Lecture 1
Readings
http://umdberg.pbworks.com/w/page/104122763/Example%3A%20Degrees%20of%20freedom
Thermodynamic Equilibrium
When an object is in equilibrium with its surroundings, it is still exchanging energy with its surroundings
- But equal amounts of energy go in each direction.
- Object -> Surroundings = Surroundings -> Object
Systems naturally move toward equilibrium
As a system ( system = multiple objects, or object plus its surroundings ) moves toward equilibirum, there is a net direction of energy flow, and this flow can be used
Like water flowing down a slope
- You can put in a water wheel and generate mechanical energy by the spinning of the wheel
- But water in a still lake won't work
Equipartition of Energy
Always applied to molecular scale, so not a baseball
When a system is in thermodynamic equilibrium, each degree of freedom will have thee same amount of energy, on average.
Degrees of Freedom: A molecule in 3-D space can move in 3 possible directions
Think x,y,z axes
- So 3 degrees of freedom for moion
Kinetic energy will be evenly divided among these 3 directions
Notice the statement says on average.
- So average kinetic energy due to motion of molecules in x-direction is the same as the average kinetic energy due to motion of molecules in the y-direction.
But molecules have a distribution of kinetic energies:
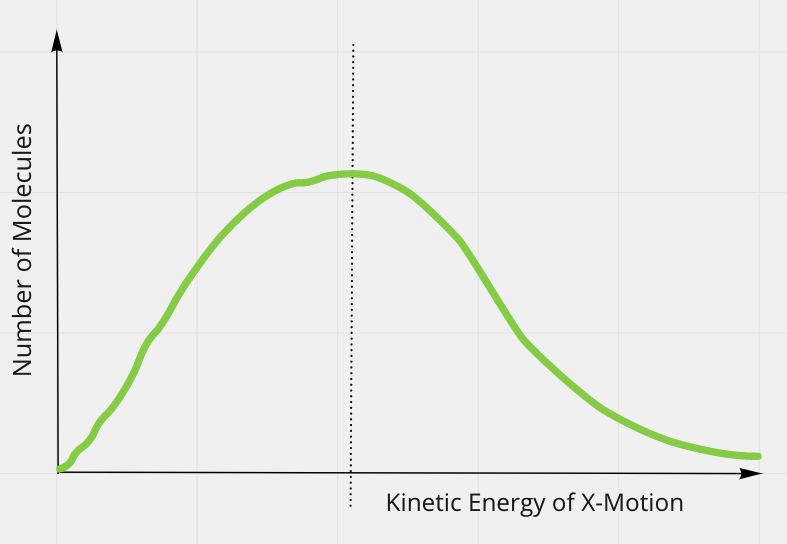
Degrees of Freedom
Basically a counting game.
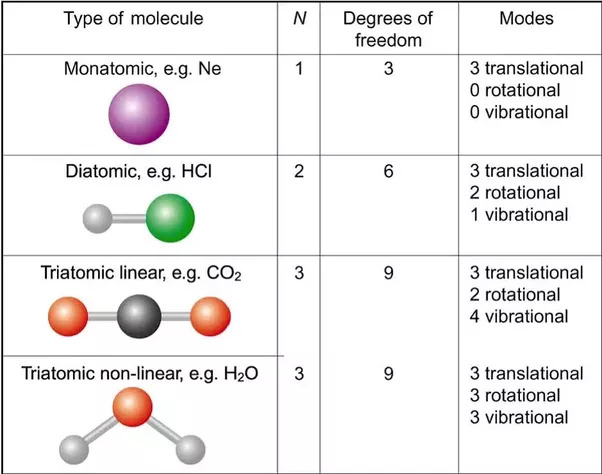
Add up which of the following motions can occur for your molecule
Translational kinetic energy - velocity in a direction
- x,y, and z if 3-D motion possible
Rotational kinetic Energy = spin about an axis
Spin about x,y, and z.
- But watch out for symmetry, see below\
Vibrational Energy
- Kinetic Energy of Spring
- Potential Energy of Spring
Each option above adds
Example: N molecules of ideal gas have x,y,z translational, nothing else.
- So 3 degrees of freedom
If, when a molecule rotates, it doesn't "change locations" ( due to symmetry ), that rotation doesn't count , Like spinning a rod
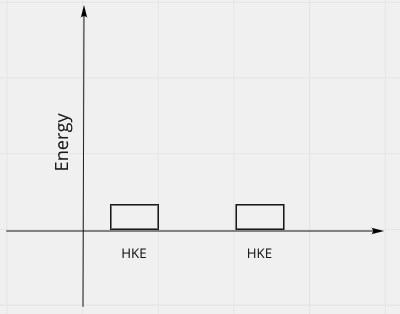
Energy Bar Charts
- Prepare for kinesin problem
- Consider the formation of a bond
- Before, have 2 free atoms, far apart, each with very little kinetic energy:
- After, the Hydrogens are bonded:
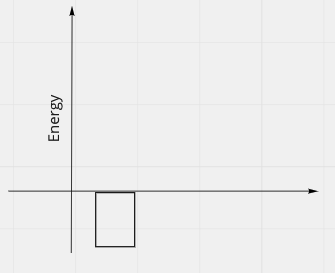
- We'll discuss why energy is below the line and negative, in the next section
- Where did energy go?
- In the first picture, total energy was positive
- In the second picture, total energy is negative.
- But total energy can't be created or destroyed.
- There must be some positive energy in the second picture.
- So after picture should look like:
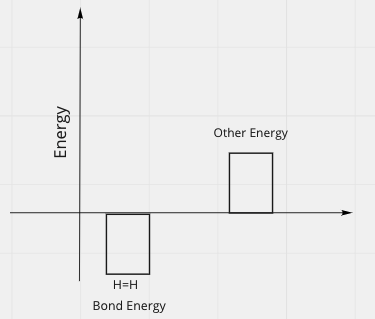
Other energy could be:
- Motion (kinetic energy) pf H=H molecule
- Or it could be motion of surrounding molecules = heat released by reaction
Chemical Bonds
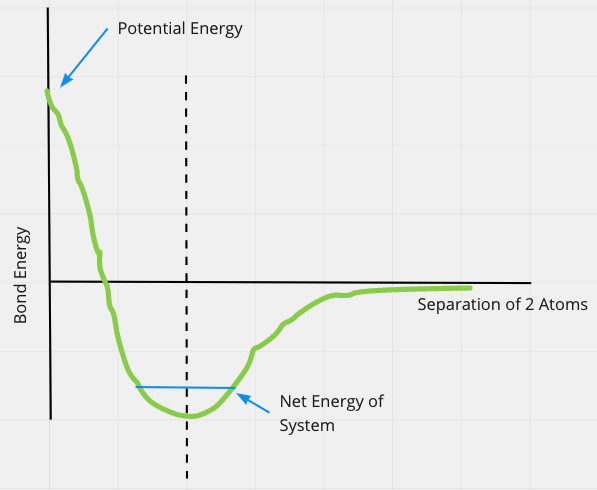
Define the zero energy to be when the 2 atoms are far apart
When bond exists, net energy is negative
Need to add energy to break a bond
- Need to bring net energy back up to at least zero
Need to remove energy to form a bond
- Unbound atoms/molecules have zero or more energy on this scale
Basic Problems
- How many degrees of freedom fro 25 molecules of
- 3 Translation + 2 Rotational = 5 total Degrees of Freedom per molecule
- No vibrational because of low temperature
- =
- How many degrees of freedom for 25 molecules of
3 Translation + 2 Rotational + 2 Vibrational = 7 total Degrees of Freedom per molecule
=
- If you added the same amount of heat energy (say 10 Joules) to both 25 molecules of
- More degrees means more places to put the energy
At Low Temperature:
At High Temperature:
is the same
- So at high temperatures,
is smaller