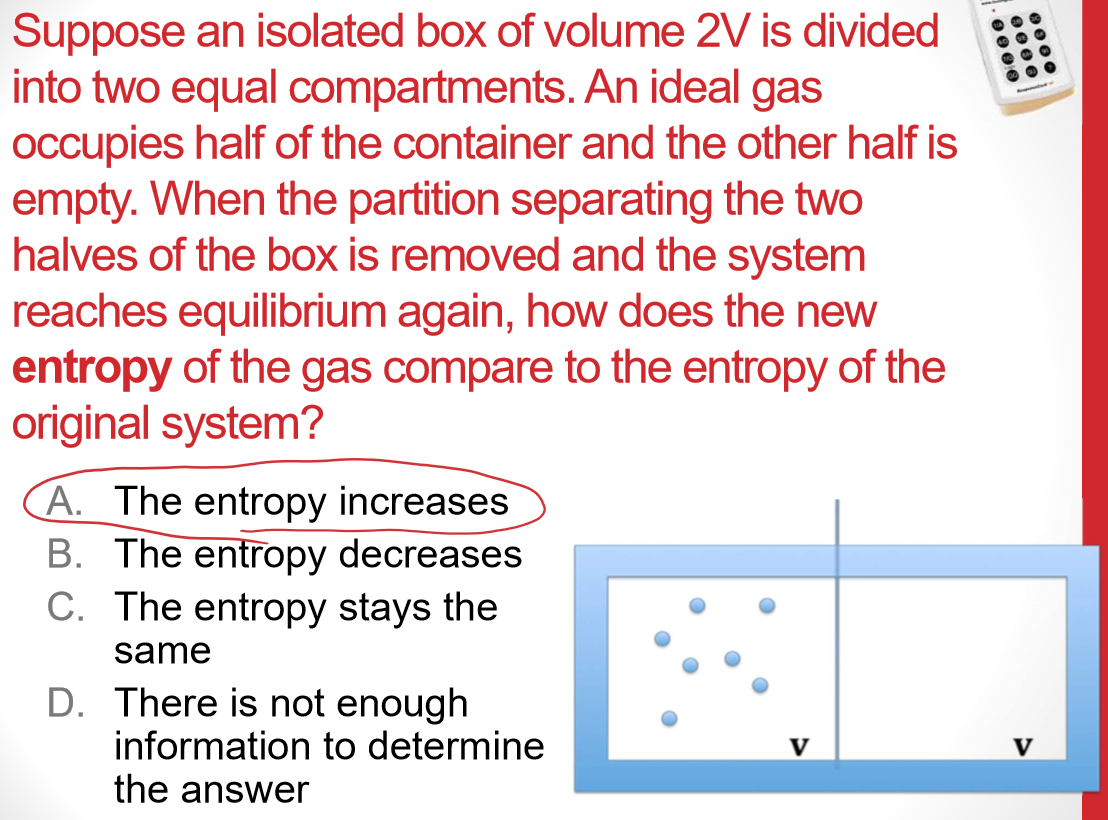
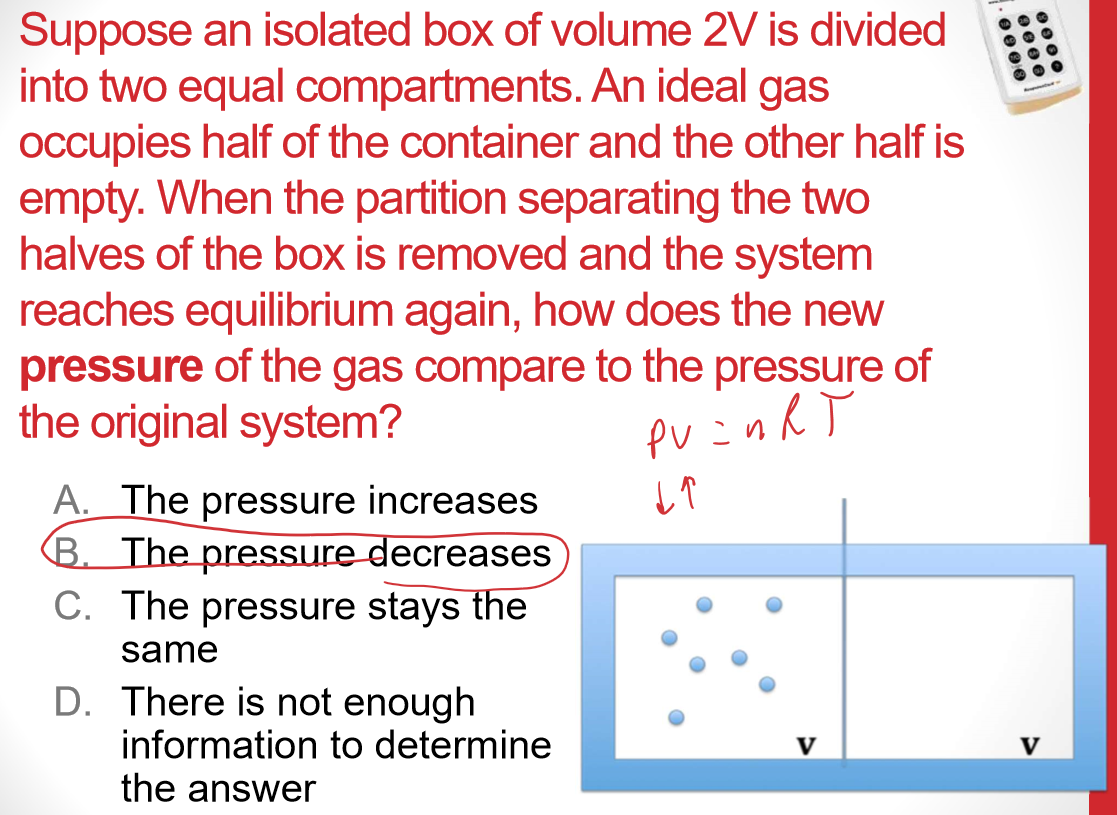
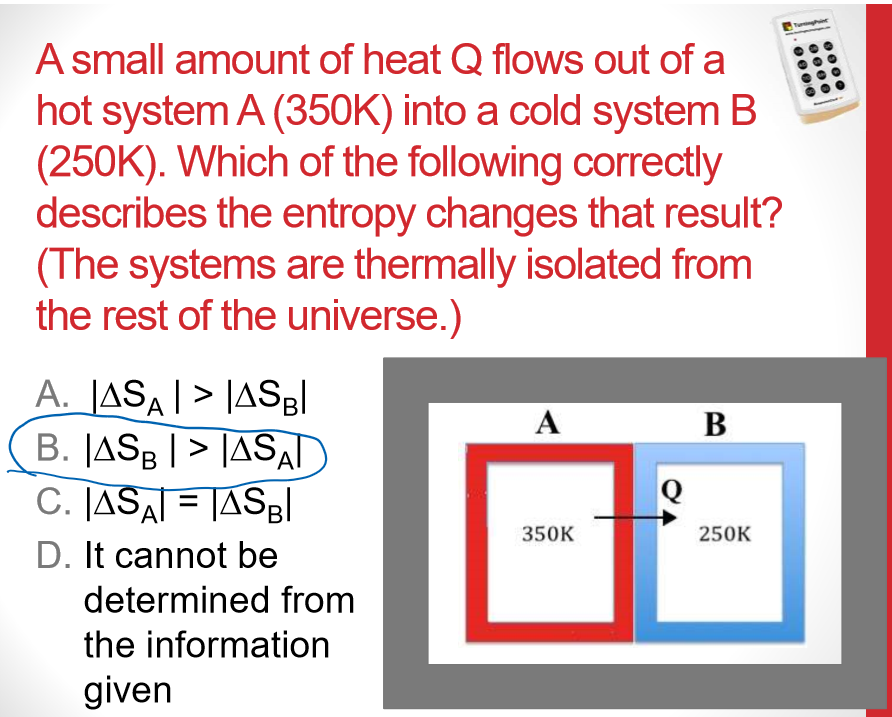
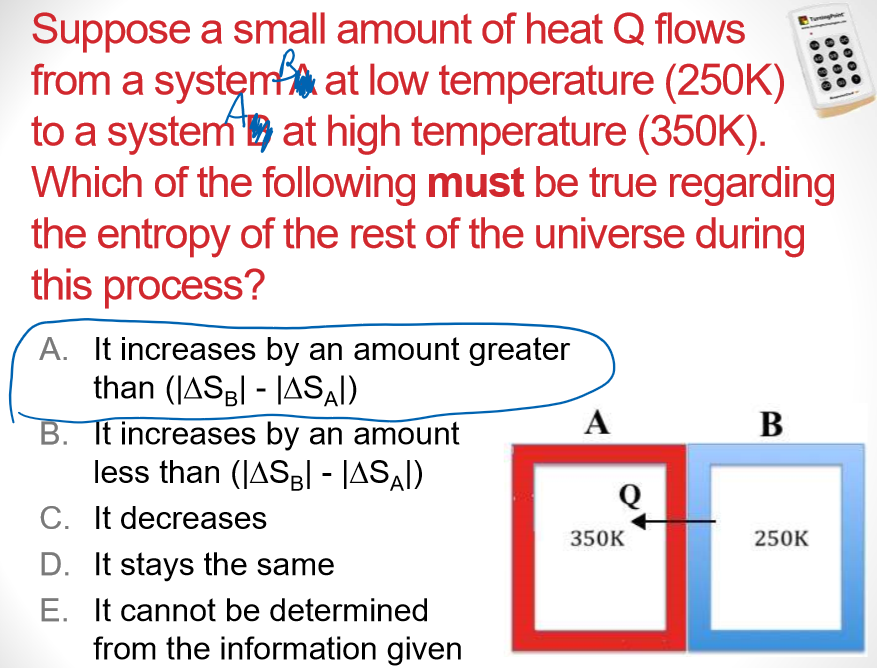
Universe S must have increased more than
- 1st law = energy is conserved
- entropy of AB decreases
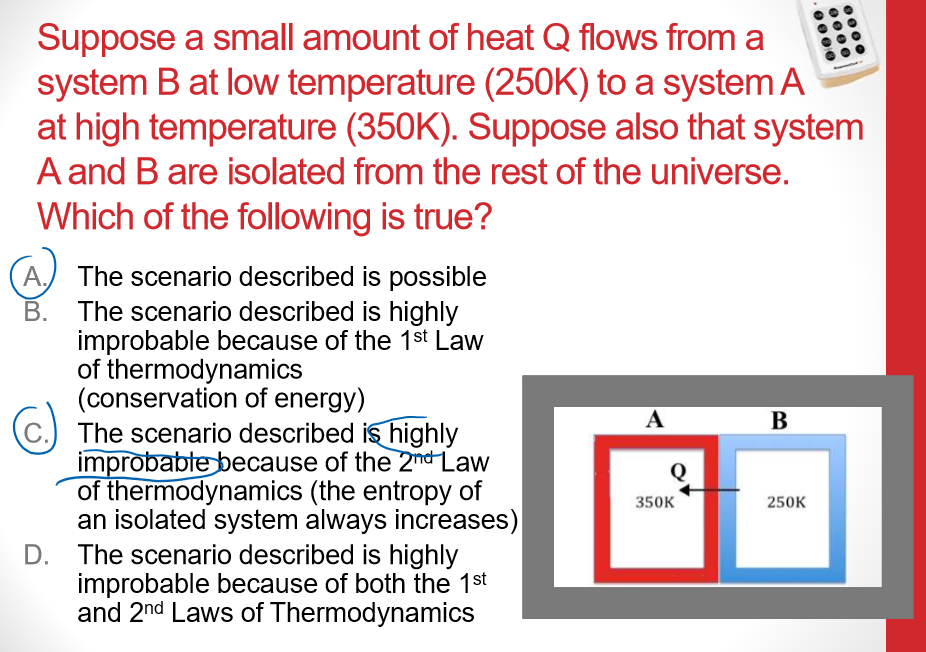

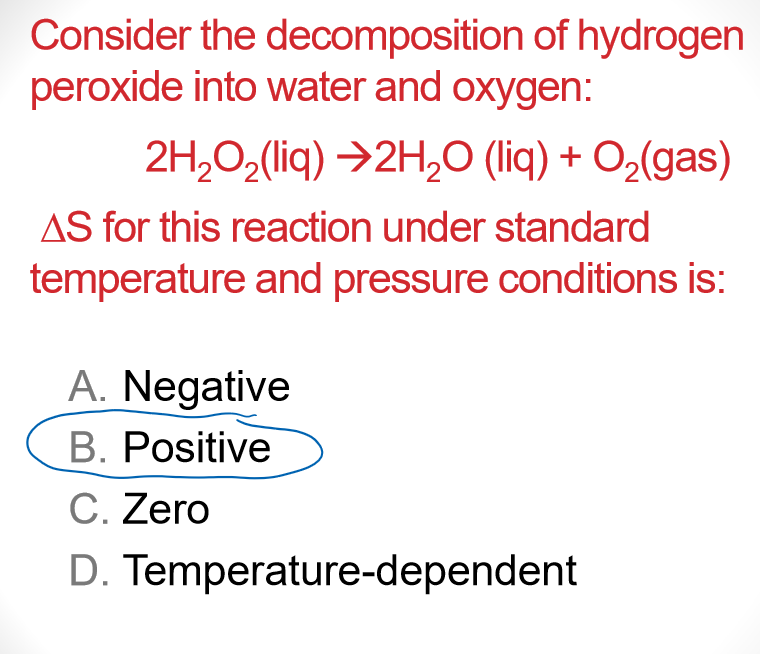


- Assumption ,
- If