Lecture 6
Readings
http://umdberg.pbworks.com/w/page/73084328/Example%3A%20Entropy%20and%20heat%20flow%20(2013)
http://umdberg.pbworks.com/w/page/63328761/Motivating%20free%20energy%20%282013%29
2nd Law
Direction things chagne is so entropy of the total system increases
- Total system = either the whole universe, or else an isolated system
- Its really a
- If heat energy of an object goes up,
- If heat energy of an object goes down,
- Its really a
- For a hot object near a cold object,
- But because
Gibb's Free Energy
- Combines conservation of energy ( 1st Law ) with the direction of change ( 2nd Law ) , as we'll see in next lecture
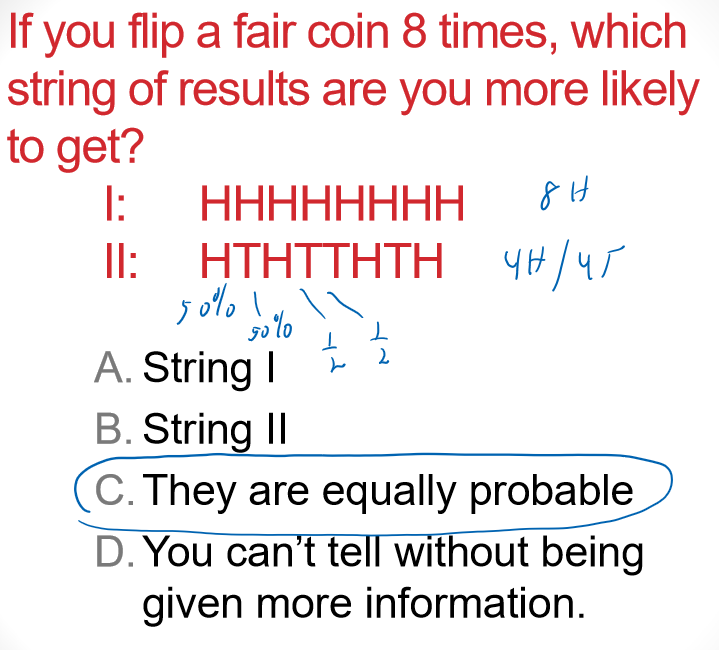
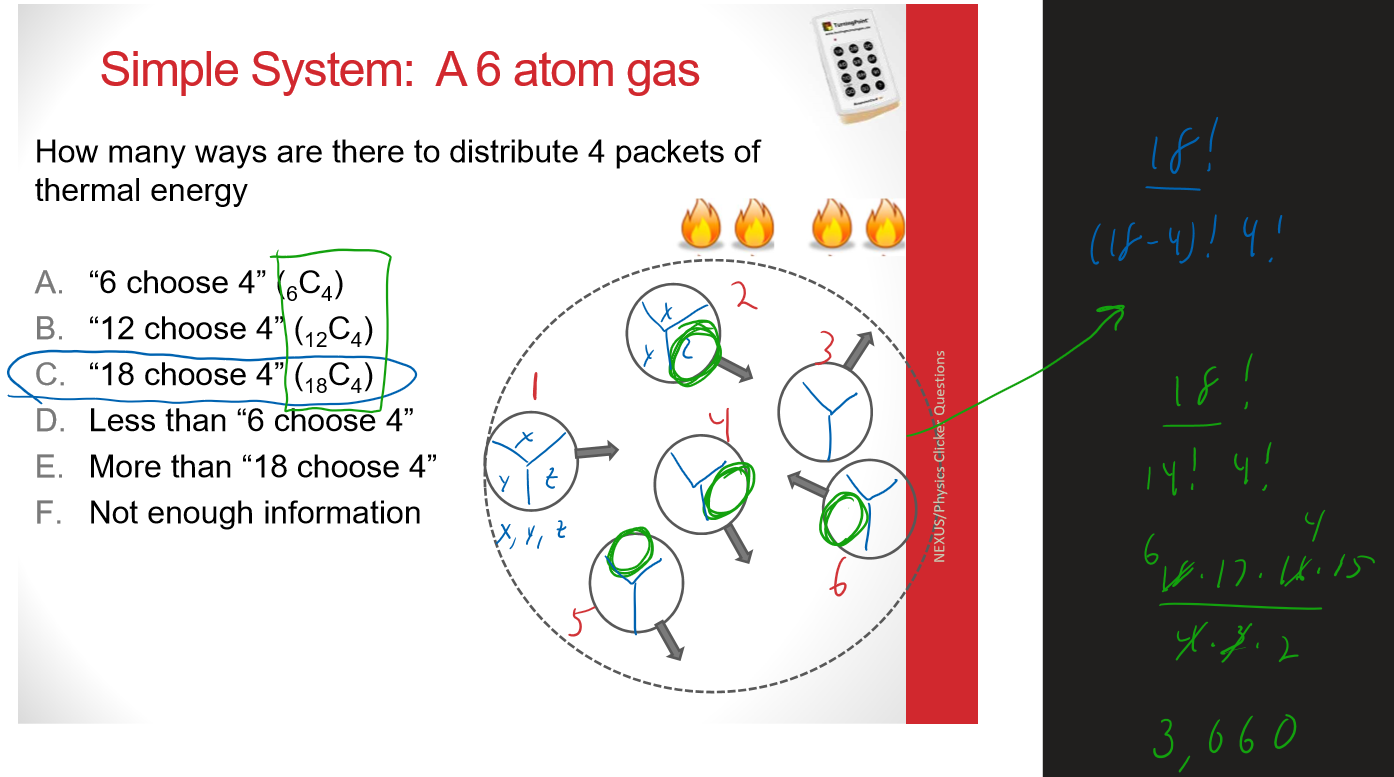
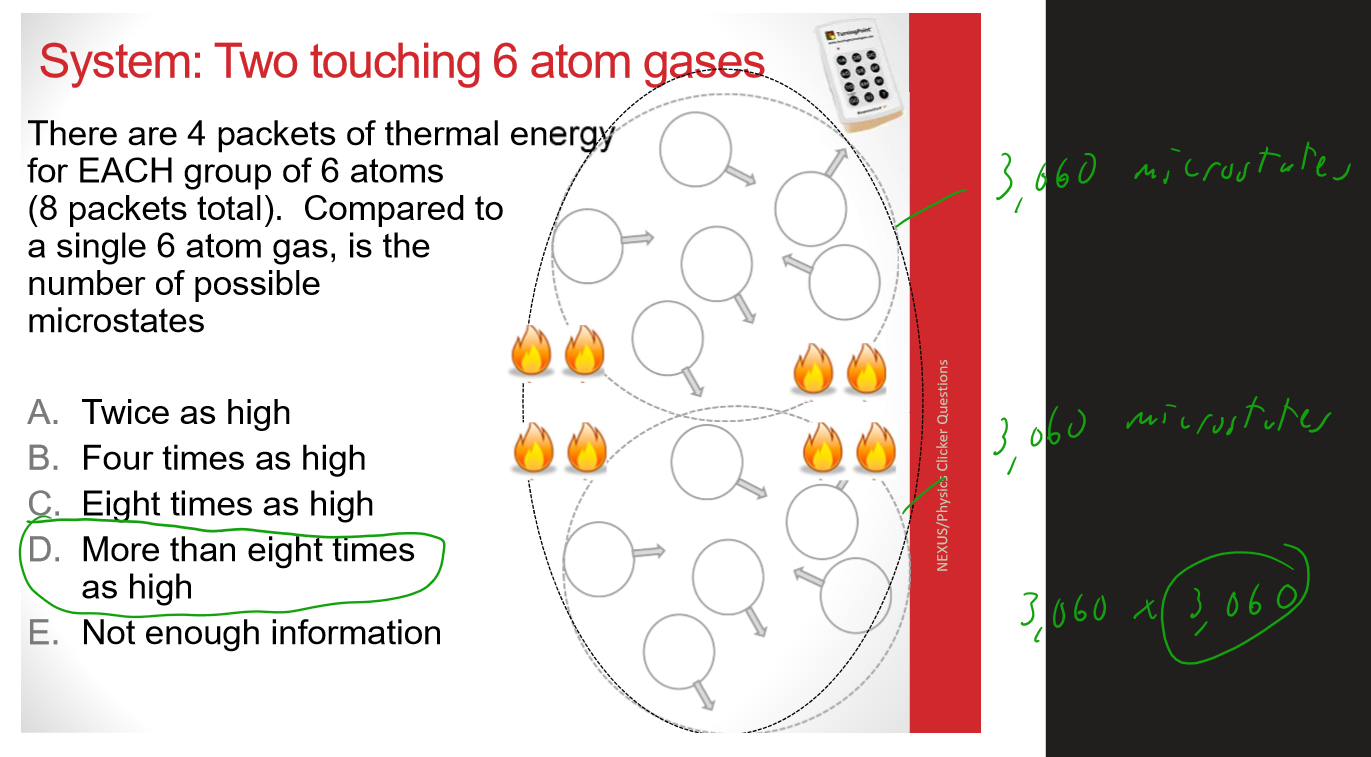
Multiple Choice