Lecture 7
Readings
http://umdberg.pbworks.com/w/page/50493326/Gibbs%20free%20energy
http://umdberg.pbworks.com/w/page/104491345/Example%3A%20Free%20energy%20of%20an%20expanding%20gas
Gibb's Free Energy
Sign tells if the change can happen spontaneously
- Negative = spontaneous
- Positive = non-spontaneous
Magnitude is the amount of energy "available" to do other work
For a system:
- Energy, Joules, Bond Energy, Expansion Energy, Volume Change
- Not an energy
- Units =
Works for constant pressure and temperature
Example,
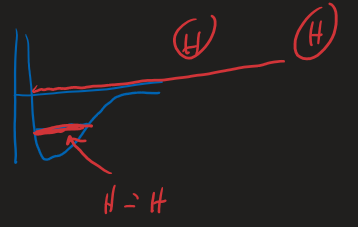
- Worked out the enthalpy before
- Define the system - just the
- System can interact with the rest of the universe, (it's not isolated), so the system can pass heat energy to and from the universe
- Enthalpy:
Reminder: the
So
What is
- Exact number is difficult, but probably negative since less molecules in a smaller volume
Note: Since
- Net entropy for the entire universe can never decrease
So
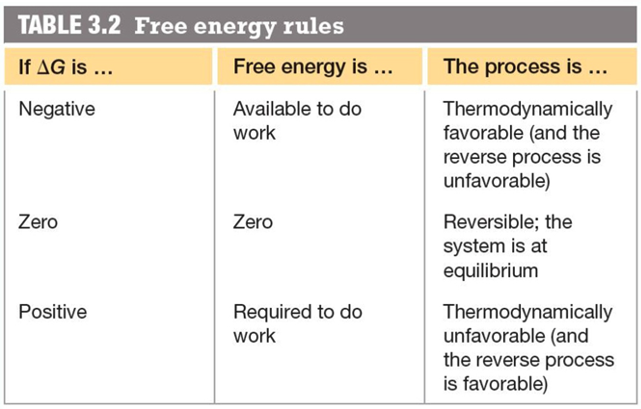
So does the reaction occur spontaneously or not?
It depends
If
if
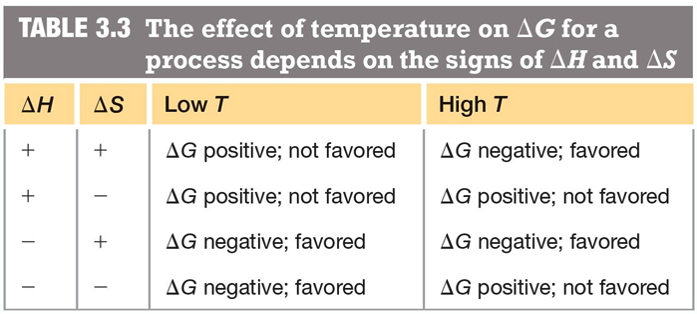
At low temperatures, the forming of bonds is favored,
At high temperatures, the thermal energy is high, molecules move faster, collisions are more violent, and bonds are likely to be broken
- So
- So
Like boiling water
- At room temperature, steam condenses to form liquid
- Heat it enough and liquid
- At room temperature, steam condenses to form liquid
Bonding is favored based on 1st law of thermodynamics, but sometimes entropy ( 2nd law ) doesn't allow it