Lecture 5
Readings
http://umdberg.pbworks.com/w/page/104869513/Example%3A%20Arranging%20energy%20and%20entropy
Arranging Energy and Entropy
The 2nd law says the system will move toward the macrostate with the greatest number of microstates
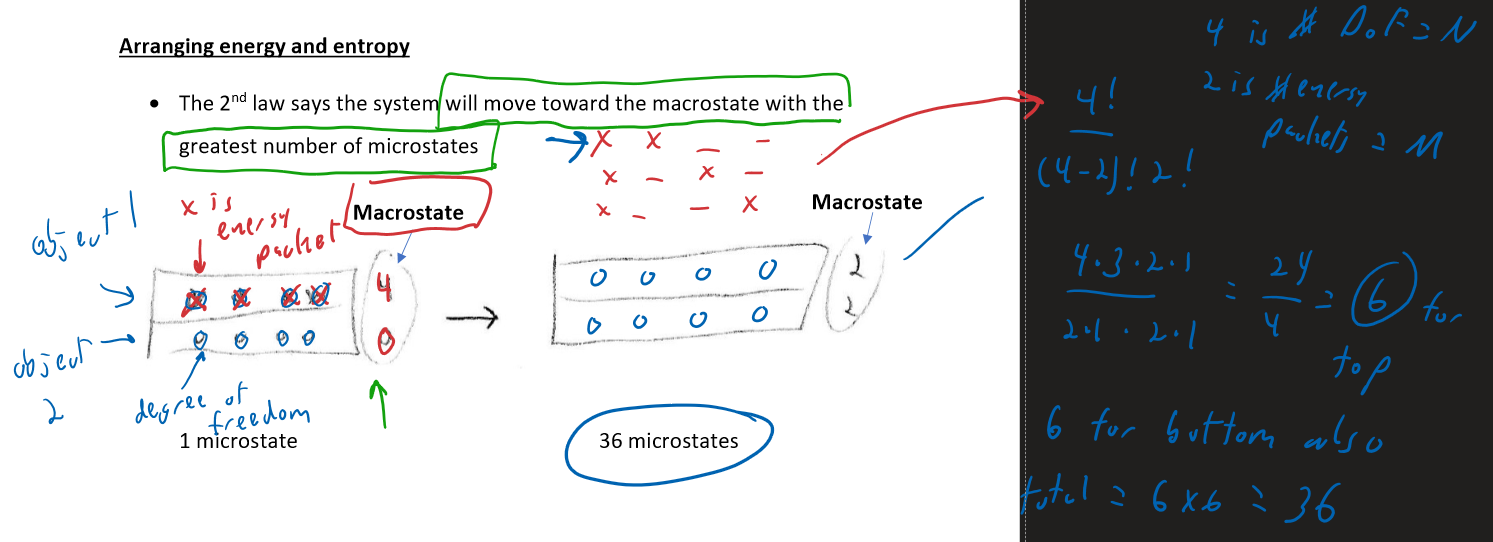
So the system will move toward the situation ( the macrostate on the right )
Rule: Microstates multiply
Rule: Entropy adds
How many microstate for:
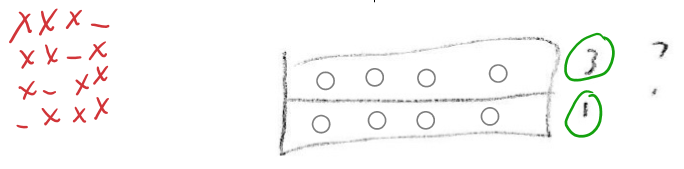
- For top material: 4 ways to arrange 3 energy into 4 degrees of freedom
- For bottom material, 4 ways to arrange 1 energy into 4 degrees of freedom
- So total number of microstates =
Entropy and Biology
Thermal equilibrium = death
Maintaining differences - temperature, pH , concentrations - is usually a matter of maintaining a lower entropy, a disequilibrium
- I say usually because sometimes, apparent order is favored by the 2nd law, such as when oil and water separate
Local entropy can decrease ( for example for an organism ), but only if the entropy of the universe as a whole increases
I like the though of life existing because we dip into the flow of energy from the sun, using that energy to maintain our lower entropy