Lecture 4
Readings
http://umdberg.pbworks.com/w/page/73084145/Why%20entropy%20is%20logarithmic%20%282013%29
Logarithmic Entropy
If you combine 2 systems, you can add entropy
THe number of possible microstates for combined system is the product of the numbers of microstates (W) of the 2 separate systems.
Choose
How many different sets?
Obtain 3 heads out of 7 flips
Number of ways to end up with 3 steps to right out of 7 total steps
Number of ways for 3 balls to end up in 7 boxes
Entropy as Sharing
Entropy is like tempearture, pressure, and concentration
It's a course, overview idea that doesn't care about specific molecules at specific times
The following four statements are all equivalent due to 2nd Law:
A system wants to move towards thermodynamic equilibrium
A system wants to move toward the state with the greatest entropy
A system randomly choses a macrostate with lots of microstates
- Because there's so many of these particular microstates
A system wants to spread energy evenly among all its degrees of freedom
Counting Probability vs. Distributions
In this class, we look at probabilites for 3 different systems:
- Coin flips
- Diffusion of molecules
- Putting energy packets into degrees of freedom
In each system, there is a "probability" view, and a "distribution" view
Coin Flipping:
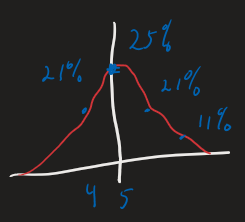
Probability View - Flip 1 coin 10 tiems.
- Microstate = specific sequence of heads and tails
- Macrostate = total number of heads
- You can calculate the probability of any particular macrostate for this 10 flips.
- The probability depends on the number of ways to get that number of heads
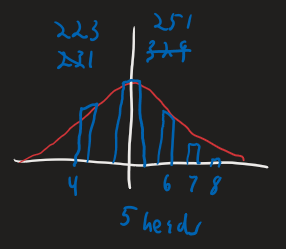
Distribution View - Flip 1000 coins, 10 times each.
Microstate = How many times heads came up on each coin
- For example, coin 1 had 4 head flips, coin 2 had 7 heads, etc.
Macrostate = a particular distribution of the number of heads
- For example, 4 heads came up on 223 coins, 5 heads came up on 251 coins, etc.
This distribution is highly likely to be similar to the probability found in the probability view.
As you increase the number of coins used, the distribution becomes fore and more like the probability view
Molecule Diffusion
Probability View - 1 molecule takes 10 steps
- Microstate = a specific sequence of steps, L R R 0 L L etc
- Macrostate = final location of the 1 molecule
- You can calculate the probability of any particular final location
Distribution View - 1000 molecules, each takes 10 steps
Microstate = final location of each molecule.
- For example, molecule 1 ended up at -3 , molecule 2 ended up at +4 , etc
Macrostate = a particular distribution of the final locations.
- For example, 147 molecules ended up at -3 , 238 ended up at +1 , etc
This distribution is highly likely to be similar to the probability found in the probability view.
As you increase the number of molecules, the distribution becomes more and more like the probability view
Energy Packets and Degrees of Freedom for 2 Objects
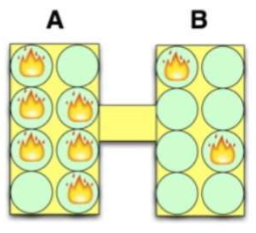
Probability View - 1 set of 2 objects with say 8 energy packets
Microstate = a particular arrangement of each of the multiple energy packets among all the degrees of freedom in the 2 objects
Macrostate = the number of energy packets in each object
- For example, 6 in the left object and 2 in the right object
You can calculate the probability of any particular macrostate by counting the number of ways to arragne the packets to create each macrostate.
- Assumption: each degree of freedom is equally likely
Distribution View - 1000 sets of 2 objects, with each set having 8 energy packets spread between the 2 objects
OR , observe 1 set of the 2 objects at 1000 different time points.
Microstate = the specific division of energy packets at each observation
- For example, 1st set/time point has 5 energy on left and 3 on right; 2nd set/time point has 4 on left and 4 on the right, etc
Macrostate = a particular distribution of the energy packet divisions
- For example, 3L and 5R occurred 265 times, 4L and 4R occurred 341 times, etc
This distribution is highly likely to be similar to the probability found in the probability view.
As you increase the number of set/time points, the distribution becomes more and more like the probability view
Main Message
We spend a lot of time calculating the probability of various arrangements for 1 molecule/set of objects.
This is because the probability matches up with what the system will look like if you have a large number of molecules and/or give the system enough time
This is the 2nd Law of Thermodynamics
In reality, you've got trillions * trillions of molecules, or trillions * trillions of energy packets, each doing their own random choices
The number of ways to get certain macrostates is much, much higher than other macrostates, so the system moves toward the likely macrostates.
And the macrostate for these trillions * trillions is a distribution
6 million energy packets on the left and 2 million energy packets on the right is an extremely unlikely macrostate.
- So the system (if they are isolated from the surroundings and in thermal contact) will head towards something close to 4 million on the left and 4 million on the right.
- It will fluctuate and won’t always be exactly 4 million each.
Whenever you observe the system, the macrostate you find will be randomly chosen from all possible macrostates, but according to the probability of each.
- But the sun will go out before you ever find the system with 6 million on the left and 2 million on the right.