Biochemistry 4230 - Exam 3 - Study Highlights
01 - Lipid Structures

Which of the following populations of fatty acids would most likely be a solid at room temperature?
A saturated fatty acid with 20 carbons
- The saturated fatty acid population with 20 carbons will have the most intermolecular interactions occurring between acyl tails of fatty acids, causing them to be more stable and therefore more likely solid at room temperature.
- Saturated fatty acids have more interactions possible than unsaturated fatty acids with the same number of carbons.
- Trans fatty acids are unsaturated and have more possible interactions than cis fatty acids, assuming the number of carbons is the same.
- The omega fatty acids have a cis bond that causes “kinks” in the acyl tail and thus impedes acyl tail interactions.
- Also, longer-chain acyl tails have more interactions than shorter acyl tails.
02 - Fatty Acid Oxidation
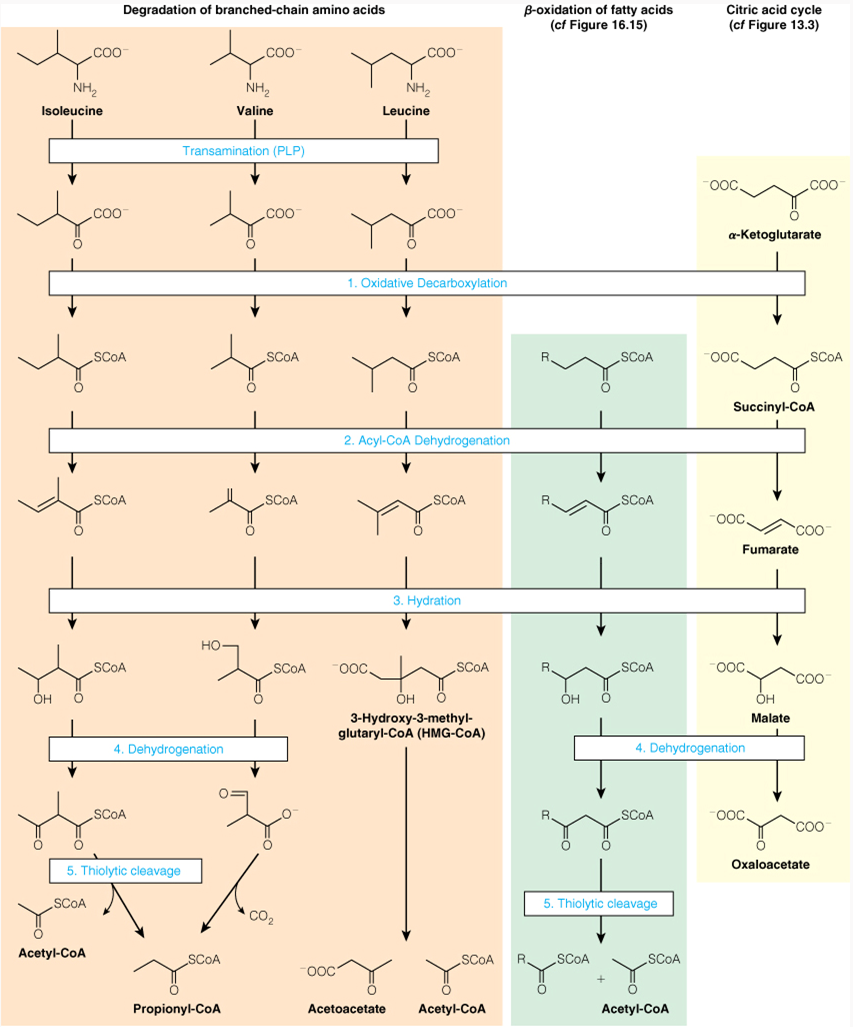
Branched-chain Amino Acid Oxidation, Fatty Acid β-oxidation, and the Citric Acid Cycle Share A Common Chemical Strategy
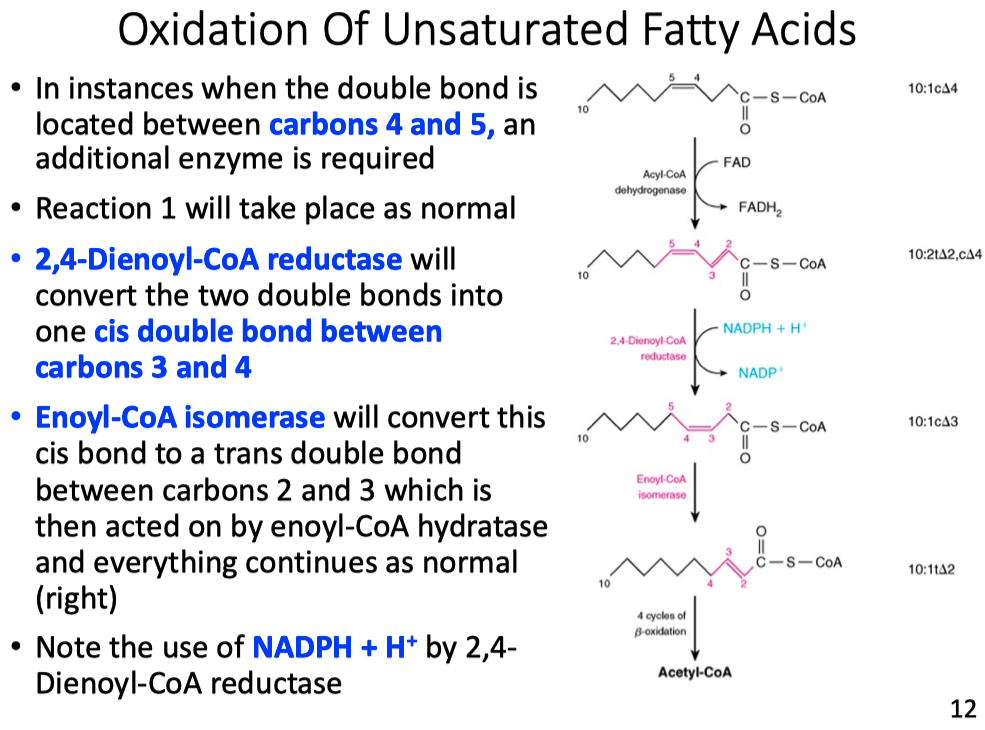
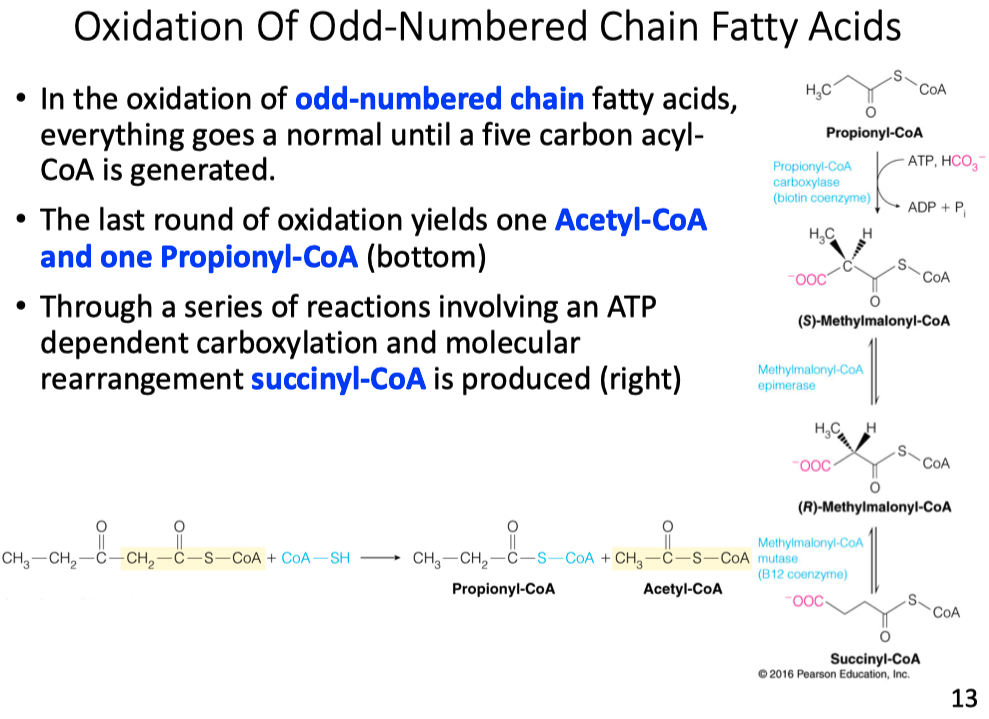
In a healthy person, if we labeled palmitate with the label shows up in glucose. This means that in the strictest sense of the word carbon atoms from a fat are incorporated into glucose. However, biochemists say, "You cannot make glucose from fat." How can you reconcile this apparent contradiction?
you can make glucose that has the label from oxaloacetate
The fatty acid is what is labeled
Biochemists say you can't make glucose from fat
Theoretical Idea:
as you are breaking off acetyl-CoAs through -oxidation , you are making a two carbon chain aka acetyl-CoA
When Acetyl-CoA enters into the TCA cycle with oxaloacetate , it combines to form citrate
- However in steps 3 and 4 in TCA cycle we are loosing a carbon dioxide
The fatty acid contributed two carbons, but as it goes into the TCA cycle, two carbons are lost
The general idea is you can't make sugar by oxidizing fat because your "net" gain is zero carbons
However,
The carbons that are lost as carbon dioxide are originally from oxaloacetate
So theoretically the carbons that came from acetyl-CoA could be turned into malate
- malate can leave the mitochondria and can then be incorporated into glucose via gluconeogenesis.
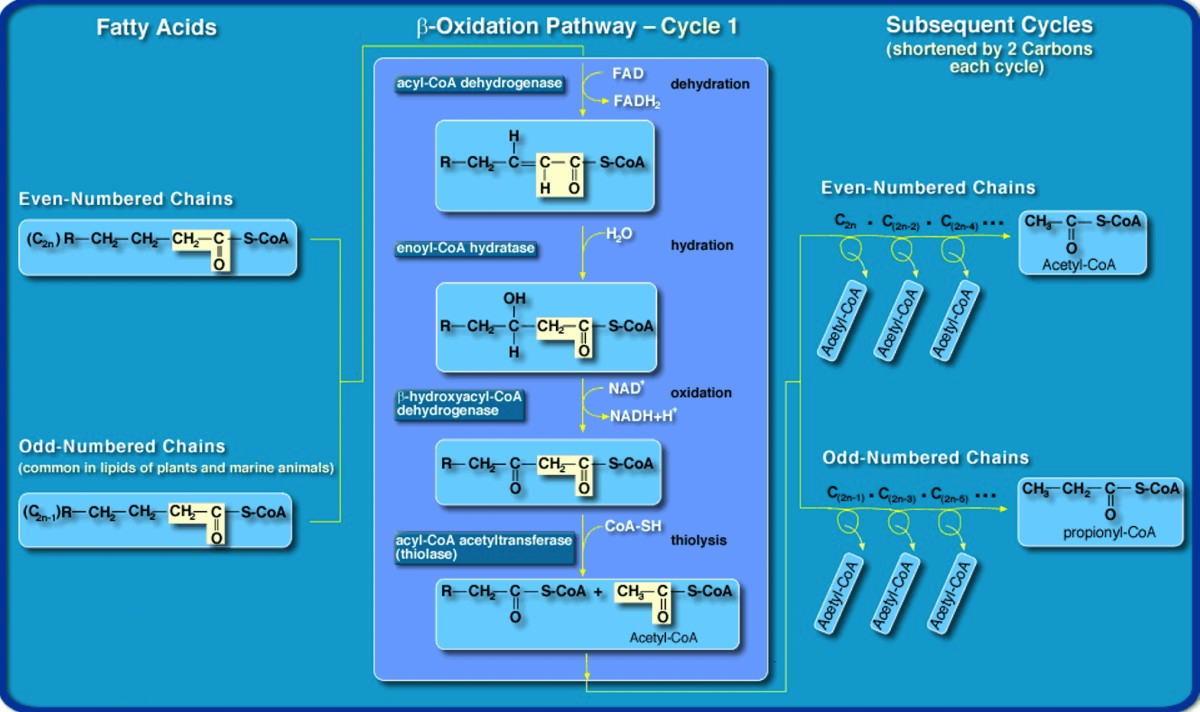
Explain why glucose could be synthesized by metabolism of a C17 carbon fatty acid.
- Odd number fatty acid chains produce a propionyl-CoA
- propionyl-CoA gets converted into succinyl-CoA
- succinyl-CoA is then converted into oxaloacetate
- oxaloacetate is a substrate in gluconeogenesis
How many moles of glucose could theoretically be produced from one mole of C17:0 fatty acid?
- Glucose has 6 carbons
- The fatty acid gives only (1) propionyl-CoA
- 2 oxaloacetates are needed to make 1 glucose
- so 0.5 moles of glucose could be made from an odd chain fatty acid
Muscles are made up of cells called fast twitch and slow twitch. Fast twitch muscle generally appears white while slow twitch is brown due to additional mitochondria with their ETC proteins. Muscles burn fat all the time, especially slow twitch muscle. During periods of high demand fat can only be burned so fast because there are only so many copies of the protein and enzymes involved in ETC and oxidative phosphorylation. As a result, muscle burns glucose as we have many more copies of these enzymes. Explain why it is important that we have muscle cells that have many copies of the enzymes for glycolysis.
- You have different types of energy production for different types of muscle fibers
- Fast twitch fibers have very few mitochondria, therefore they rely on lactic acid fermentation.
- Slow twitch muscle fibers have mitochondria and can afford the time to run through glycolysis and ETC to generate energy.
Inhibitors of fatty acid degradation are used to relieve angina ( heart pain ) resulting from insufficient oxygen. Why does this make sense?
If we prevent fatty acid degradation or fatty acid oxidation
we are not going to produce acetyl-CoA
acetyl-CoA is not going to go through the TCA cycle to make NADH and FADH2
- its not "consuming" oxygen as the final electron acceptor in the ETC
So it will provide more oxygen for the tissues because its not being consumed in ETC, but less energy
As you may know, camels don't need to consume much water, which is why they are used in desert conditions. Where does the water come from? Be sure to consider water as a reactant and product in your answer.
- The hydrocarbons stored as fats in the camel's hump are degraded and combined with oxygen to create
Briefly explain why the values calculated in ATP yield per carbon for glucose and palmitate using theoretical in vivo values for ATP production vs standard free energies differ.
Standard energies and ATP yield energies are looking at in in vitro vs in vivo
When you are looking in vivo :
- we have to consider all the other molecules and concentrations in the system
- you have to consider the concentration of ATP already inside the cell
Standard free energies are just a theoretical in vitro value
Intracellular concentrations are different than values used to calculate standard free energy
ATP/ADP ratio in vivo is times larger than equilibrium ATP hydrolysis altered in reaction coupled to it
What do these results say about the relative efficiency of oxidizing carbohydrates and fats?
- No matter what, Fatty Acids are going to give you a higher percentage of ATP per carbon molecule
- Fats are much more efficient at giving us energy per carbon molecule
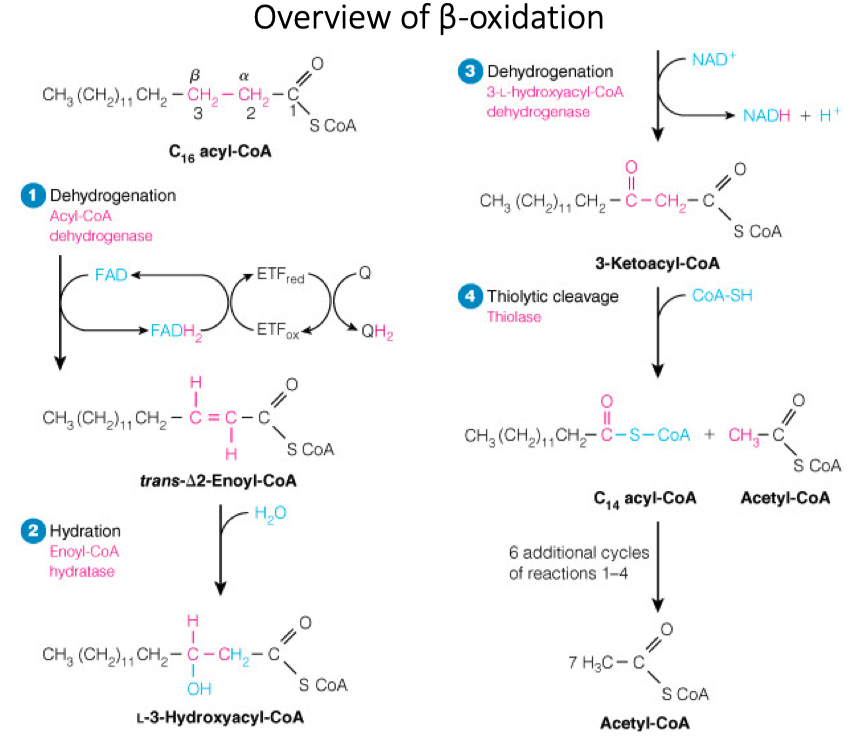
What is oxidized in each round of beta-oxidation?
The beta carbon of the acyl chain
What is reduced in each round of beta-oxidation?
NAD+ and FAD
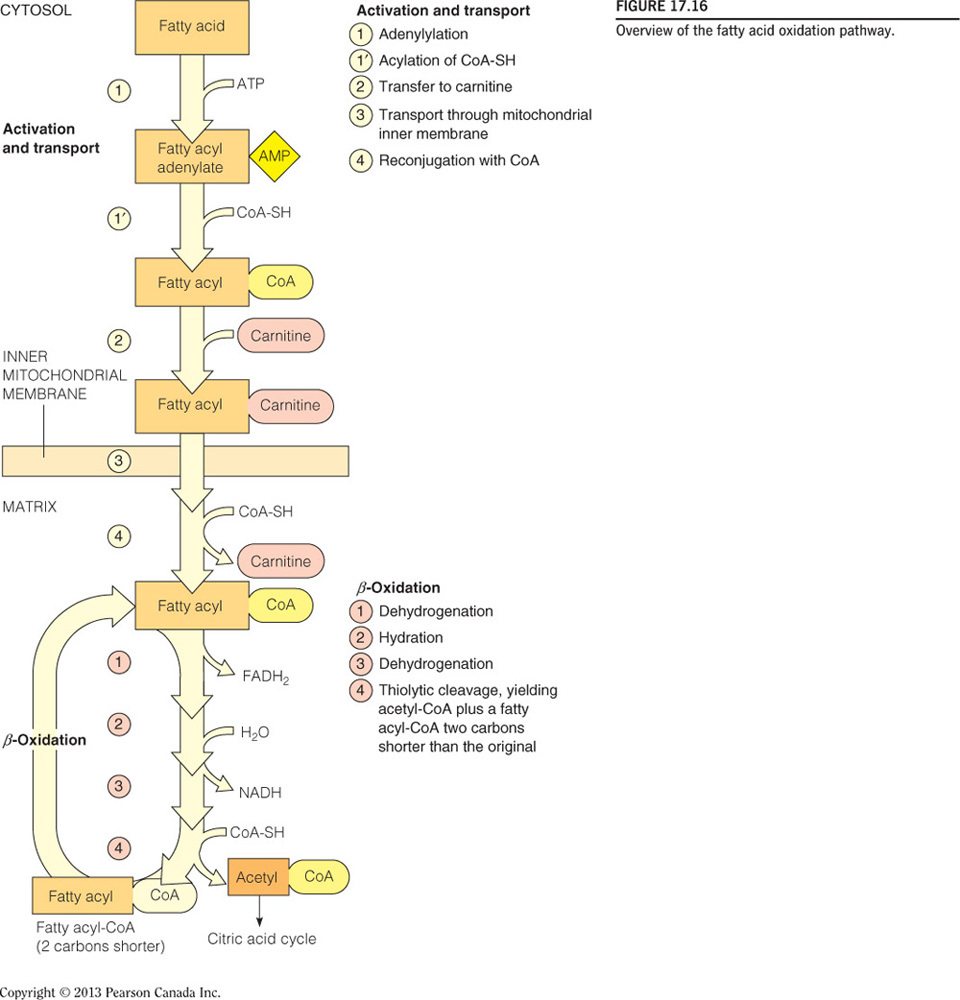
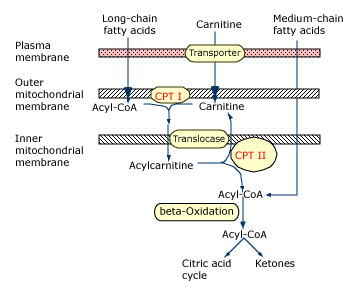
Which of the following is the correct sequence of events (from start to finish) in cellular fatty acid oxidation?
Fatty acid activation → carnitine shuttle → beta-oxidation
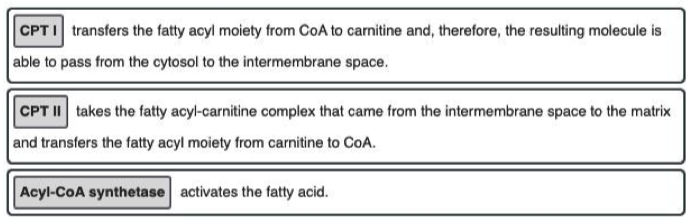
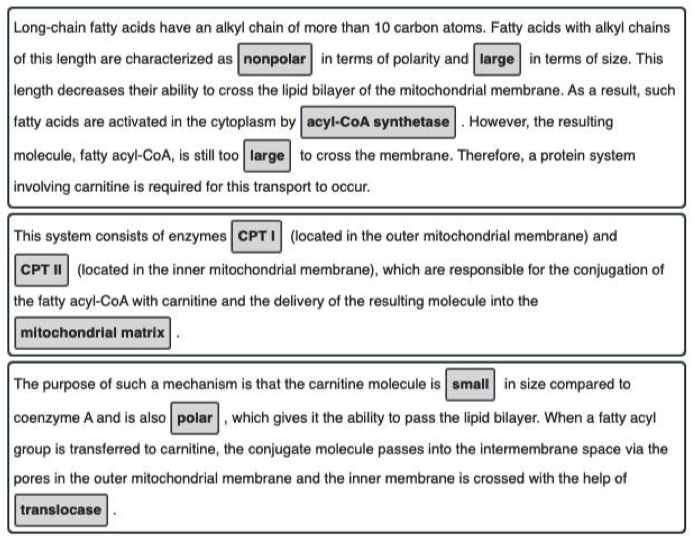
- The three phases of fatty acid oxidation in the cell, in order, are fatty acid activation → carnitine shuttle → beta-oxidation (as seen in the figure below).
- Fatty acid activation takes the initial fatty acid in the cytosol and adds a CoA to yield acyl-CoA on the inner membrane.
- The acyl chain is then passed to a carnitine to become acyl-carnitine.
- Acyl-carnitine can enter the mitochondrial matrix where the acyl group is then committed to undergo complete oxidation via beta-oxidation and the citric acid cycle.
- Beta-oxidation partially oxidizes the acyl group, releasing some NADH and FADH2, and also releasing acetyl-CoA.
- Acetyl-CoA then goes to the citric acid cycle for final oxidation.
Which of these catalyzes the “committing-step” in fatty acid oxidation?
Carnitine shuttle
02 - Fatty Acid Oxidation - Things To Know
Fatty Acid Oxidation
Free Fatty Acid Activation with CoA
- Fatty acid activation takes the initial fatty acid in the cytosol and adds a CoA to yield acyl-CoA on the inner membrane.
Oxidation Occurs in Mitochondrial Matrix
Which Molecules Can Diffuse and Which Cannot:
Can Diffuse:
- Nonpolar Molecules: , , hydrocarbons with less than 10 carbons
Cannot Diffuse:
- Polar molecules
- Ions
Free Fatty Acid Transport
- The acyl chain is then passed to a carnitine to become acyl-carnitine
- Acyl-carnitine can enter the mitochondrial matrix where the acyl group is then committed to undergo complete oxidation via beta-oxidation and the citric acid cycle.
Steps in Beta Oxidation
Steps:
- Dehydrogenation / Oxidation
- Hydration
- Dehydrogenation / Oxidation
- Thiolytic Cleavage
Products:
- acetyl-CoA
- NADH
- FADH2
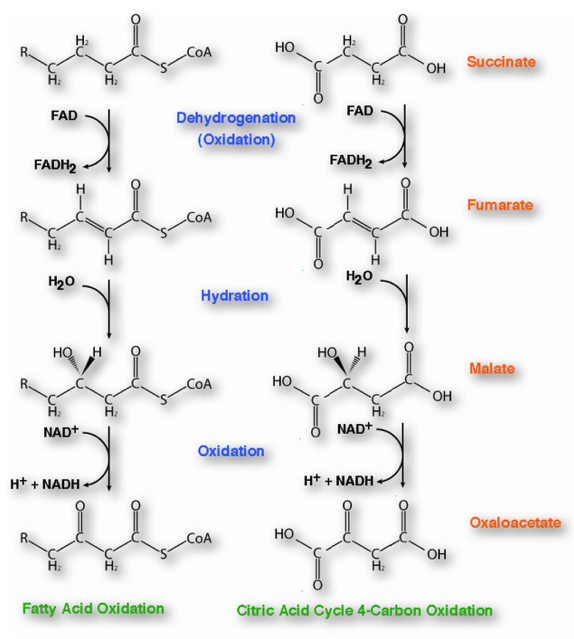
Similarities to Citric Acid Cycle:
- Both Operate in Mitochondria
- Both have Dehydrogenation , Hydration , then Dehydration Steps
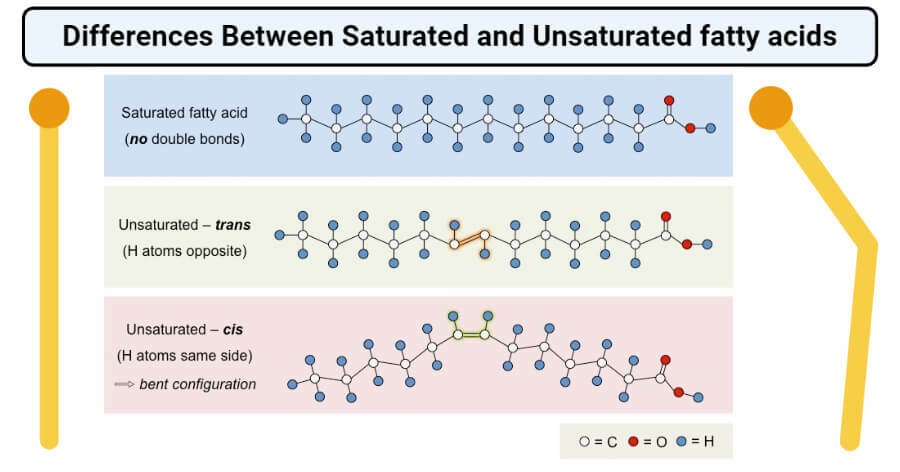
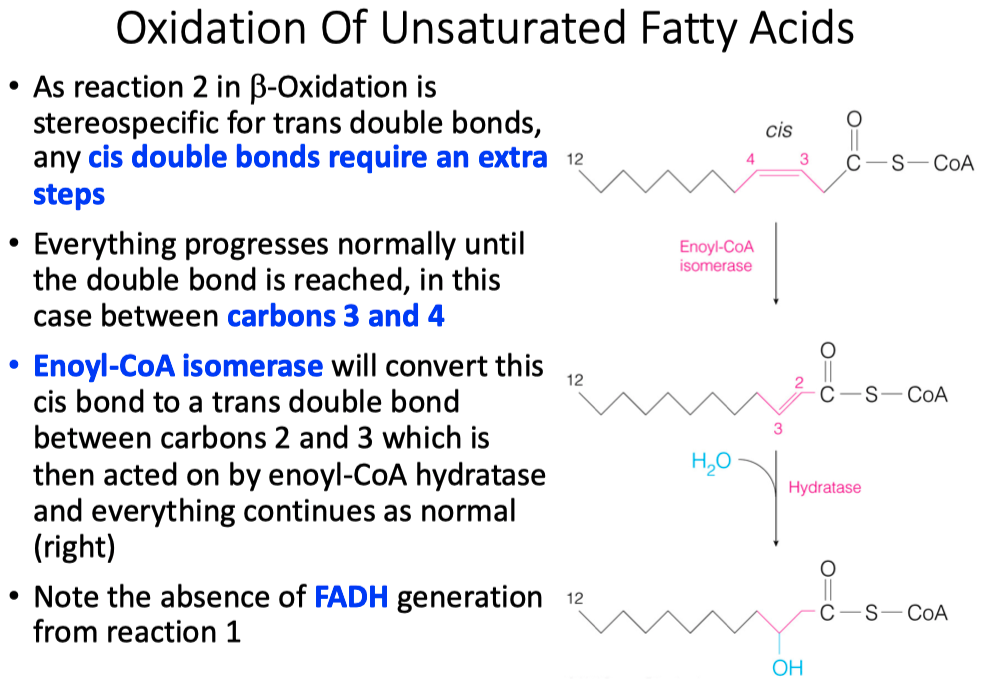
Saturated vs Unsaturated
Melting Point ( Lowest to Highest )
- Unsaturated < Cis Bonds < Trans Bonds < Saturated
Odd-Number Chain
- Odd number fatty acid chains produce a propionyl-CoA
- propionyl-CoA gets converted into succinyl-CoA
- succinyl-CoA is then converted into oxaloacetate
- oxaloacetate is a substrate in gluconeogenesis
Energy Generation for All Possible Combinations
- Fatty Acid's no matter what provide more energy per carbon than sugars
03 - Lipid Synthesis
Fatty Acid Synthesis vs β-Oxidation
| Synthesis | Degradation | |
|---|---|---|
| Greatest Flux Through Pathway | After Carbohydrate-Rich Meal | In Starvation |
| Hormonal State Favoring Pathway | High Insulin / Glucagon Ration | Low Insulin / Glucagon Ration |
| Major Tissue Site | Primarily Liver | Primarily Mitochondria |
| Subcellular Location | Primary Cytosol | Primarily Mitochondria |
| Carriers of Acyl /Acetyl Groups Between Mitochondria and Cytosol | Citrate ( mitochondria to cytosol ) | Carnitine ( Cytosol to Mitochondria ) |
| Phosphopantetheine-Containing Active Carriers | Acyl Carrier Protein Domain , Coenzyme A | Conenzyme A |
| Oxidation / Reduction Coenzymes | NADPH ( reduction ) | NAD+ , FAD ( oxidation ) |
| Two-Carbon Donor / Product | Malonyl CoA: Donor of 1 acetyl group | Acetyl-CoA: Product of β-Oxidation |
| Activator | Citrate | |
| Inhibitor | Long-Chain Fatty acyl-CoA ( inhibits acetyl-CoA carboxylase ) | Malonyl CoA ( inhibits carnitine palmitoyltransferase-1 ) |
| Product of Pathway | Palmitate | Acetyl-CoA |
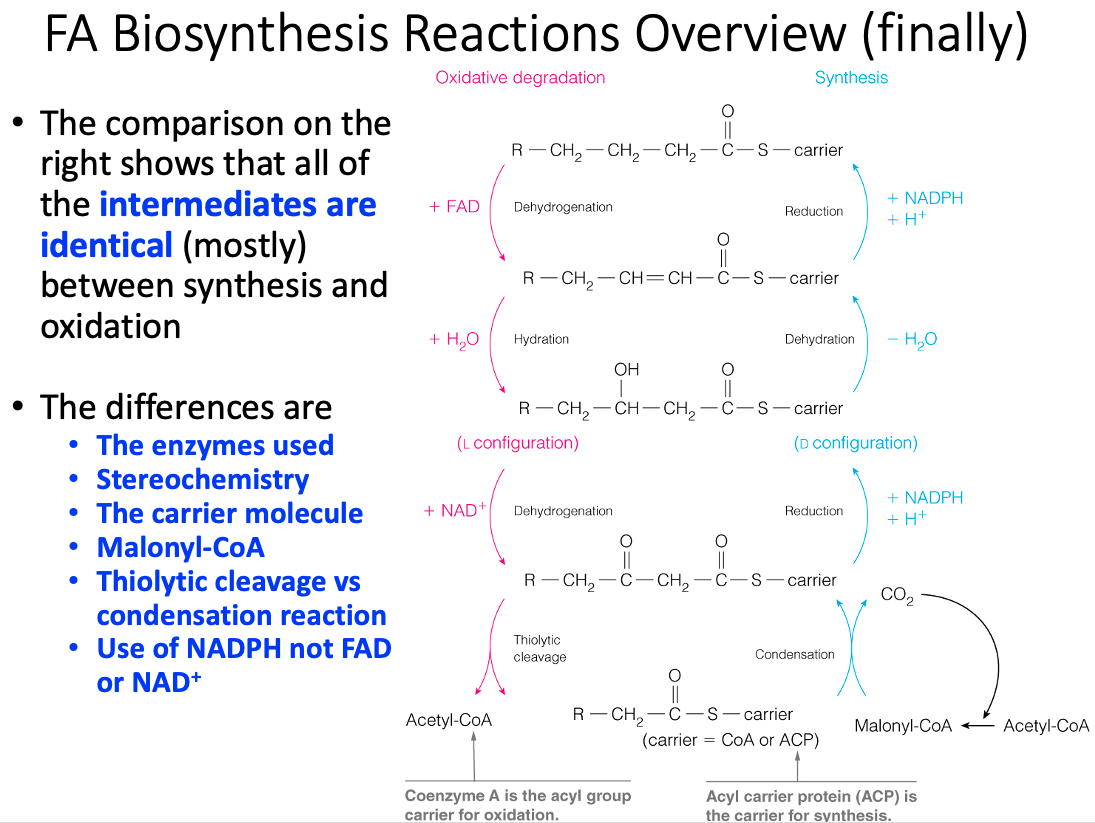
| Repetitive Four-Step Process | Condensation ,Reduction , Dehydration , Reduction | Dehydrogenation , Hydration , Dehydrogenation , Thiolysis |

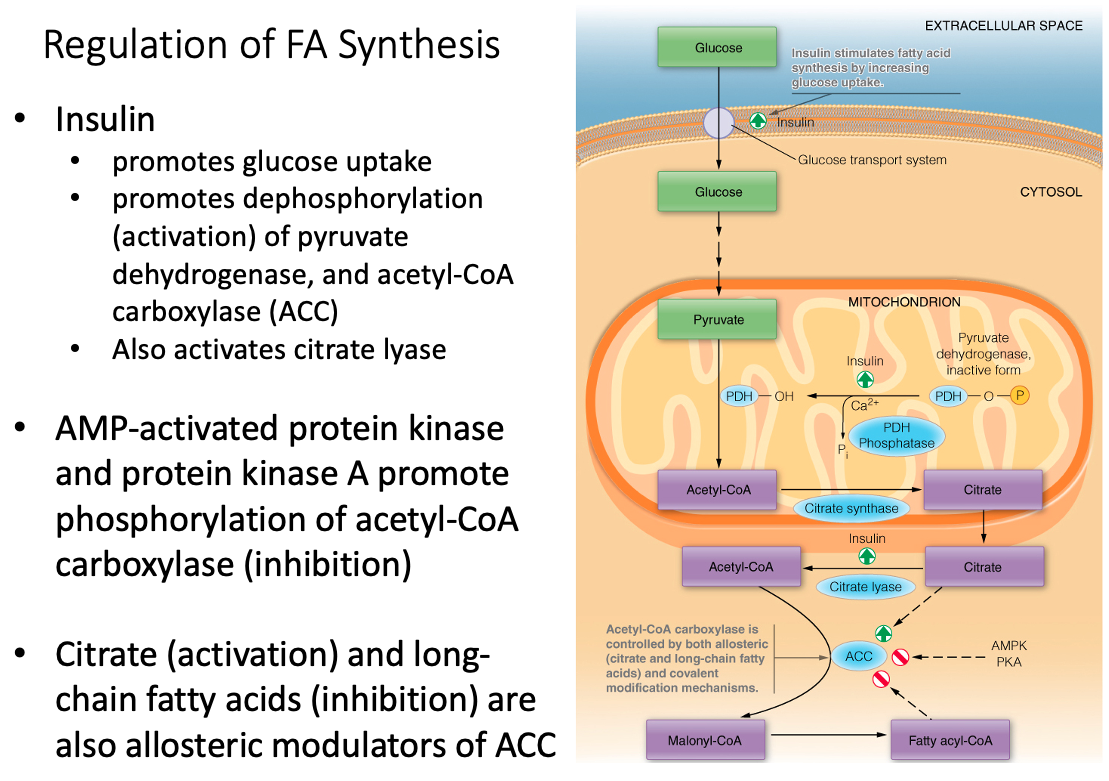
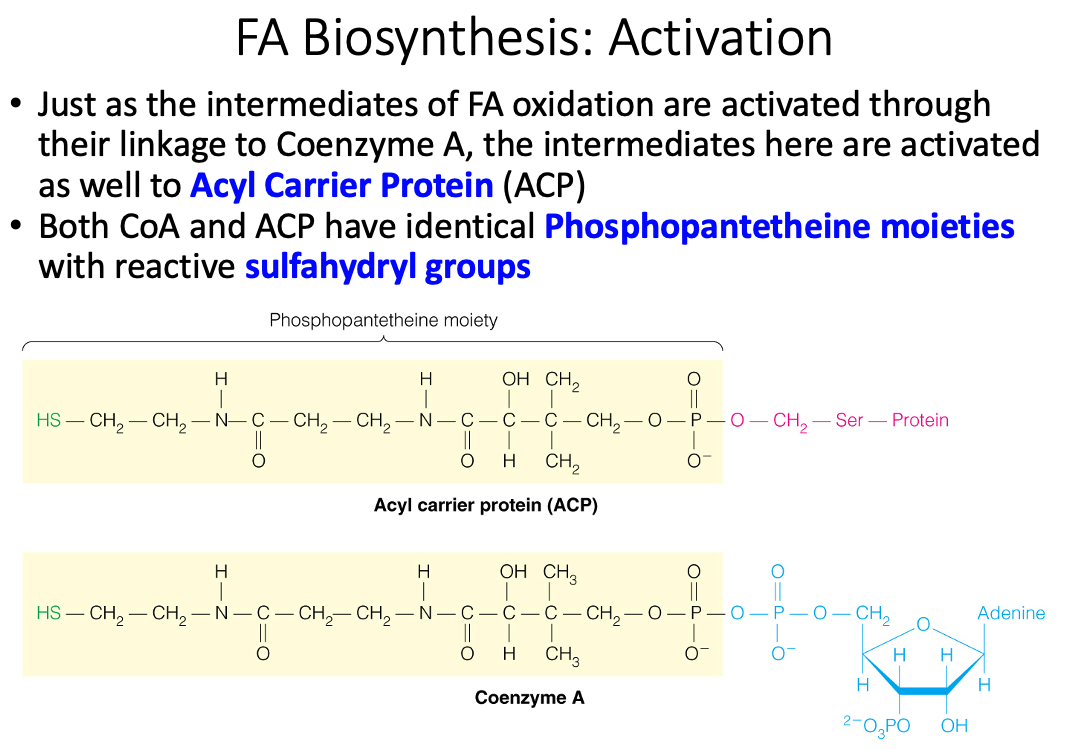
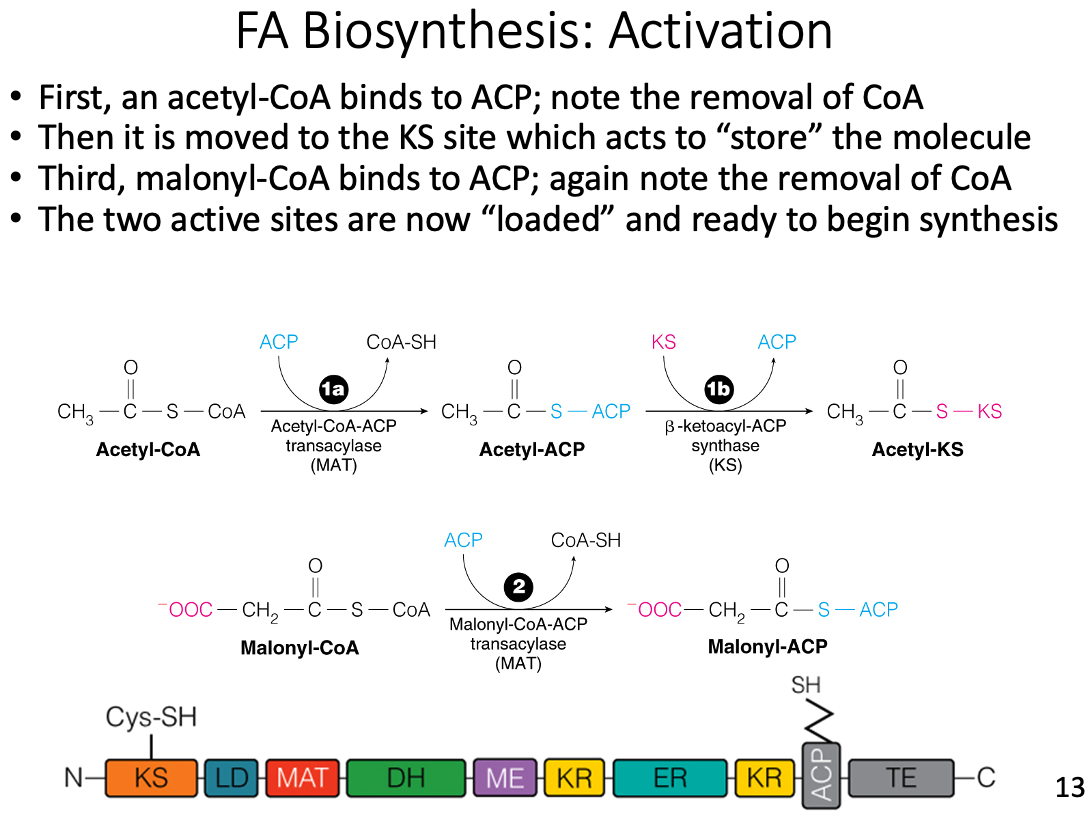

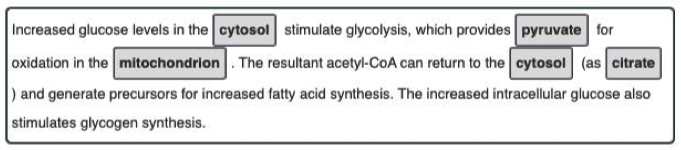
Discuss the metabolic rationale for phosphorylation of acetyl-CoA carboxylase by AMP-activated protein kinase (AMPK) and cyclic AMP-dependent protein kinase (PKA)
| -oxidation | Fatty Acid Synthesis |
|---|---|
| Catabolic | Anabolic |
| Location = mitochondria | Location = cytoplasm |
| Separate soluble enzymes are used | 1 multifunction enzyme is used |
| Acyl Carrier = CoA | Acyl Carrier = Protein bound phosphantethiene |
| NAD , FAD | NADP |
| Precursors = Fatty acyl CoA Products = Acetyl CoA | Precursors = Acetyl CoA and Malonyl CoA Products = Fatty Acid Chains |
| Regulation = Substrate Availability | Regulation = Allosteric |
Would it be possible to synthesize a fatty acid in mammalian cells starting from a C-16 or C-18 carbon fatty acid? Explain
- No , fatty acid takes place in plants
- Mammals are not able to introduce double bonds in fatty acid beyond carbon 9
- Mammals only have , , 5, and
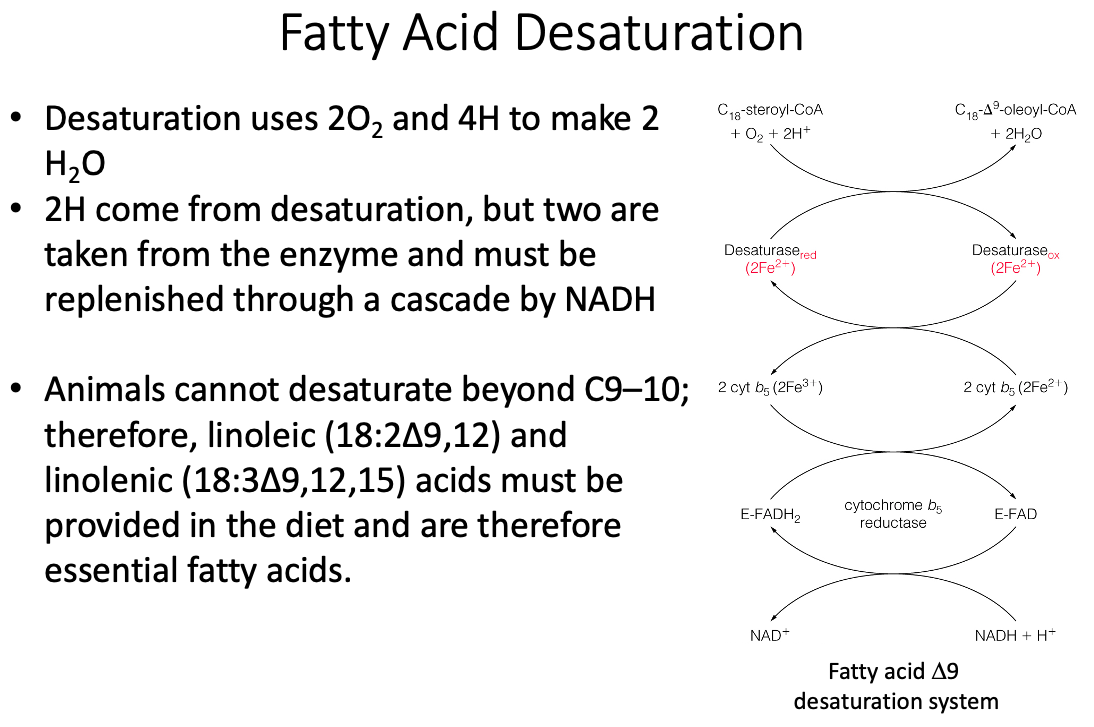
What is the system name for a C-18 fatty acid? Is such a fat essential? How do you know?
- delta-x =
- omega-x =
- Linoleic Acid
- Essential Fatty Acid
- We can't make double bonds past carbon 9, so therefore it is essential.
What is the system name for a C-18 fatty acid? Is such a fat essential? How do you know?
- delta-x =
- omega-x =
- Essential Fatty Acid
- We can't make double bonds past carbon 9, so therefore it is essential.
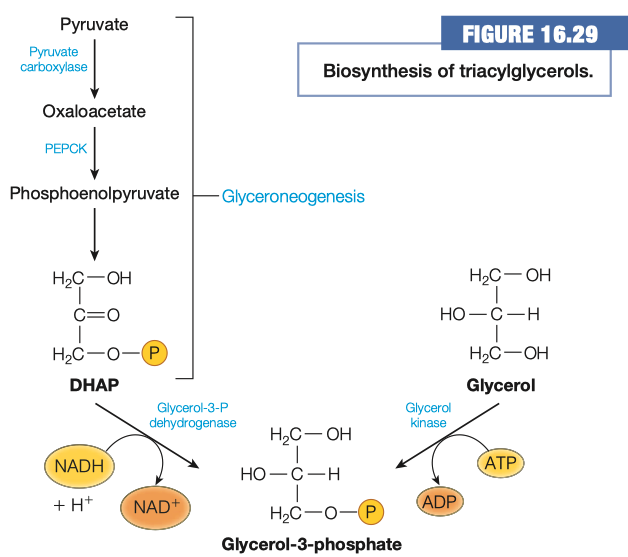
Explain why adipocytes ( fat cells ) need glucose to synthesize triglycerides.
Adipocytes don't have glycerol kinase to turn glycerol into glyceraldehyde-3-phosphate
- So they have to use the glycolytic intermediate DHAP to synthesize G3P
The 3-carbon glycerol backbone of triglycerides is derived from the 3 carbon glucose breakdown products
How does this Acetoacetate act to fuel contraction in cardiac myocytes?
- Acetoacetate and insulin stimulate the incorporation of glucose into glycogen and change the relative contribution of exogenous glucose and endogenous carbohydrate to myocardial energy metabolism, but they most likely do so by different mechanisms
- Insulin and acetoacetate have additive effects on increasing the incorporation of exogenous glucose into glycogen
- Insulin stimulation and acetoacetate oxidation affect the relative contributions of exogenous glucose and endogenous carbohydrate including glycogen to energy metabolism
Even in the presence of acetoacetate, pyruvate is required to maintain flux through the citric acid cycle. Explain
- Acetyl CoA is converted to acetoacetate ( a ketone body )
- Acetoacetate is transported by the blood to the extrahepahic tissues, where it is oxidized via the citric acid cycle to provide energy to skeletal muscles and renal cortex
- Pyruvate is required to maintain the acetyl-CoA concentration in the citric acid cycle
About ninety percent of the cholesterol used in cell membranes is synthesized in the liver. The rest is obtained from dietary sources. A single enzyme controls the entry of carbons into cholesterol biosynthesis. What enzyme is it? What molecules are given to individuals to lower endogenous cholesterol production?
Hydroxymethylglutaryl-coenzyme A (HMG-CoA)
- Regulatory enzyme for cholesterol metabolism
Cholesterol production is controlled by regulation of plasma cholesterol levels through low density and high density receptor mediated uptake.
The regulation can also be done by enzyme cholesterol acyl transferase.
The regulation of HMG-CoA and its activity levels
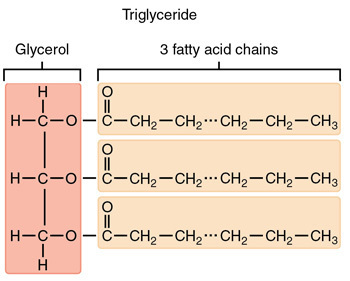
Triglyceride
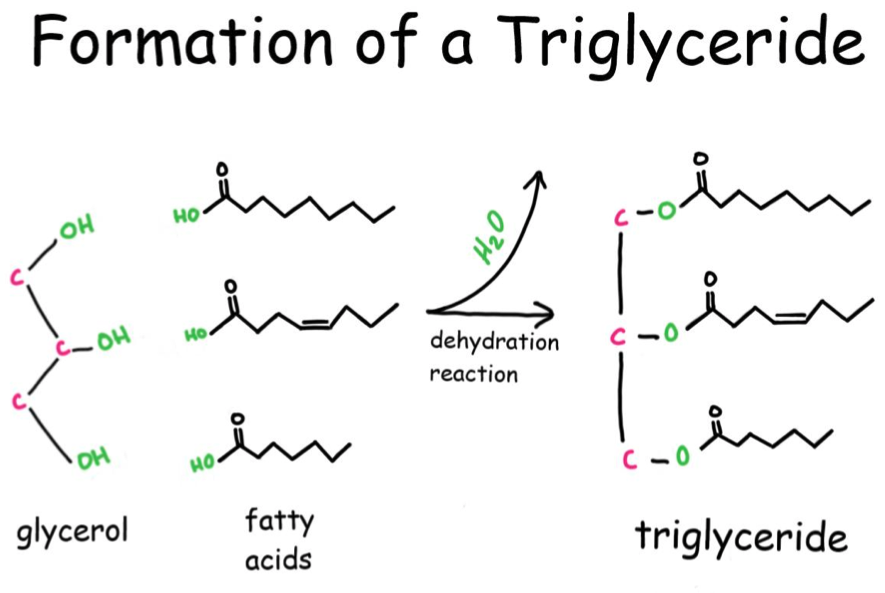
Glycerophospholipids
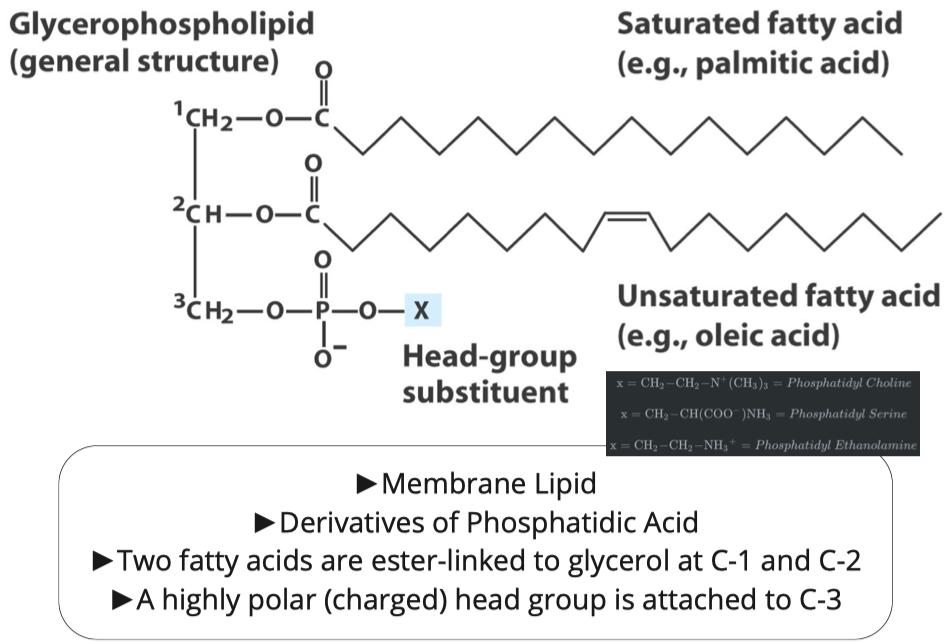
Sphinolipids
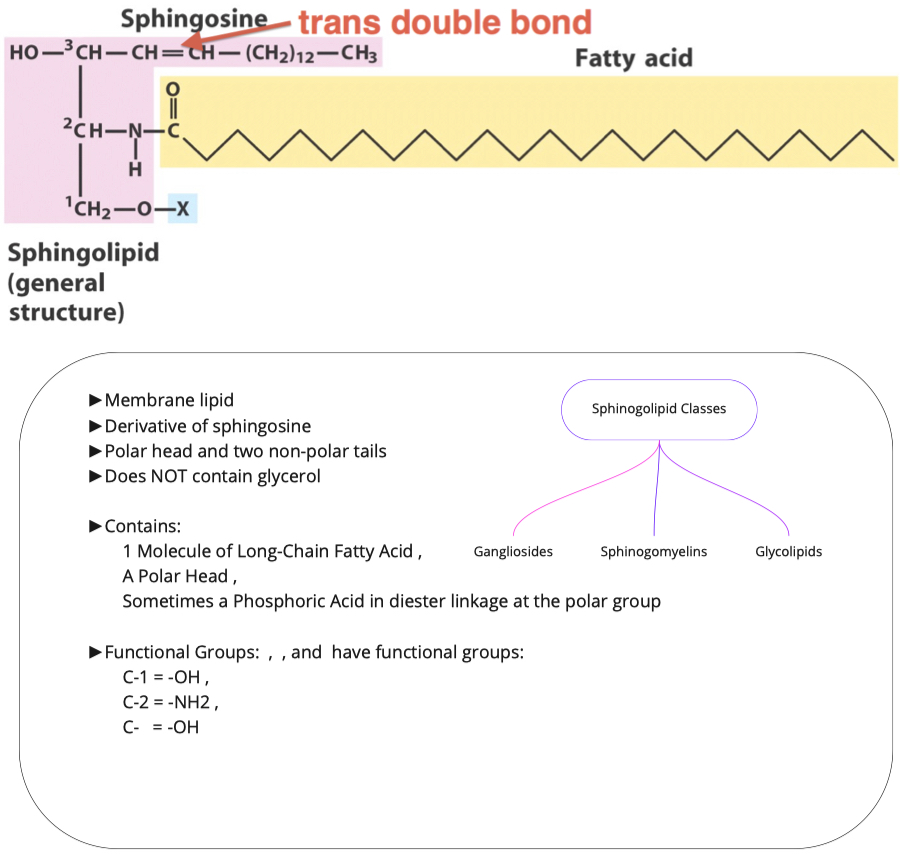
Triacylglycerols
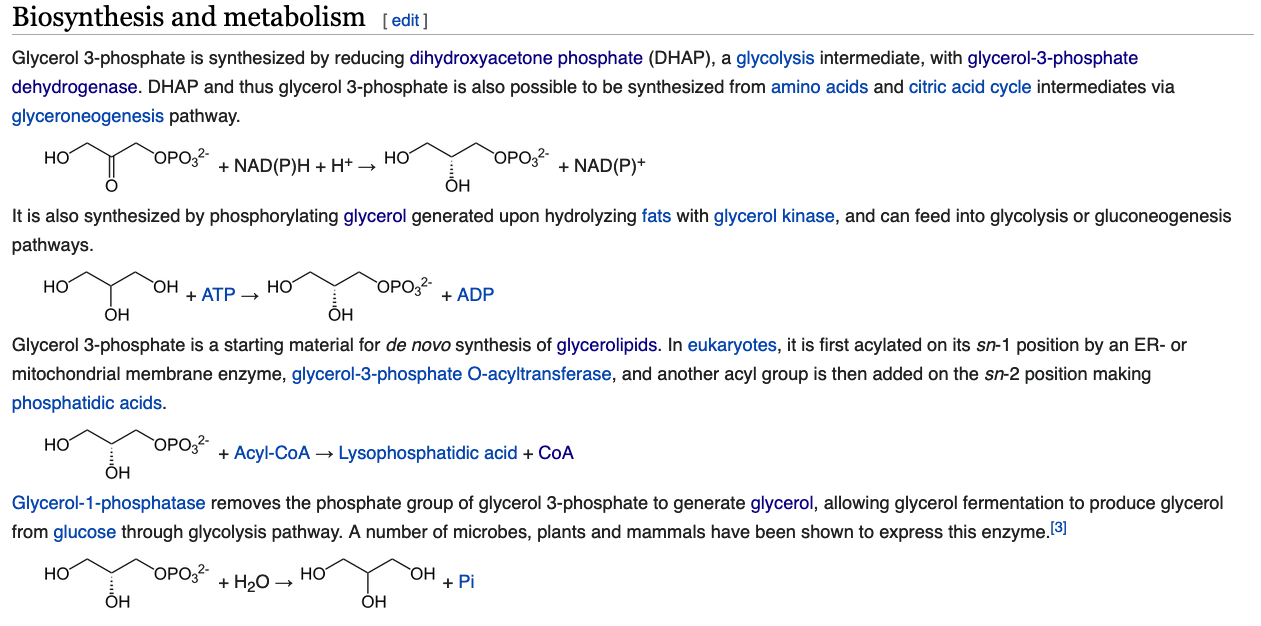
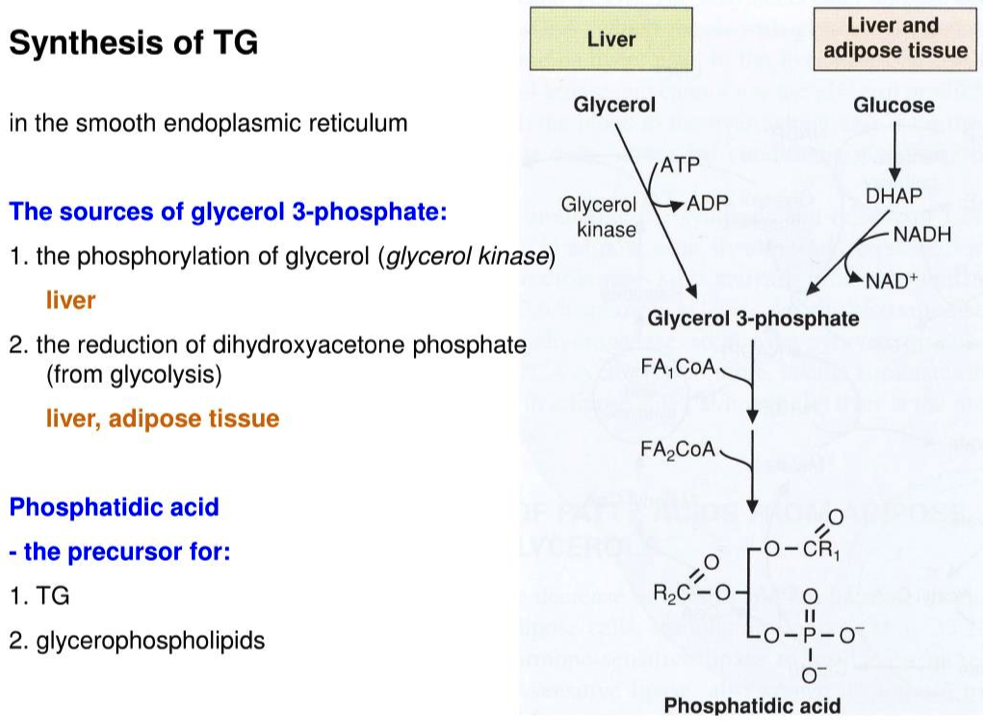
How is glycerol-3-phosphate made using intermediates in central metabolism? What metabolic pathway is this similar to? How is it different?
- Glycerol to Glycerol-3-phosphate is similar to glycolysis
- Pyruvate to DHAP is similar to gluconeogenesis
- Overall , energy consuming step and rate limiting step
Which method is used in adipocytes and why?
- Adipocytes don't have glycerol kinase
- Adipocytes get DHAP from glycolysis
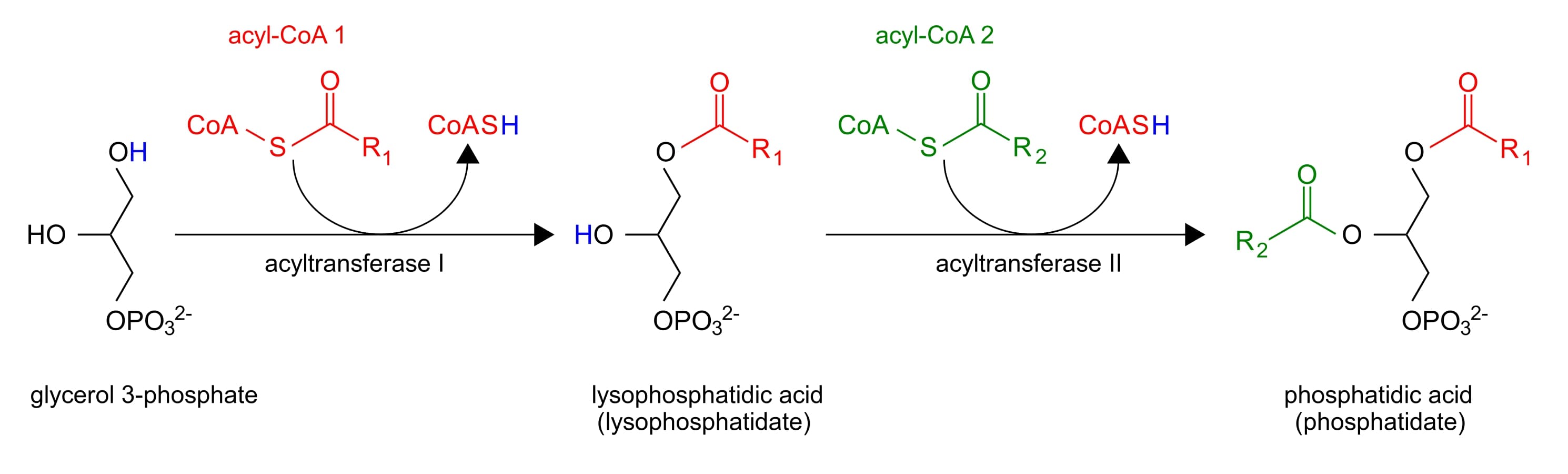
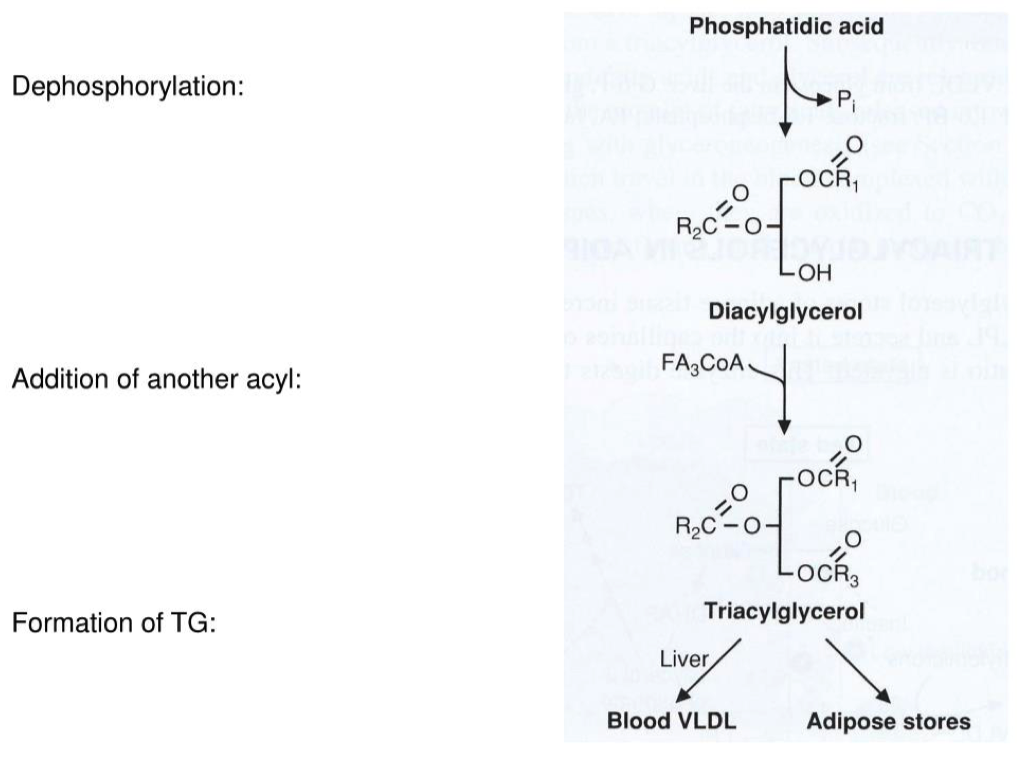
Now that we have Glycerol-3-phosphate, two fatty acids are added via esterification reactions. What class of lipids are generated in this step?
Adding two fatty acids to glycerol-3-phosphate to make a phosphatidic acid
To make a triacylglycerol:
- you have to first make a phospholipid
- You then have to remove the phosphate head and make diacylglycerol
- And then add the 3rd fatty acid to make a triacylglycerol
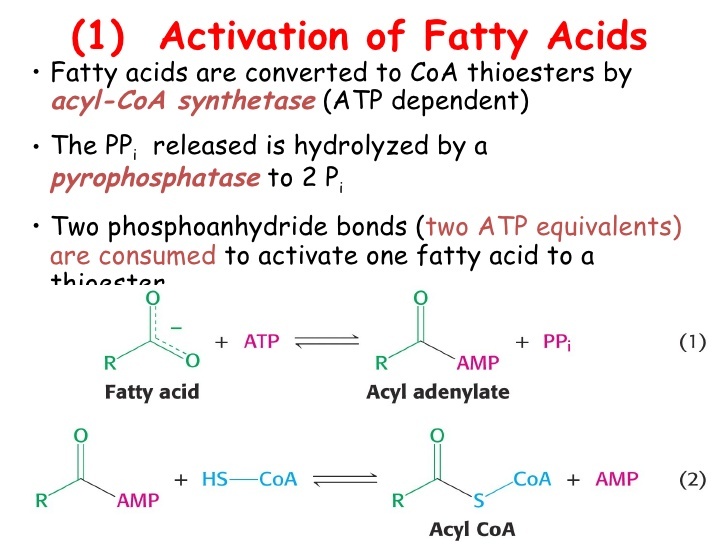
Are fatty acids Activated?
- Fatty acids first be converted to an active intermediate before they can be catabolized
- This is the only step in the complete degradation of fatty acid that requires energy from ATP
- The activation of a fatty acid is accomplished to two steps
Phosphatidic acids have two fatty acid chains, but triacylglycerols have three. What remaining steps must take place in order for triacylglycerol synthesis to be completed?
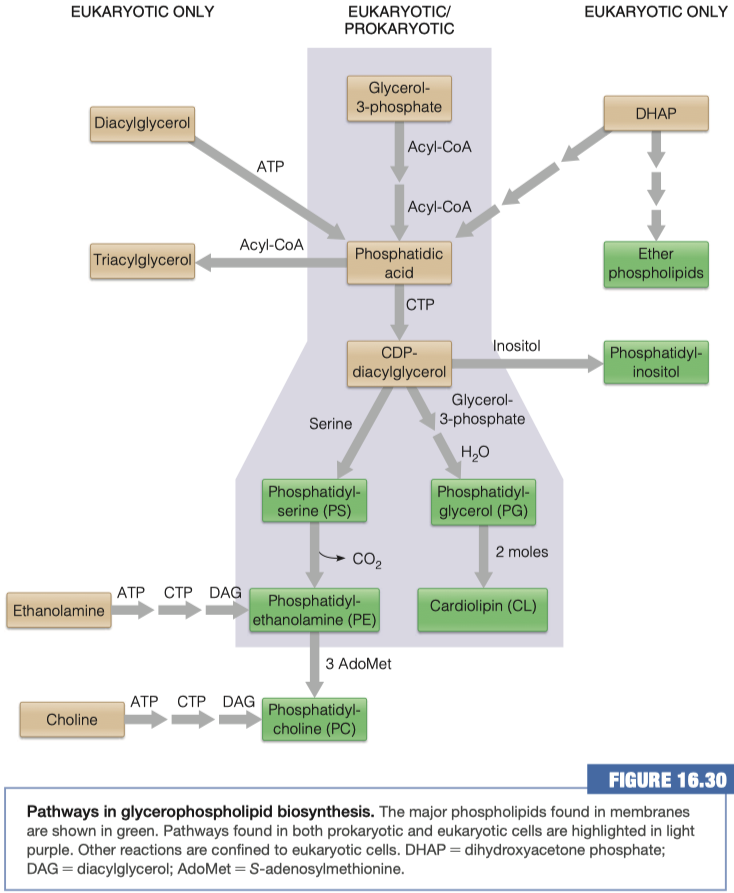
Glycerophospholipids
Similar to triacylglycerols, the glycerophospholipids use phosphatidic acid as a precursor, indeed phosphatidic acids are glycerophospholipids themselves. What are the six main phospholipid classes found in our membranes?
https://en.wikipedia.org/wiki/Membrane_lipid#Major_classes
- Phospholipids
- Glycolipids
- Fatty Acids
- Phosphoglycerides
- Sphinogolipids
- Sterols
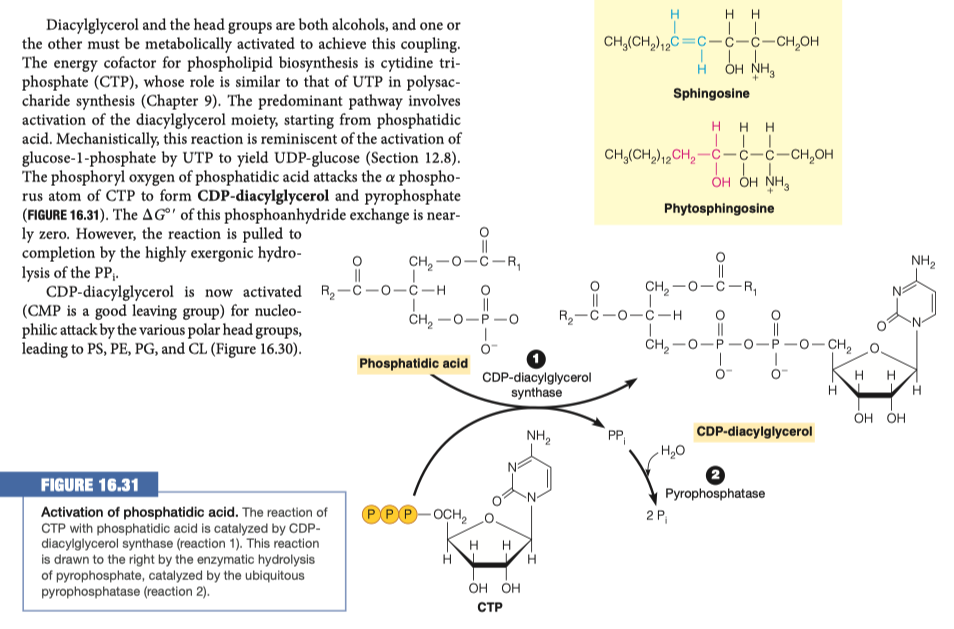
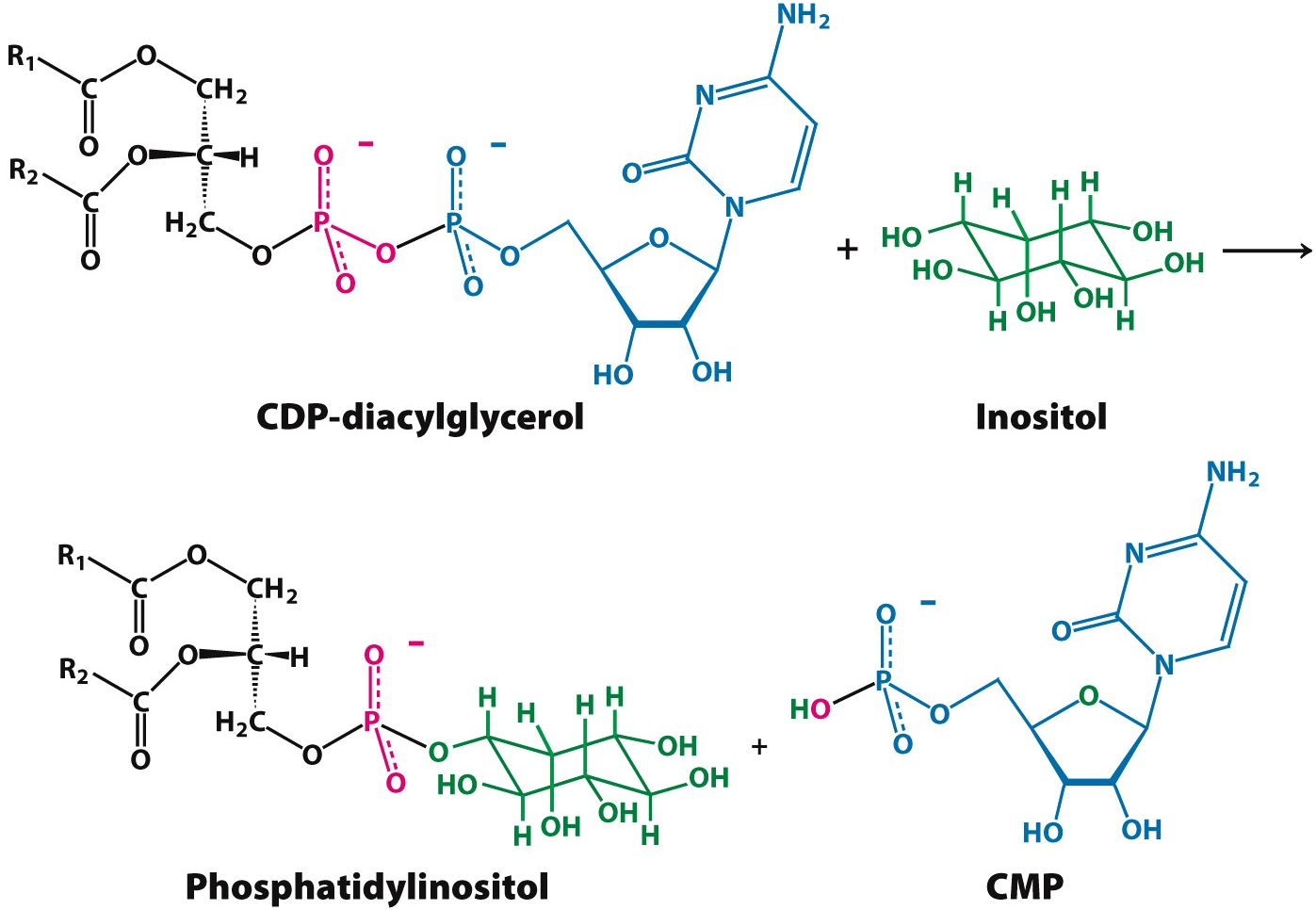
In order to modify the phosphate group on the phospholipid it must first be activated. How is the phosphatidic acid activated?
Phosphatidic acid is activated by adding CTP
- a is removed
CDP-diacylglycerol is similar to:
In the pentose phosphate pathway, adding UTP to glucose to make UDP-glucose
Glycogen synthesis
In the first steps going backward in glycogen synthesis, you have to activate your sugars
Similar idea of having something available as your leaving group to provide the energy for something to takes places
In this instance we are making CDP-diacylglycerol
- CDP-diacylglycerol is the precursor to many different glycero-phospholipids
In order to make what molecule must be added to CDP-diacylglycerol and where?
Inositol group replaces CDP-diacylglycerol
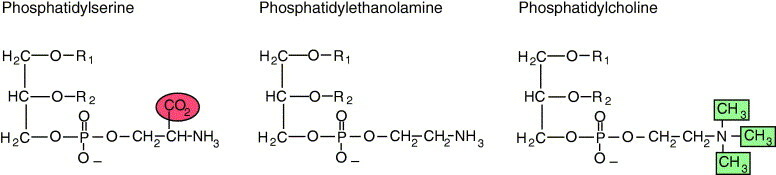
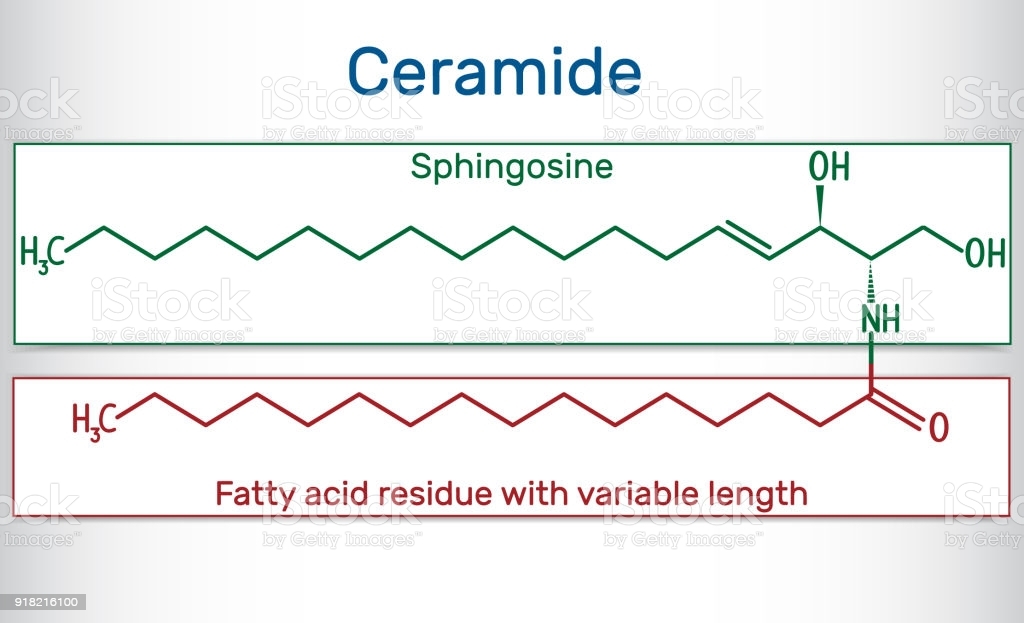
Sphinogolipids
What are the two molecules needed to make sphingosine? How is this different from triacylglycerol and phospholipid synthesis?
- palmitoyl CoA and Serine
- In Triacylglycerol synthesis we added two groups, here we are adding a serine
What must be added to sphingosine (and where) in order to make a ceramide? Is this an esterification reaction?
Ceramides:
do not have phosphates
have amide bridge
only two fatty acid chains
1 is unsaturated chain
- First fatty acid has very specific form and structure because it comes from palmitoyl
- we do processing to make it unsaturated
1 is unsaturated or saturated chain
Glycerophospholipids:
- do have phosphates
- 3 fatty acid chains
making an amide link
What is added to a ceramide to make sphingomyelin? What other group of lipids is this similar to?
Adding a phosphate group
- Specifically, adding Phosphatidylcholine
- Phosphate is left, and diacylglycerol leaves as side product to be reused somewhere
Similar to Phosphatidylcholine
Cerebroside is in the class of lipids called glycosphingolipids. What molecules must be added to ceramide to make this class of lipids?
- Ceramide + sugar = cerebroside = "glycoso-sphino-lipid"
New carbon units are loaded onto which subunit of fatty acid synthase?
- Acyl carrier protein (ACP)
- New carbon units, from malonyl-CoA, are covalently loaded onto acyl carrier protein (ACP) at the beginning of each new cycle.
What is reduced in each round of fatty acid synthesis?
The beta carbonyl carbon of the acyl chain
- In each round of fatty acid synthesis, occurring on fatty acid synthase, NADPH is oxidized to NADP+.
- The electrons stripped from NADPH are used to reduce the growing beta-carbonyl carbon fatty acyl chain.
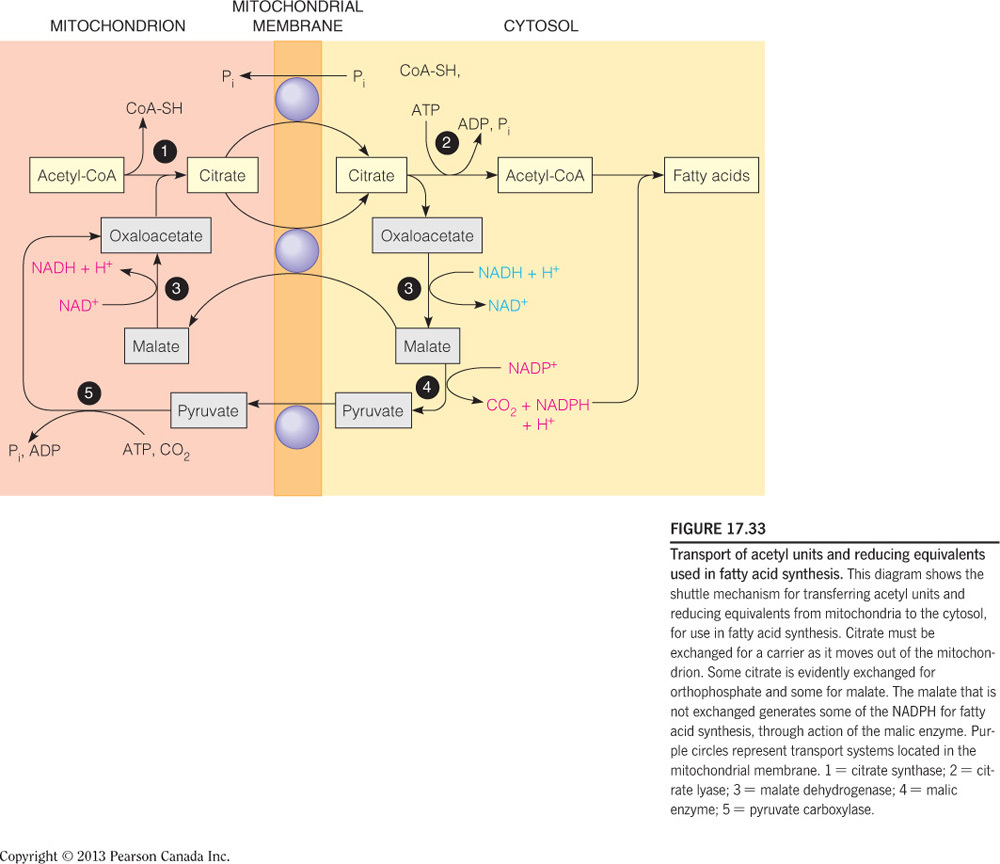
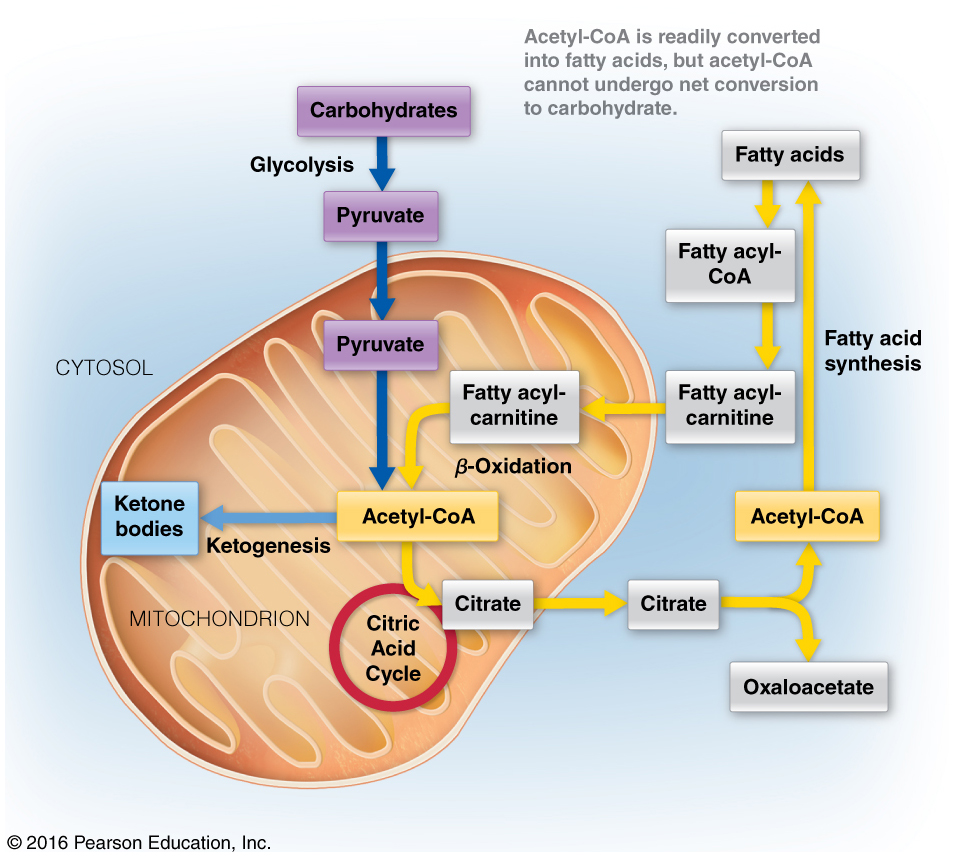
How is the two-carbon building block for fatty acid synthesis moved from the mitochondrial matrix into the cytosol?
Citrate shuttle
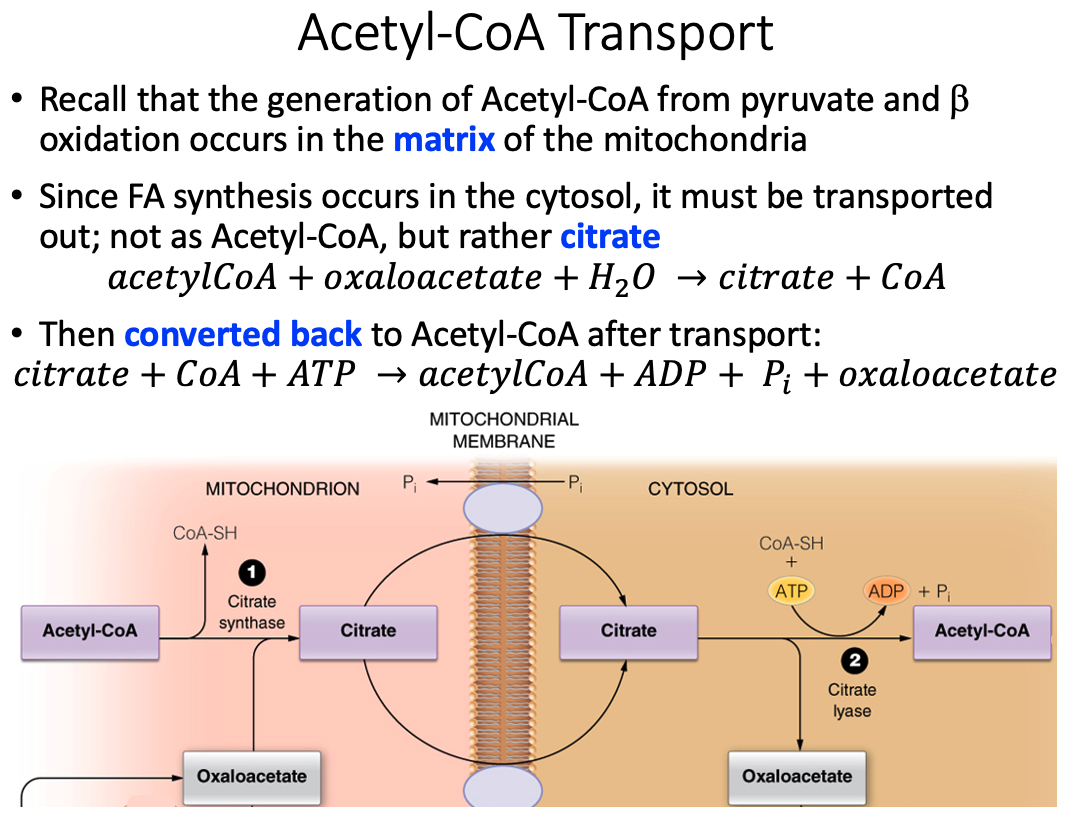
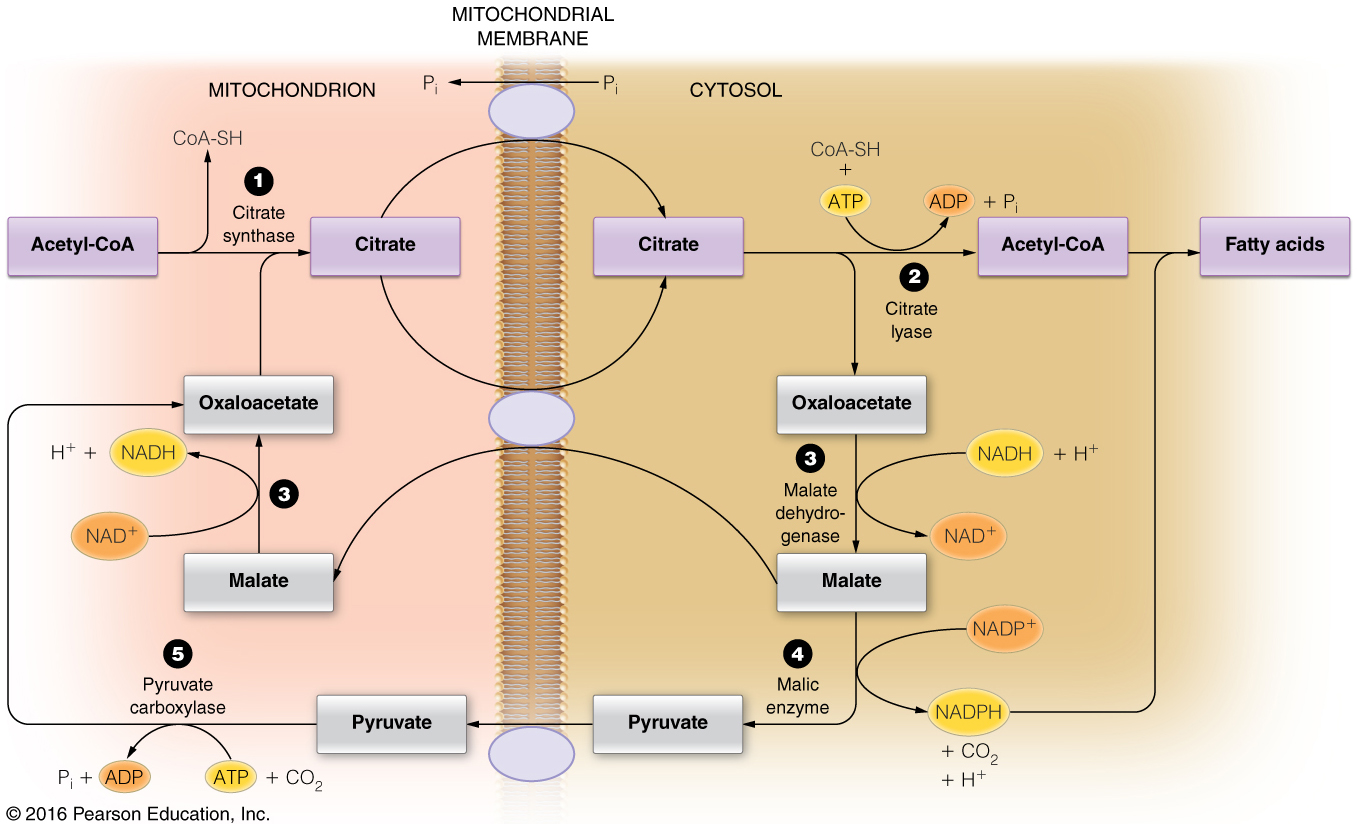
- Acetyl-CoA, the two-carbon building block of fatty acid (and sterol) synthesis is generated in the mitochondrial matrix and needs to be moved into the cytosol where the lipid biosynthesis enzymes reside.
- Acetyl-CoA, however, cannot cross the mitochondrial membranes and therefore the citrate shuttle system is required.
- In the matrix, citrate synthase combines OAA and acetyl-CoA to form citrate.
- When the citrate is high in the matrix, this molecule will be transported into the cytosol.
- In the cytosol, citrate lyase breaks this six-carbon molecule into acetyl-CoA and OAA.
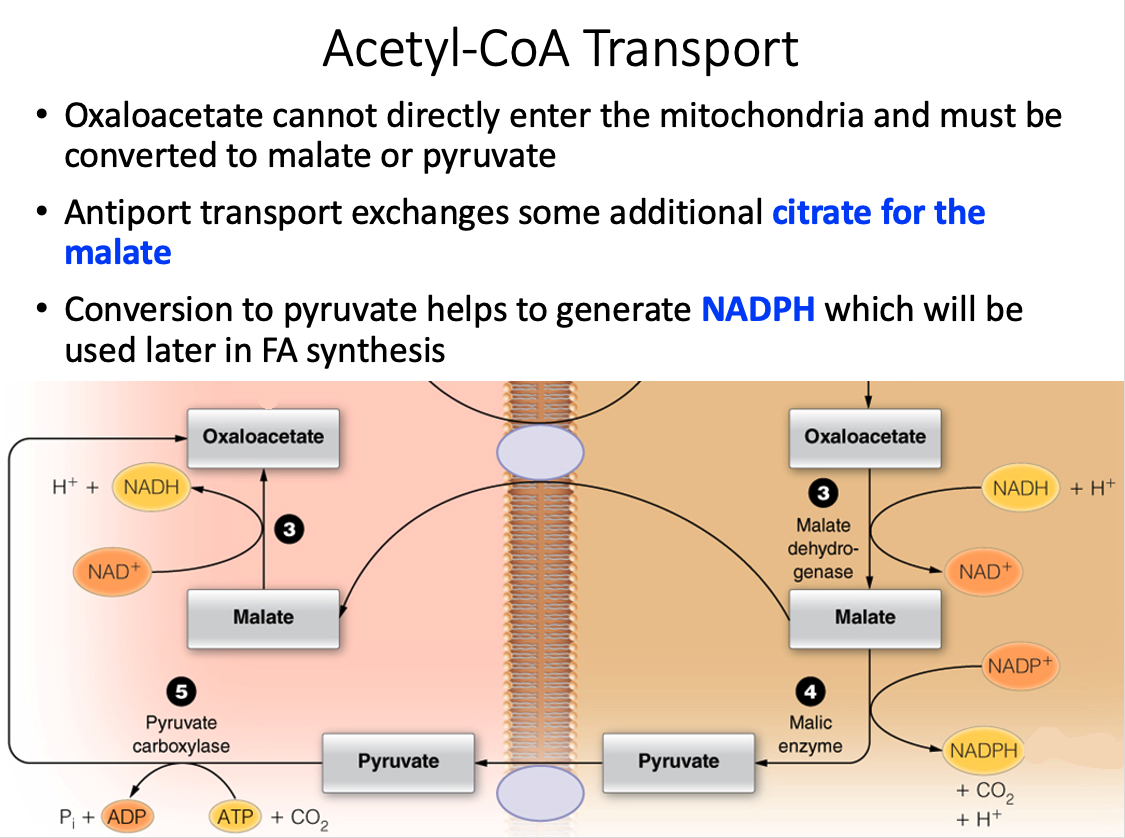
- To complete the shuttle, OAA is reduced to malate and then malate is oxidized to pyruvate, which then crosses back into the mitochondrial matrix.
- The oxidation of malate to pyruvate generates NADPH, which is the coenzyme needed by fatty acid synthase.
- Thus, the shuttle serves two purposes: (1) to move acetyl units into the cytosol and (2) to generate NADPH for fatty acid synthase.
- This process is summarized in the figure below.
Which of the following enzymes catalyzes the rate-limiting step in sterol synthesis?
HMG-CoA reductase
What is oxidized in each round of fatty acid synthesis?
NADPH
- In each round of fatty acid synthesis, occurring on fatty acid synthase, NADPH is oxidized to NADP+.
- The electrons stripped from NADPH are used to reduce the growing beta-carbonyl carbon fatty acyl chain.
Which molecule enters as the new carbon units in fatty acid synthase?
Malonyl-CoA
- Malonyl-CoA is covalently loaded onto ACP at the beginning of each new cycle.
- During condensation, the decarboxylation of malonyl-CoA generates a good nucleophile (enolate) that attacks the thioester carbonyl of the growing acyl chain.
How many cycles of fatty acid synthesis are required to synthesize a palmitate?
7
- Seven cycles of fatty acid synthesis are required to synthesize a palmitate.
- Palmitate is the 16:0 fatty acid product of fatty acid synthase.
- Each cycle of catalysis increases the carbon chain by two carbons, except the first round where two two-carbon units are condensed.
- The subtraction of one accounts for the first cycle in fatty acid synthesis that condenses two two-carbon units.
Which of the following is the correct sequence of events in cellular fatty acid biosynthesis?
Citrate shuttle → Acetyl-CoA Carboxylase → Fatty Acid Synthase
Fatty acid biosynthesis requires a two-carbon building block, acetyl-CoA.
This molecule is made in the mitochondrial matrix and cannot cross into the cytosol, where fatty acid biosynthesis occurs
- therefore, the citrate shuttle is needed to ferry two carbons across the mitochondrial membranes.
Once citrate is in the cytosol, citrate lyase breaks citrate into acetyl-CoA and OAA.
Acetyl-CoA is carboxylated by acetyl-CoA carboxylase to produce malonyl-CoA.
Malonyl-CoA is the carbon building block (in addition to one acetyl-CoA) used by fatty acid synthase.
Therefore, the correct order is: citrate shuttle → acetyl-CoA carboxylase → fatty acid synthase.
03 - Lipid Synthesis - Things To Know
Why Fatty acid synthesis?
- Because carbohydrate storage is limited by size and quantity.
- Excess carbohydrates in the diet are converted to pyruvate and eventually acetyl-CoA
Acetyl-CoA transport
- Molecular Carrier is Acyl Carrier Protein (ACP)
- Citrate ( mitochondria to cytosol )
Molecules involved
- NADP
- Precursors = Acetyl CoA and Malonyl CoA
- Products = Fatty Acid Chains
How NADPH is generated
- generated by reducing the beta carbonyl carbon on the fatty acid chain
Differences between Fatty acid oxidation and synthesis
- See Multiple Charts
How is Malonyl-CoA formed and why is it necessary?
- Acetyl-CoA + Acetyl-CoA Carboxylase = Malonyl-CoA
- Necessary for both Fatty Acid Synthesis and Fatty Acid Oxidation
What is a Phosphopantetheine moiety and how are fatty acids attached?
- Acyl Carrier = Protein bound phosphantethiene
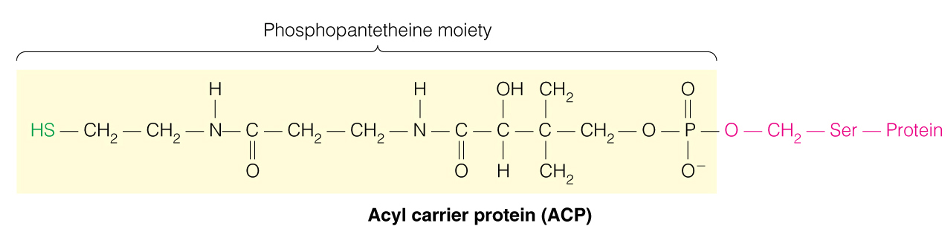
How many cycles to make palmitate
7
Quantities of reactants and products used/generated
FA Elongation
- process inside the endoplasmic reticulum (ER) that adds 2 carbons from malonyl CoA to growing Fatty Acyl CoA
Desaturation
- Adding double bonds in specific locations to make non-essential fatty acids
Essential fatty acids
- Cannot be synthesized in human body
- Can't make double bonds past carbon 9
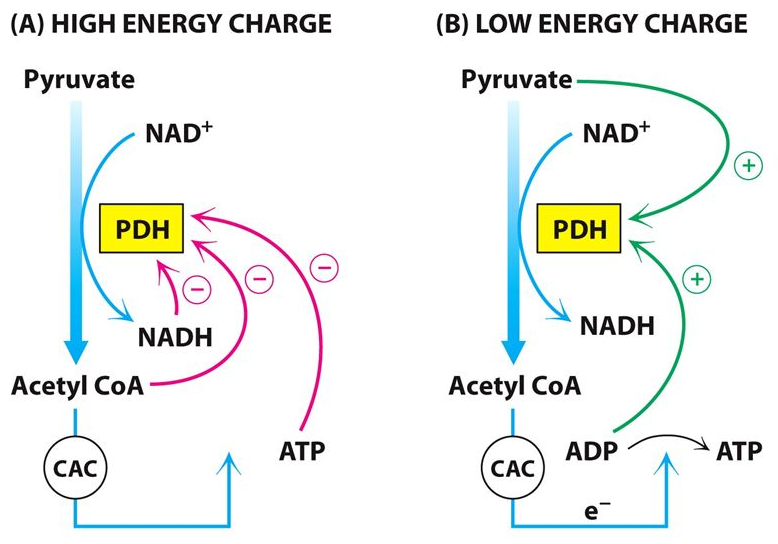
Regulation of ACC and pyruvate dehydrogenase
Acetyl-CoA Carboxylase (ACC) is Regulated By:
- allosteric ( citrate and long-chain fatty acids )
- covalent modification mechanisms
Pyruvate Dehydrogenase is Regulated By:
- Insulin promotes dephosphorylation (activation)
04 - Cholestrol Formation
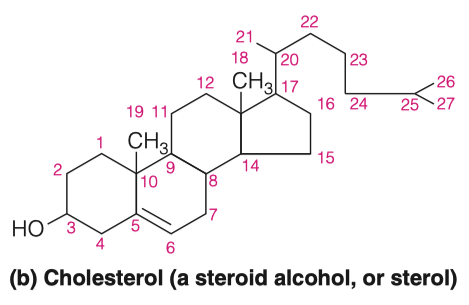
Find the structure of cholesterol and determine its properties
Cholesterol differs from the basic ring system in having:
- an aliphatic chain at C-17
- axial methyl groups at C-10 and C-13
- a double bond in ring B
- a hydroxyl group in ring A
The alcoholic functional group and the carbon chain at C-17 make cholesterol a sterol
- which is the generic term used to identify steroid alcohols
Much of the cholesterol in lipoproteins and intracellular storage droplets is esterified at this position with a long-chain fatty acid
- which makes the resultant cholesterol ester much more hydrophobic than cholesterol itself
Ketones
What do you suppose would happen in a cell when -oxidation levels far exceed that of the limits of the citric acid cycle? Where would this occur?
- Massive buildup of acetyl-CoA
- Under conditions in which further oxidation of acetyl-CoA through the citric acid cycle is limited, acetyl-CoA is used to synthesize ketone bodies, which are excellent energy substrates for brain and heart.
- Occurs in the liver
How will this affect the rate of -oxidation?
Thermodynamics is regulated by concentration levels of products and reactants
If we get a huge build up of acetyl-CoA, we are no longer able to generate more acetyl-CoA through -oxidation
Which means we start loosing our sources of NADH
- Without our sources of NADH, we can't produce ATP as readily
The body has created a system where it generates ketone bodies instead
High levels of acetyl-CoA = make ketones
- To circumvent the issues from question 1, the body can use acetyl CoA to make ketone bodies (Figure 16.19)
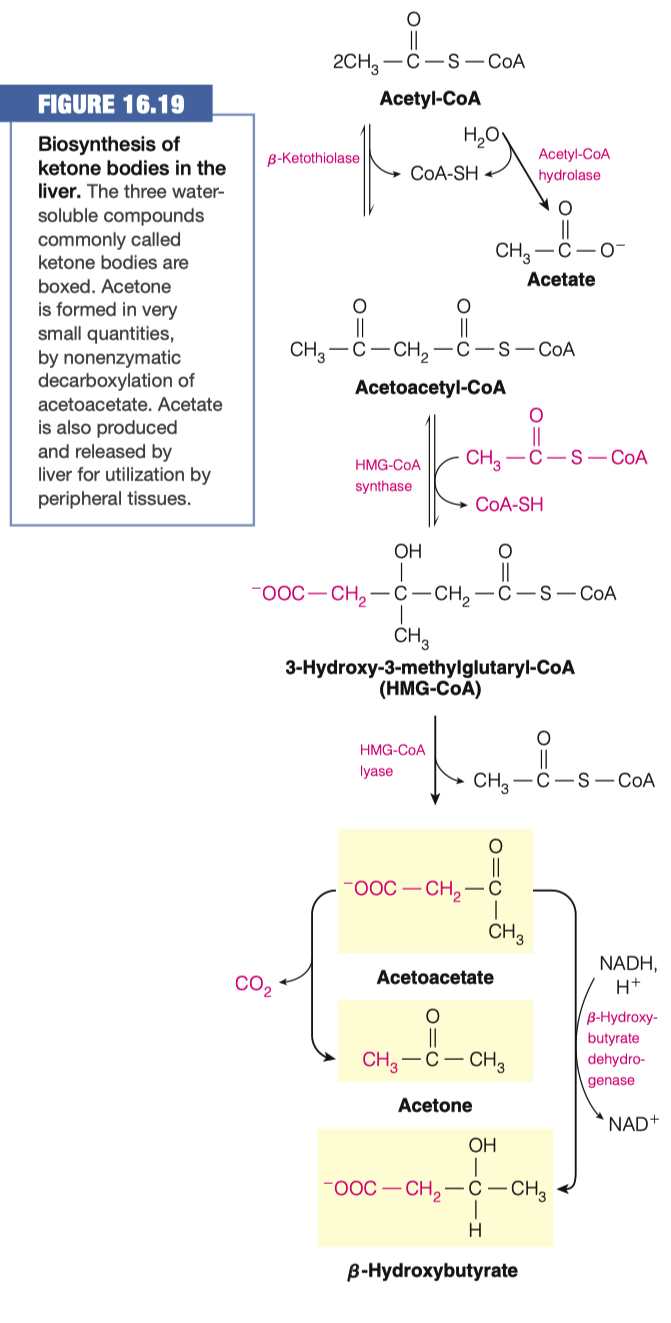
Hypothesize why we add an Acetyl-CoA to Acetoacetyl-CoA, to make HMG-CoA if we just then remove the Acetyl-CoA to make Acetoacetate
HMG-CoA synthase = regulated enzyme by glucagon (activates) and insulin (inactivates).
- It appears that this extra step was added so the pathway can be hormonally regulated.
We made HMG-CoA because its used in a lot of different things other than the production of ketone bodies
So we went through this side step to make HMG-CoA
- Only when we don't need any HMG-CoA will make ketone bodies
1st acetyl-CoA is attached "backwords"
- its a different directionality
- the second one is in the other direction (right hand side)
- "we are kind of shifting the molecule into a different structure in the way we are doing it"
- We need HMG-CoA for cholesterol synthesis
Cholesterol Synthesis
Cholesterol is a member of a group of lipids known as isoprenoids (generated from isoprene or its derivatives) and is subcategorized in the steroid family (based on a saturated tetracyclic hydrocarbon structure)
Ketogenesis happens in the liver, inside the mitochondria
-Oxidation happens in the liver
- so we might as well make ketones there because that is where we are getting our massive amounts of acetyl-CoA
Cholesterol is also made in the Liver , inside the endoplasmic reticulum (ER)
This is how we regulate which process should take place
If we have excess acetyl-CoA , it can be moved into the cytosol or stay in the mitochondria
If its in the cytosol, it can be made into cholesterol
if stays in the mitochondria, it will be turned into ketone bodies
- Ketone bodies go out to blood stream and to tissues
Why is cholesterol considered a steroid alcohol?
What other features make cholesterol different from a standard saturated tetracyclic hydrocarbon?
- Can esterify with long chain fatty acids to form cholesterol esters such as cholesteryl
- Metabolic precursor of steroid hormones
- Aliphatic chain on C-17 , axial methyl groups at C-10 and C-13, a double bind in ring B , and a hydroxyl group ion ring A.
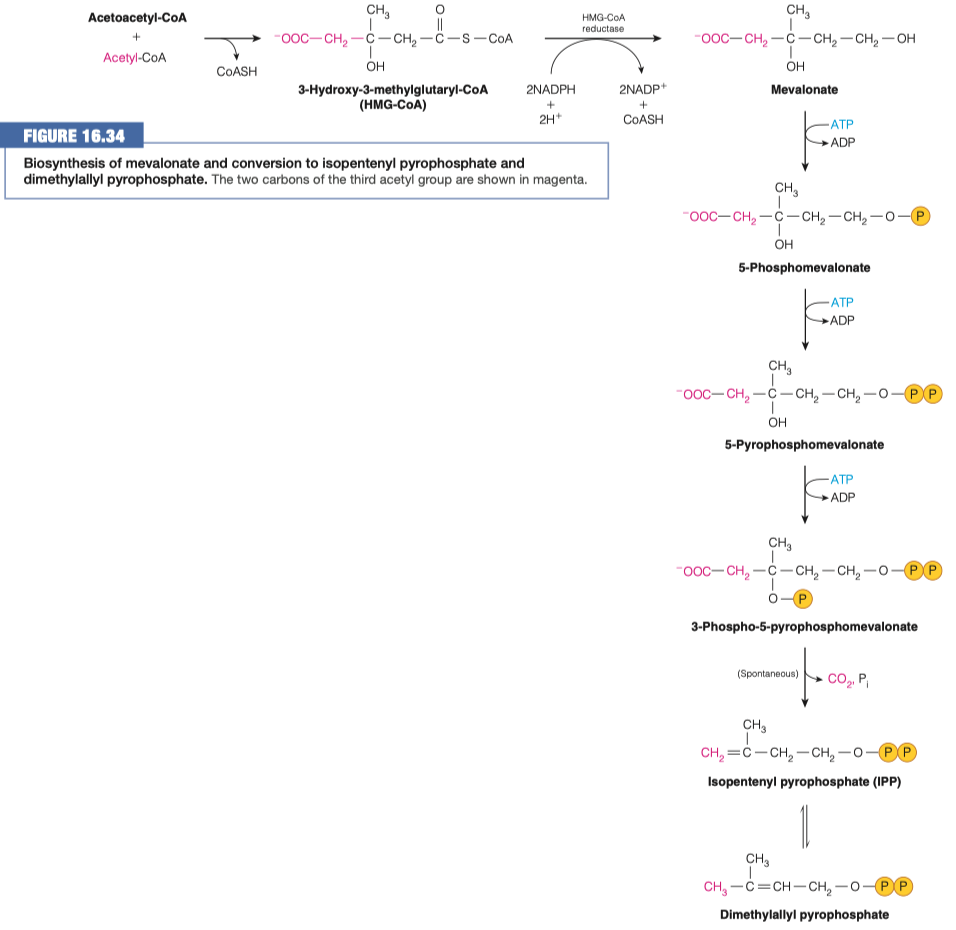
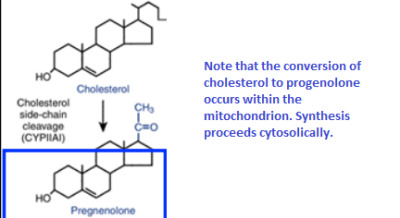
What other process is this similar to? What is different about it?
identical to reactions used in ketogenesis
It occurs in a different cell compartment.
- Ketogenesis occurs in mitochondria, whereas cholesterol biosynthesis occurs in the cytosol and the endoplasmic reticulum (ER).
step 1 is similar to ketogenesis
step 2 is different because it uses NADPH instead of NADH
- anytime we are doing a synthesis we tend to use NADPH
step 2 is also different because uses a different enzyme
- in cholesterol synthesis we use HMG-CoA reductase
- here, we are using HMG-lyase
What is the role of HMG-CoA reductase? How is it different from HMG-CoA lyase?
- HMG-CoA lyase that cleaves HMG-CoA is missing from the ER, where cholesterol biosynthesis begins
- HMG-CoA reductase an integral membrane protein in the ER catalyzes the reduction of HMG-CoA to mevalonate
- This multistep reaction requires two equivalents of NADPH (four electrons) to reduce the thioester to an alcohol.
- This is the major step that regulates the overall pathway of cholesterol biosynthesis.
- HMG-CoA lyase broke off an acetyl-CoA
- HMG-CoA reductase only broke off a CoA
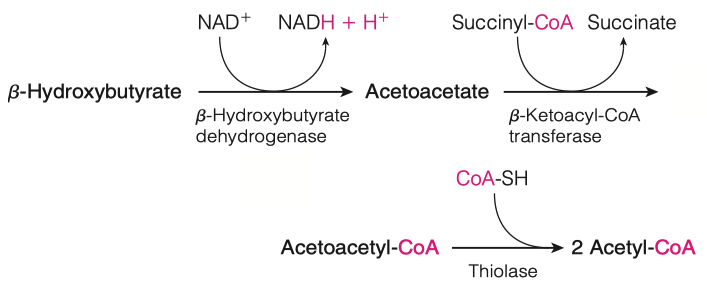
What happens to the ketone bodies once they are generated in the liver?
- Ketone bodies are transported from liver to other tissues, where acetoacetate and -hydroxybutyrate can be reconverted to acetyl-CoA for energy generation.
- The reconversion involves enzymatic transfer of a CoA moiety from succinyl-CoA to acetoacetate, yielding acetoacetyl-CoA and succinate
Isopentenyl pyrophosphate (IPP) will go through an isomerization reaction to form dimethylallyl pyrophosphate.
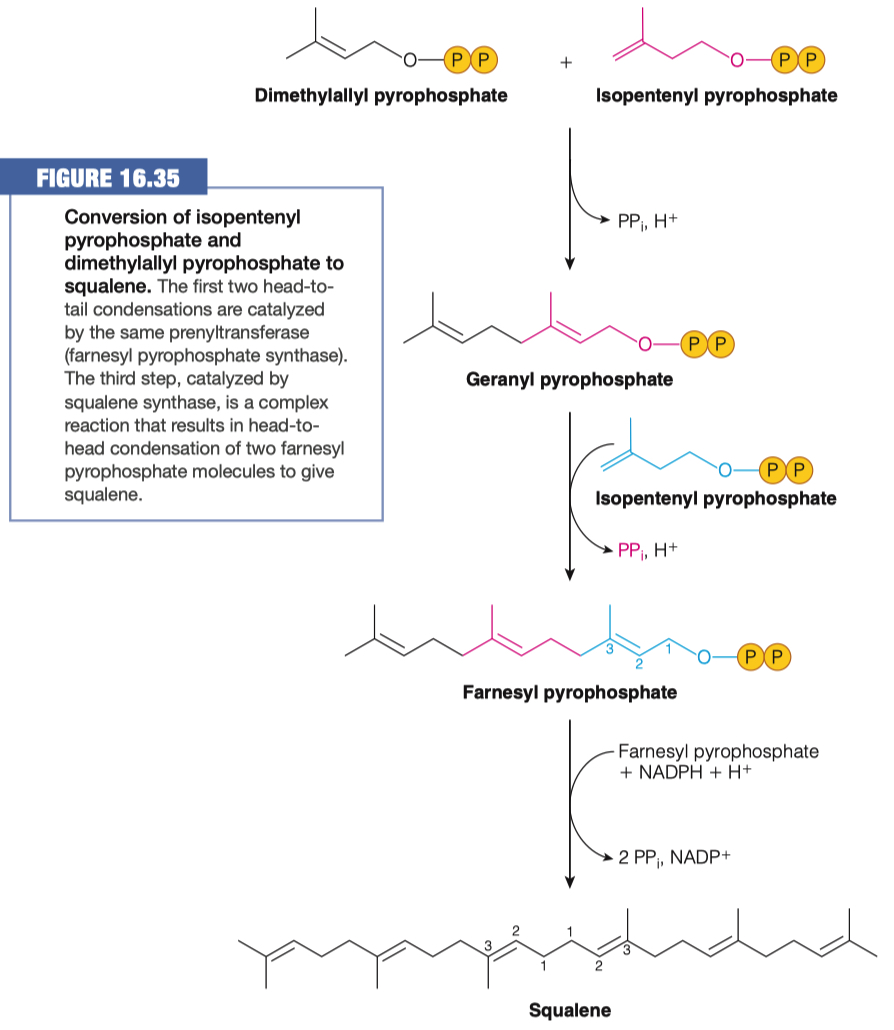
Squalene is cyclized to give rise to cholesterol
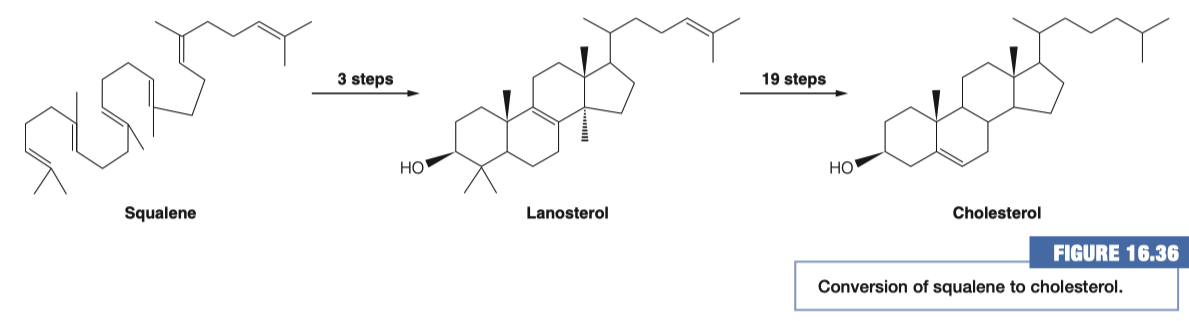
Where do these reactions take place?
- Endoplasmic Reticulum ( ER )
- everything previous to this step was done in the cytosol
How many demethylation must take place? Does this make sense based on the # of carbons in squalene and cholesterol?
3 Demethylations
Cholesterol has 27 carbons
Squalene has 30 carbons
- Therefore 3 demethylations must take place
Cholesterol Synthesis Regulation
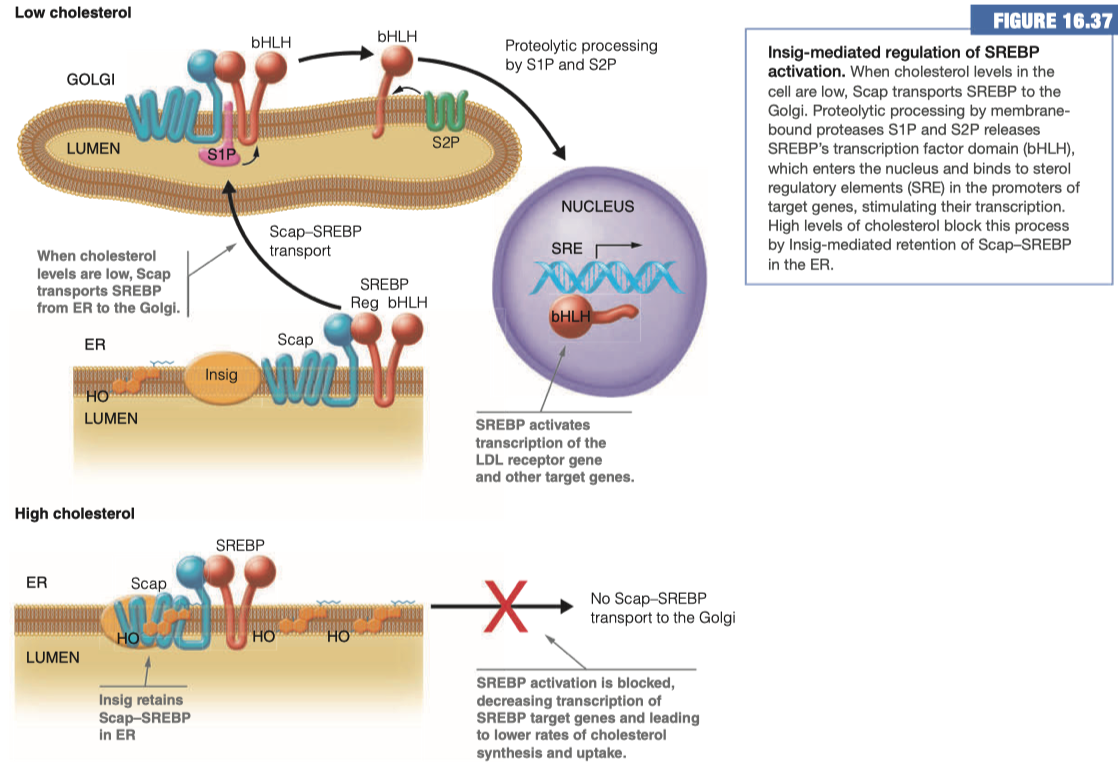
We have "Insig" , "Scap" , and "SREBP" all as "players" here
In a HIGH cholesterol situation, Insig attracts Scap and secludes it at the membrane and prevents its interaction with SREBP
This means no Scap-SREBP transport to the Golgi Apparatus
- Which means no connection of the beta helix loop domain into the nucleus to promote the activity of any of our various cholesterol enzymes
In a LOW cholesterol situation the Insig does not interact with Scap.
When Scap and SREBP interact with each other, they are transported over to the Golgi
- Once in the Golgi, Scap-SREBP is chopped in half, and then it goes to the nucleus where it comes in contact with its receptor elements and promotes the activity or the expression of the multiple enzymes involved in cholesterol synthesis and/or uptake
In our body, we are determining how much cholesterol is in each cell.
Then we are regulating how much cholesterol is brought into the cell via these mechanisms, SCAP, SREBP, ect
- And we can decide if we need to make more, use more, or bring more into the cell
What genes are being regulated by the process above?
SREBP target genes
- LDL receptor gene
- Other target genes
Any of the enzymes associated with cholesterol synthesis
HMG-CoA
Any of the enzymes that make:
- IPP
- Dimethylallyl pyrophosphate
- Geranyl pyrophosphate
- Ect
What are statins? Which particular enzyme do they target and why?
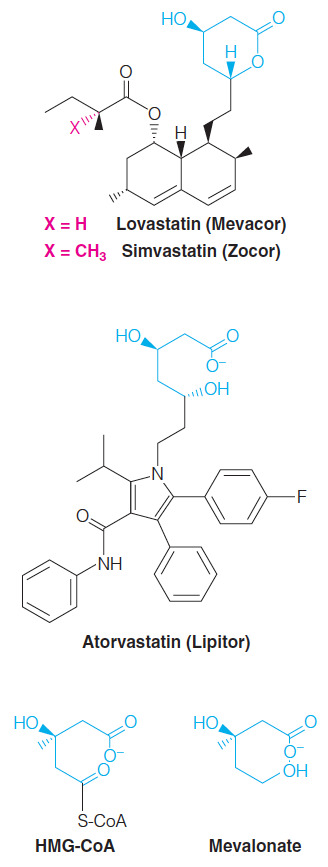
Competitive HMG-CoA reductase inhibitors
- Each statin carries a mevalonate-like moiety (blue), explaining the competitive nature of its activity
Inhibition of HMG-CoA reductase depresses de novo cholesterol biosynthesis and, hence, intracellular cholesterol levels.
This in turn leads to increased production of LDL receptors, allowing more rapid clearance of extracellular cholesterol from the blood
- Thus lowering blood cholesterol levels.
They prevent the transition from HMG-CoA to mevalonate
Very similar structure to HMG-CoA
The enzyme that normally uses HMG-CoA will use statins instead
This means it gets "confused" and doesn't end up converting HMG-CoA into mevalonate
If you are not making mavalonate:
- then you are not making IPP or dimethylallyl pyrophosphate
- you also cannot go through any of the remaining steps to make squaline or cyclize it into cholesterol
Statins are competitive inhibitors that we take to lower cholesterol levels inside our bodies through competitive inhibition
Statins inhibits HMG-CoA reductase, in the sterol biosynthesis pathway, via which mode of inhibition?
Competitive inhibition
- Statins are structurally similar to HMG-CoA which is the substrate of HMG-CoA reductase.
- All statins contain the mevalonate connectivity (seen in blue in the figure below) but not the CoA.
- Since the statin inhibitors look similar to the substrate, these molecules bind to the active site of HMG-CoA reductase and, since the binding is reversible, these inhibitors are competitive inhibitors.
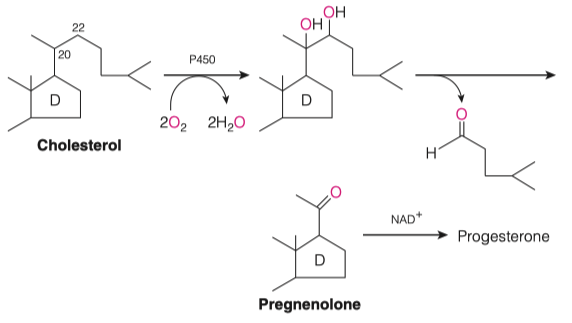
Cholesterol Role in Steroid Hormone Production
How are cholesterol and pregnenolone similar to one another?
Similar ring structures
Both cyclic hydrocarbons
Majority of the reaction takes place on ring structure "D"
Some hydroxylation event which facilitates the cleavage of the majority of the "tail" off of cholesterol
- This produces a carbonyl oxygen functional group, aka pregnenolone
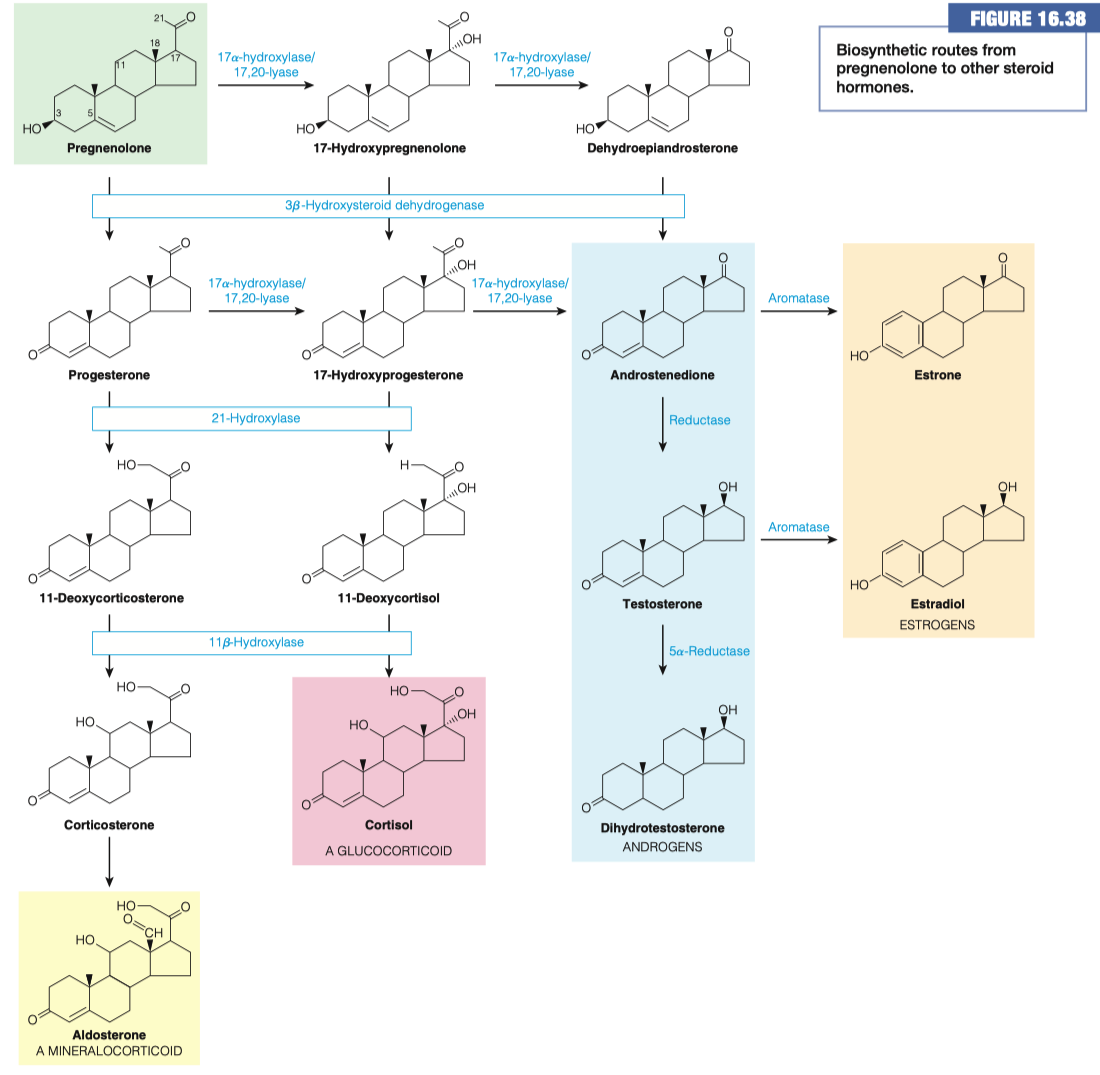
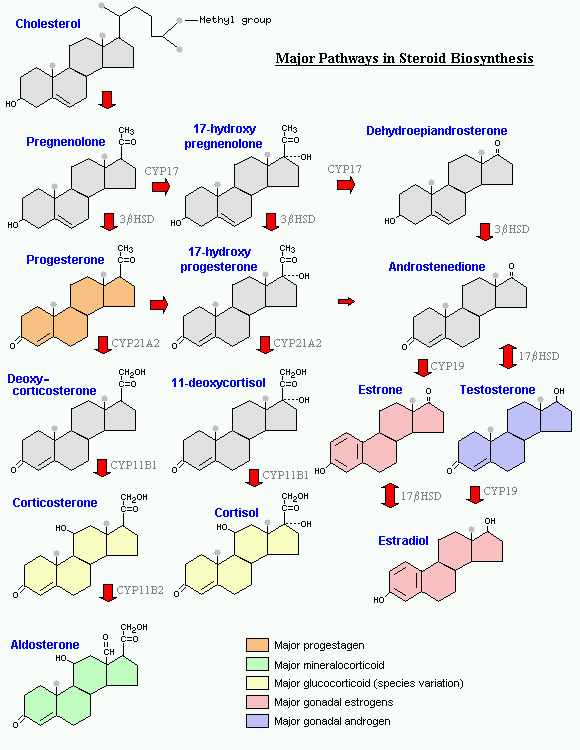
Cholesterol is made into pregnenolone
- Pregnenolone is a precursor for all of the different steroids in our body
"Cholesterol is how we get our hormones"
The risk of using statins is they inhibit the production of cholesterol, which would lead to lower levels of steroids and hormones in the body
- Could cause major growth problems in younger populations
05 - Lipid Transport
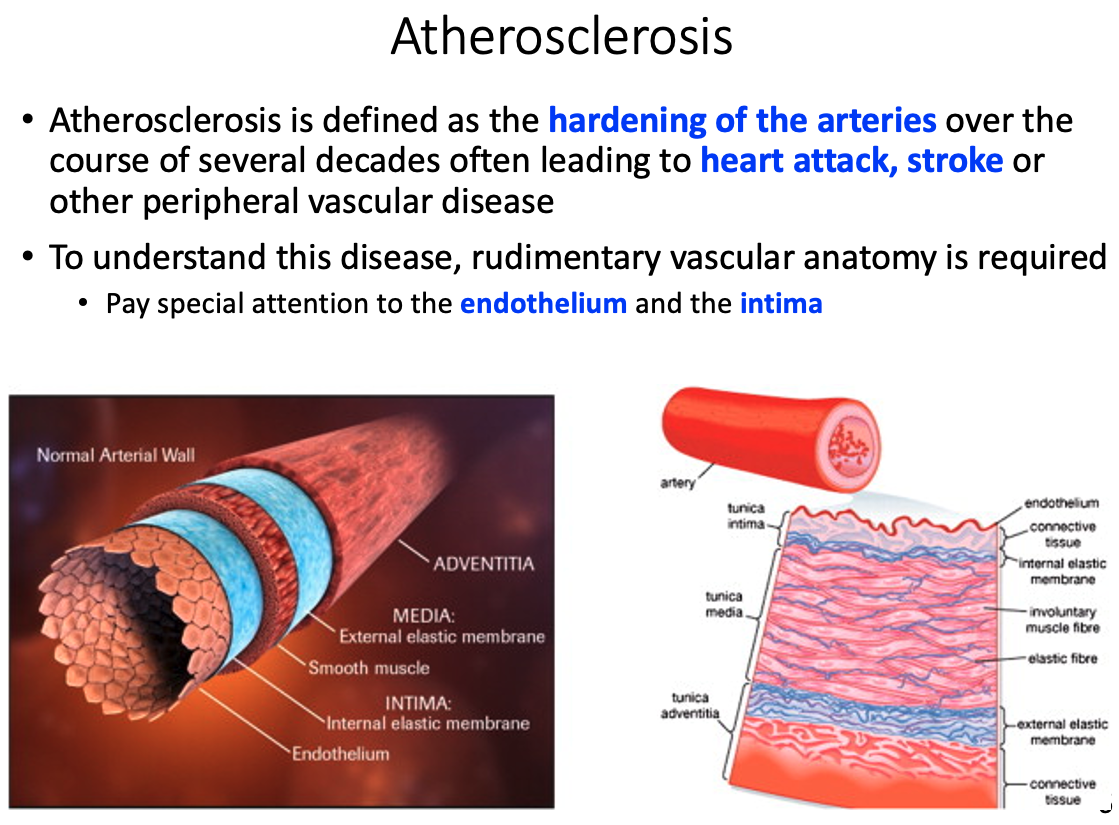


What are the three main sources of available fats (those that can be used in metabolism or serve as components of our membranes) for your body?
- diet
- synthesis
- storage from adipocytes
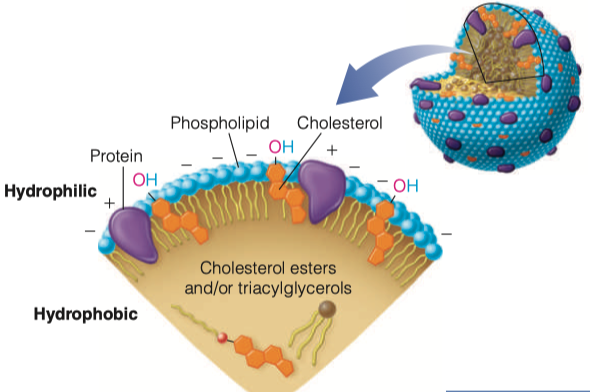
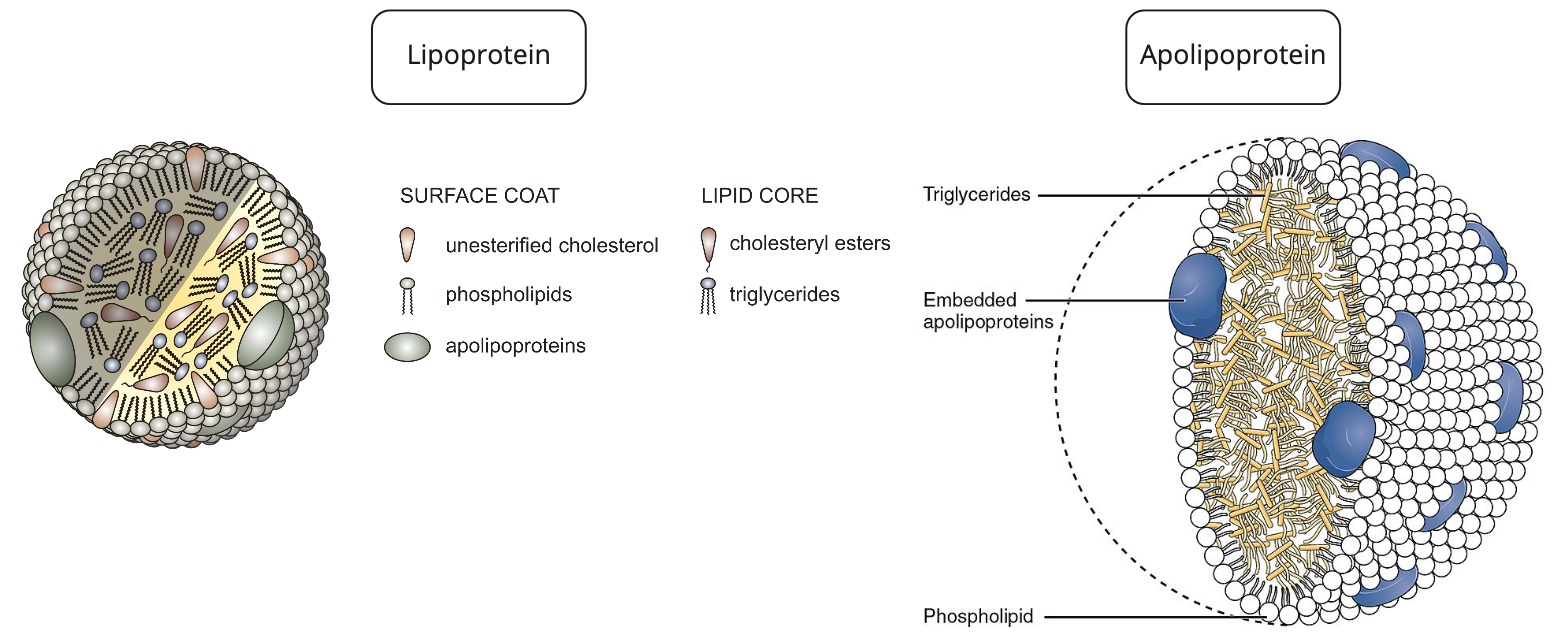
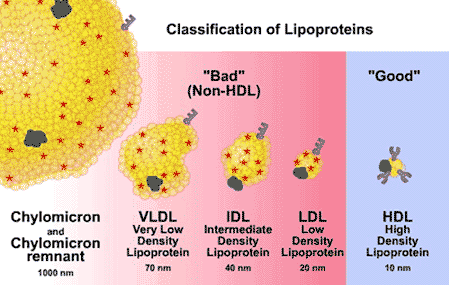
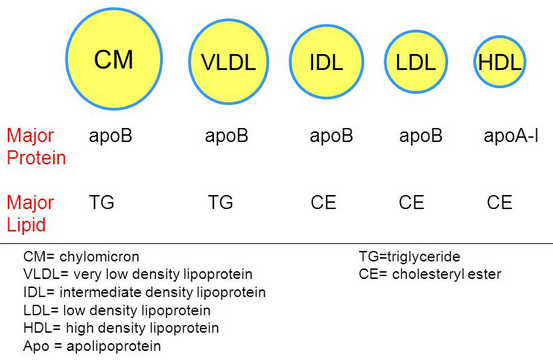
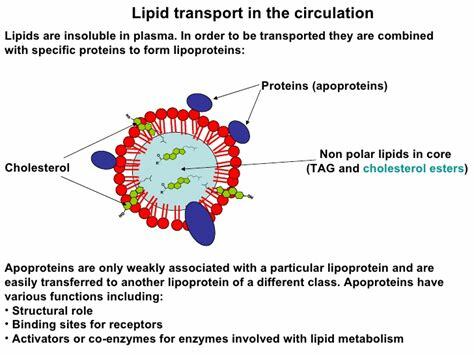
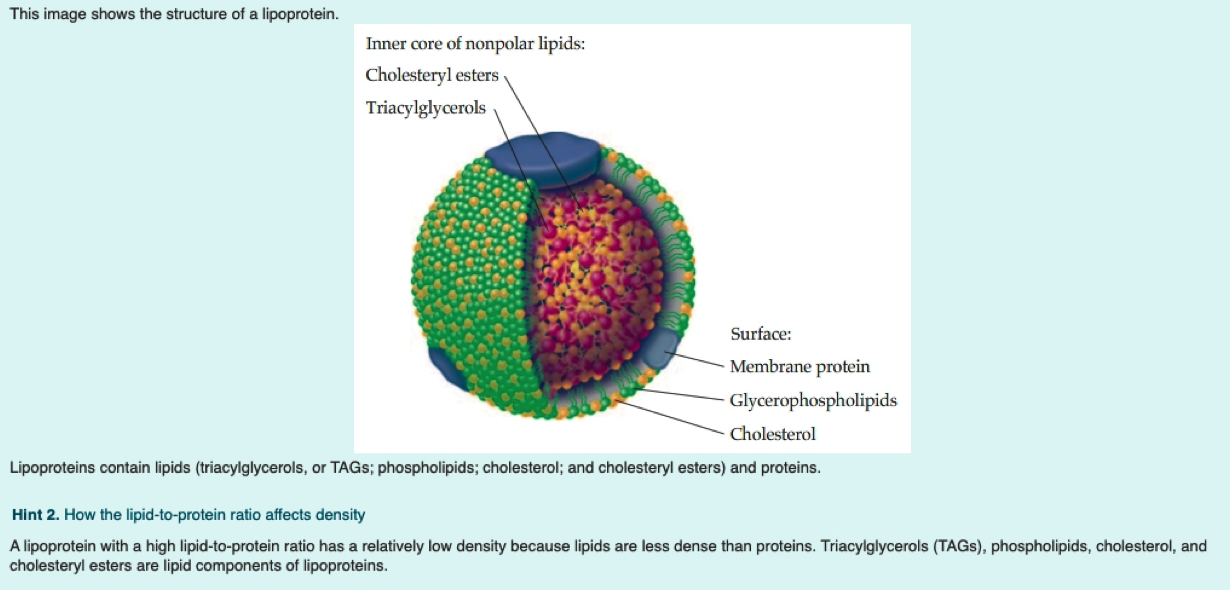
What is a lipoprotein (be general)?
= Fats + Protein
spherical particle
has a hydrophobic inner core (yellow) composed of cholesterol esters and triacylglycerols
- surrounded by a hydrophilic surface formed by the polar head groups of phospholipids and free cholesterol.
the apolipoproteins orient with their hydrophobic regions in the inner core and their hydrophilic regions at the surface.
How are apolipoproteins different from lipoproteins?
- apolipoprotein = the specific proteins that constitute the protein fraction of lipoproteins; they mediate the interactions of lipoproteins with tissues.
- lipoprotein = any lipid–protein conjugate. Specifically refers to lipid–protein associations that transport lipids in the circulation. Each consists of a core of hydrophobic lipids surrounded by a skin of amphipathic lipids with embedded apolipoproteins. Different kinds of lipoproteins play different roles in lipid transport.
- https://www.differencebetween.com/difference-between-lipoprotein-and-vs-apolipoprotein/
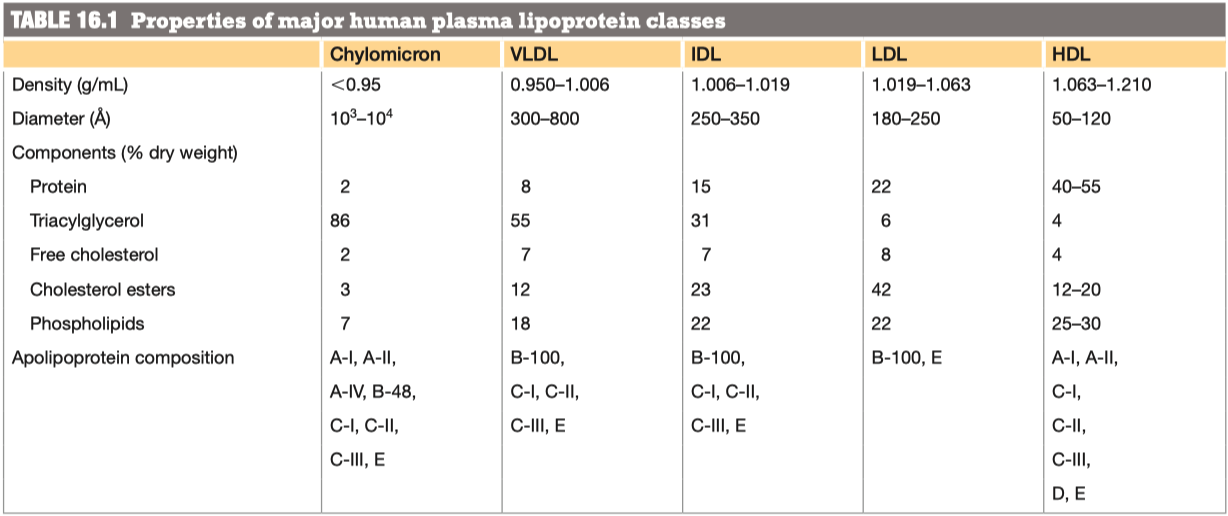
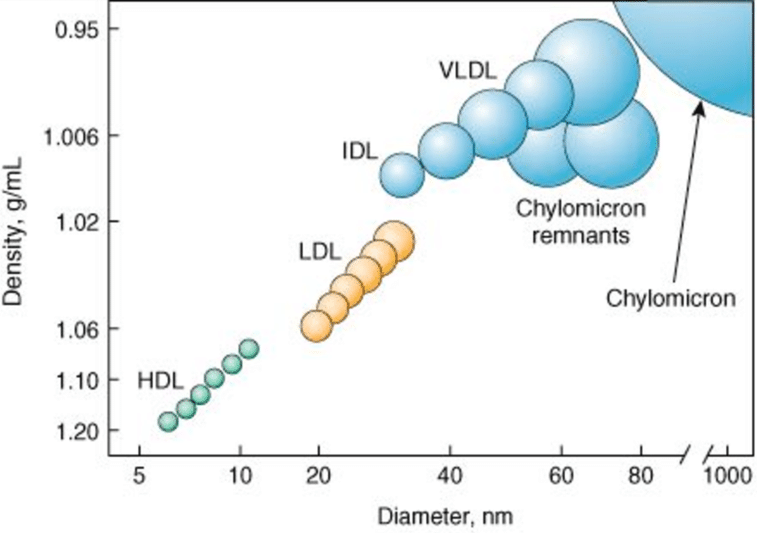
Why/How is density affected in these lipoproteins?
lipid content of a lipoprotein class is inversely related to its density
- the higher the lipid abundance, the lower the density
Digestion Absoption
We will start with identifying how fats are obtained from your diet. Lingual lipase and gastric lipase are enzymes that start the hydrolysis of short and medium chain fatty acids from their backbones, though this affect is minimal compared to the digestion that occurs in the small intestine
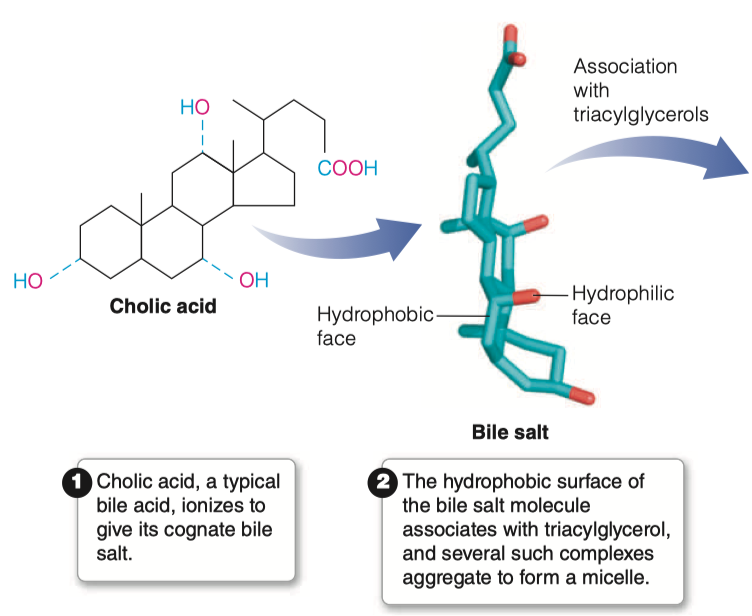
What are bile salts and where do they come from?
Bile Acids are derived from cholesterol
Bile acids are conjugated with taurine or glycine residues to give anions called bile salts
Bile Salts = detergent substances synthesized in liver and stored in gallbladder
- Essential to digestion of lipids and their absorption through intestinal mucosa
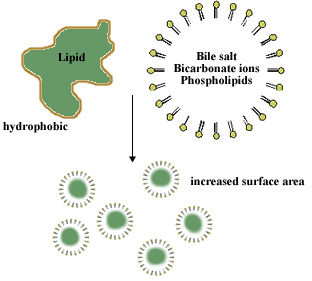
What characteristics of bile salts make them essential for lipid digestion?
A bile salt molecule has both hydrophobic and hydrophilic surfaces
This amphipathic character allows bile salts to orient at an oil–water interface, with the hydrophobic surface in contact with the apolar phase and the hydrophilic surface in contact with the aqueous phase.
- This detergent action emulsifies lipids and yields micelles, allowing digestive attack by water-soluble enzymes and facilitating the absorption of lipid through intestinal mucosal cells.
Bile salts emulsify fats, thereby promoting their hydrolysis in digestion
What does emulsify mean? How doe this relate to lipid digestion?
- Conjugated bile acids are almost always in their deprotonated (A-) form in the duodenum, which makes them much more water-soluble and much more able to fulfil their physiologic function of emulsifying fats
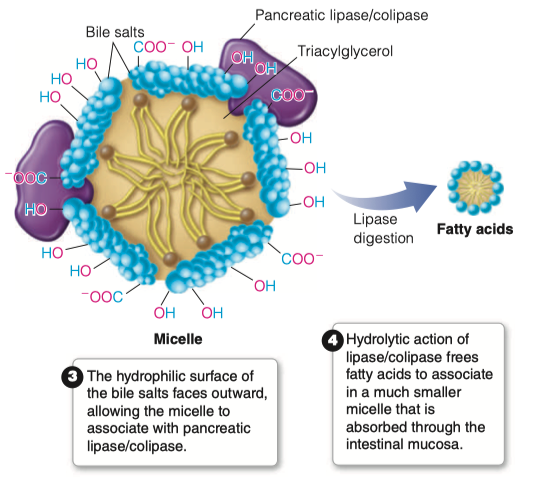
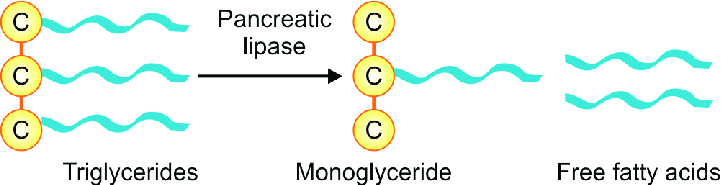
What is the role of pancreatic lipase?
An unusual calcium-requiring enzyme that catalyzes reactions at an oil–water interface.
The substrate being cleaved is in an apolar phase, and the other substrate, of course, is water.
Pancreatic lipase also functions in a 1:1 complex with colipase, a 90-amino acid protein that aids in the binding to the lipid surface.
Bile salts will come in contact with pancreatic lipase
Pancreatic lipase/colipase then catalyzes the digestion of lipids
Breaks down all of the triacylglycerols into individual fatty acids
- Individual fatty acids are small enough to be then absorbed by intestinal mucosa
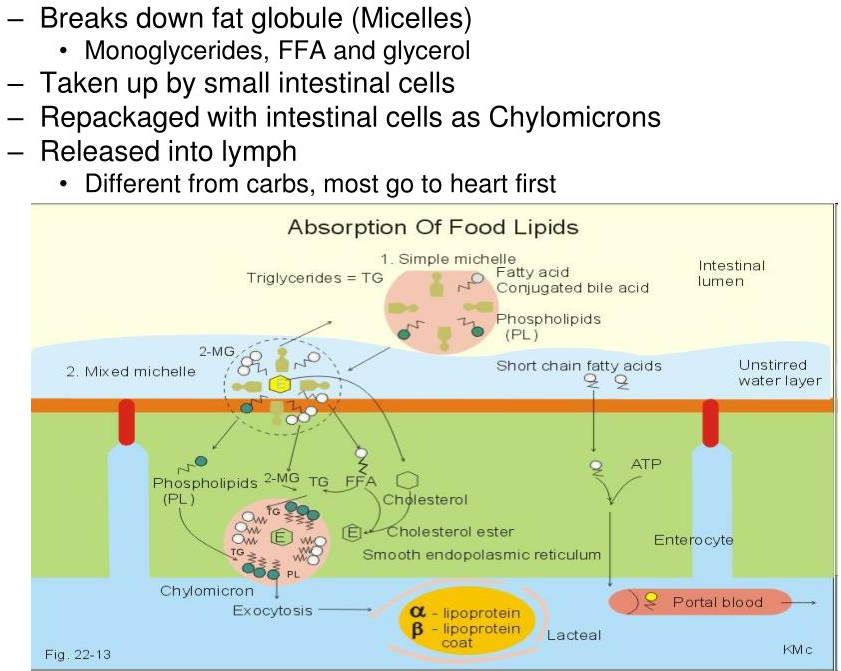
Where are lipids absorbed in the intestine?
- The products of fat digestion in the intestine comprise a mixture of glycerol, free fatty acids, monoacylglycerols, and diacylglycerols.
- During absorption through intestinal mucosal cells, much resynthesis of triacylglycerols occurs from the hydrolysis products.
- This resynthesis occurs in the endoplasmic reticulum and Golgi complex of mucosal cells
What happens to the lipids once they are absorbed?
Once fatty acids are absorbed into intestinal mucosa they are resynthesized back into triacylglycerols
- We break them down so we can get them into the mucosal cells
- Then the mucosal cells synthesize everything back into triacylglycerols
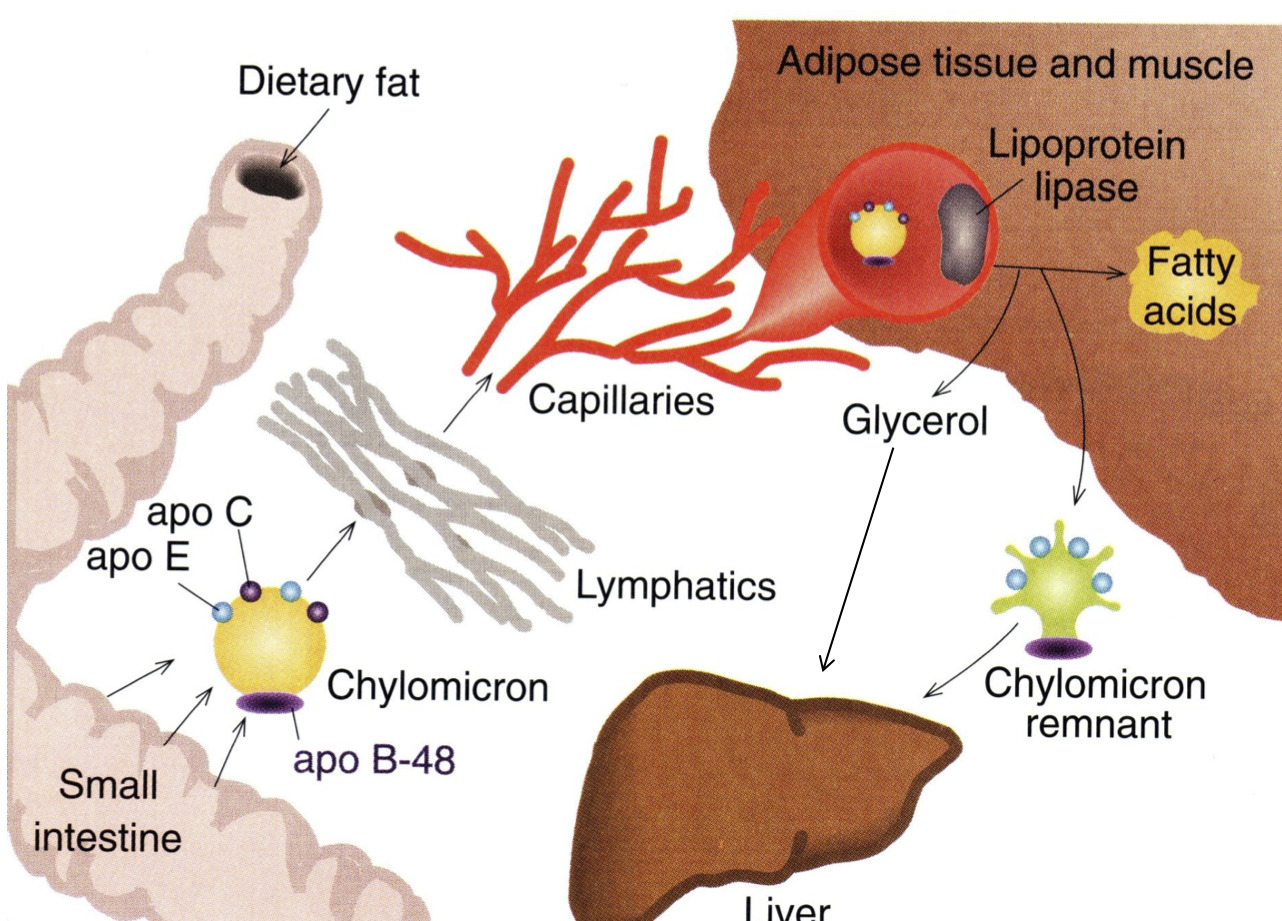
Once triacylglycerols have been resynthesized, they are assembled into chylomicrons
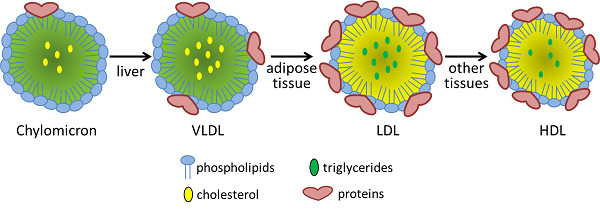
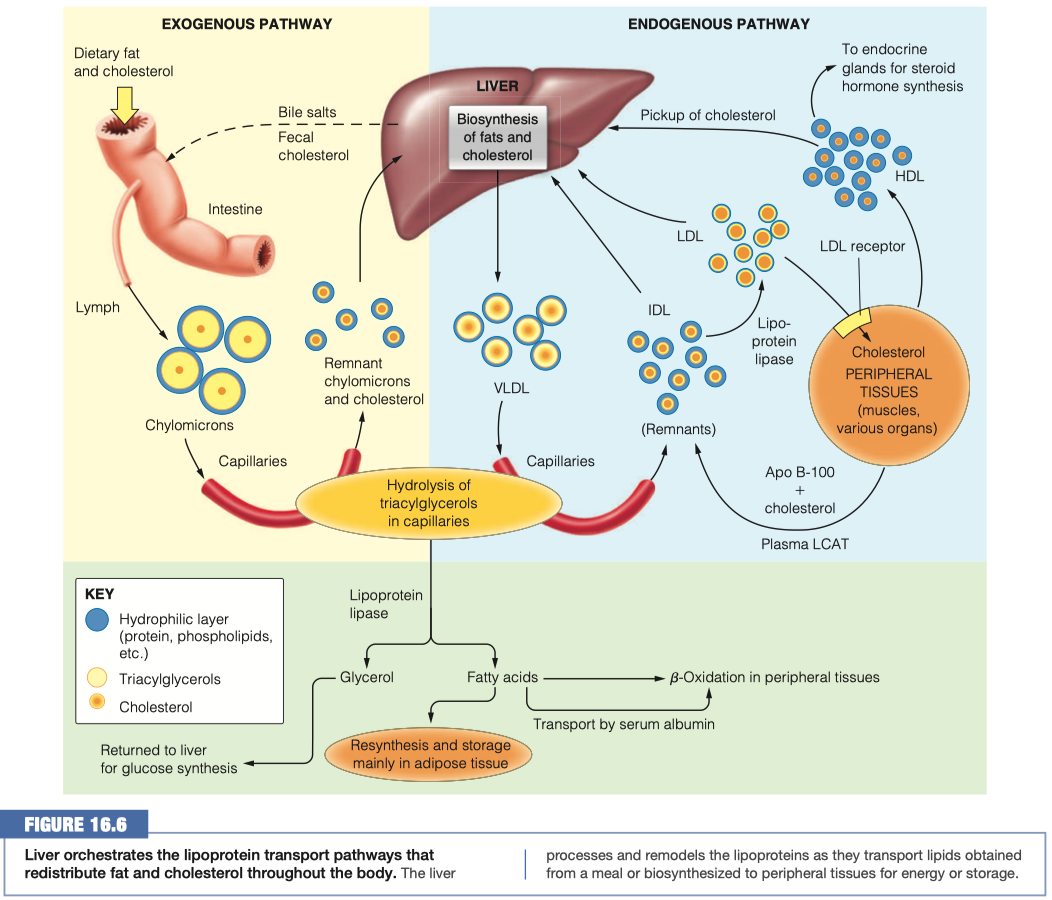
Now that the lipids are present in our cells, they must be transported to other areas of the body via the blood When the lipids leave the mucosal cells, which lipoprotein is formed in the lymph? How does this compare to the other lipoproteins?
Chylomicrons
- Least dense, massive amount of triacylglycerols
Chylomicrons are pushed into the lymphatic system and then into blood vessels / capillaries
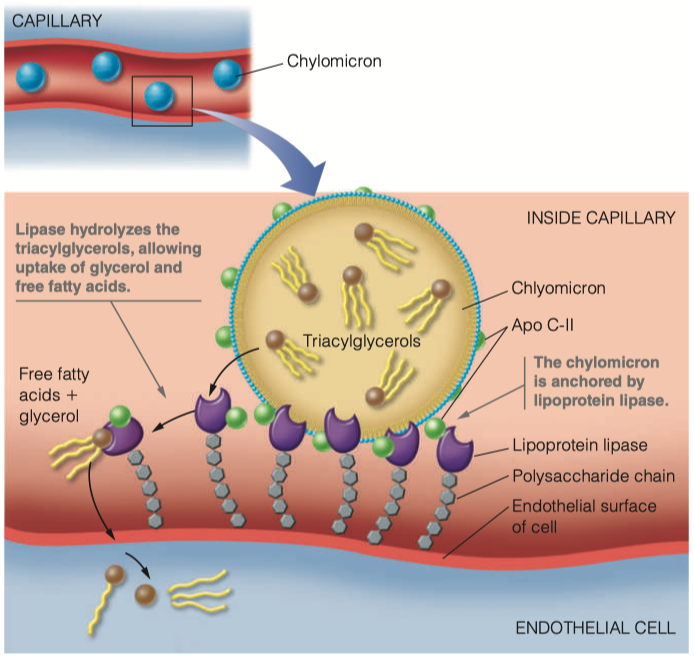
The lipoproteins are transferred form the lymph to the blood. Once they are in the blood, how are they recognized by lipoprotein lipase?
- The chylomicron is anchored by lipoprotein lipase, which is linked by a polysaccharide chain to the lumenal surface of the endothelial cell.
- When activated by apolipoprotein C-II (Apo C-II), the lipase hydrolyzes the triacylglycerols in the chylomicron, allowing uptake into the cell of the glycerol and the free fatty acids.
We have proteins ( Apo C-II ) on the outside of the chylomicron that functions as the ligand for lipoprotein lipase
Once lipoprotein lipase has connected with Apo C-II , it will grab the triacylglycerols from the chylomicron and bring them inside the endothelial cell
- Then triacylglycerols will begin to become hydrolyzed back into individual fatty acids
What is the function of lipoprotein lipase? Where do the products of the enzymatic reaction end up?
- The lipase hydrolyzes the triacylglycerols in the chylomicron, allowing uptake into the cell of the glycerol and the free fatty acids.
- We've gone from digestion, where we used pancreatic lipase to hydrolyze triaclyglycerols
- We brought them into the mucosal cells, resynthesized triaclyglycerols, and then assembled them into chylomicrons
- Chylomicrons then float around in capilaries
- The cells which need fat, will have a lipoprotein lipase receptors which then identify and attach to Apo C-II
- They then bring triaclyglycerols into the endothelial cell
- Once all the the triacylglycerols are gone, it becomes a "chylomicron remnant"
Once all of the triacylglycerols have been removed from the chylomicron, what is left? What name do we give this overall structure? Where does it ultimately go?
- Chylomicron Remnant
- Goes back to the Liver
Packaging of Existing Lipids
The remnant lipids and those synthesized in the liver must travel through the bloodstream to reach cells that need them. The liver packages these lipids into VLDL lipoproteins similar to how the intestinal mucosal cells make chylomicrons.
Compare and contrast the diameter, lipid components and apolipoprotein components of chylomicrons and VLDL lipoproteins
VLDL has more protein , and less triacylglycerol
Chylomicron Remnant has a lot less "fat"
Chylomicron vs VLDL Similarities:
- VLDL has more protein, and less triacylglycerol
Chylomicron vs VLDL Differences:
- Chylomicron has a much higher triacylglycerol level than VLDL
- VLDL has more cholesterol esters and phospholipids
How do you suppose triacylglycerols in VLDLs are hydrolyzed? Support your conjecture
Triacylglycerols in VLDLs are hydrolyzed to glycerol in the inner surface of capillaries in peripheral tissues
- Uses LPL and Apolipoprotein C-II on the VLDLs
- Uses the same system, now we are working with endogenous pathway
Instead of chylomicron remnants, what is left of the VLDL after triacylglycerol hydrolysis? Compare and contrast the diameter, lipid components and apolipoprotein components of VLDL and this lipoprotein.
- Instead of chylomicron remnants, VLDL turns into IDL
- Main thing thats different is we are missing a bunch of the triacylglycerols because they have been absorbed into the endothelial cells
- Cholesterol percentage is "up"
- All of the proteins are identical
- One just has already gone through the process of having its triacylglycerols removed
IDLs can be reabsorbed into the liver as were the chylomicron remnants, but they also have an alternate path here, conversion into LDL. Compare and contrast the diameter, lipid components and apolipoprotein components of IDL and LDL lipoproteins and summarize the changes you see.
We could send the cholesterol right back to the liver and say "nobody needs it"
- Or it could be used by other cells
IDL has more triacylglycerol per protein ( more dense )
LDL has more cholester esters "percentage wise"
Its not actually more, its just more percentage wise because we have gotten rid of everything else
Difference in some of the apolipid proteins
- We got rid of Apo A-I , Apo C-II, and Apo C-III
- We still have Apo B-100 and Apo E
LDL Uptake and HDL
How do LDLs interact with a cell that requires additional lipids?
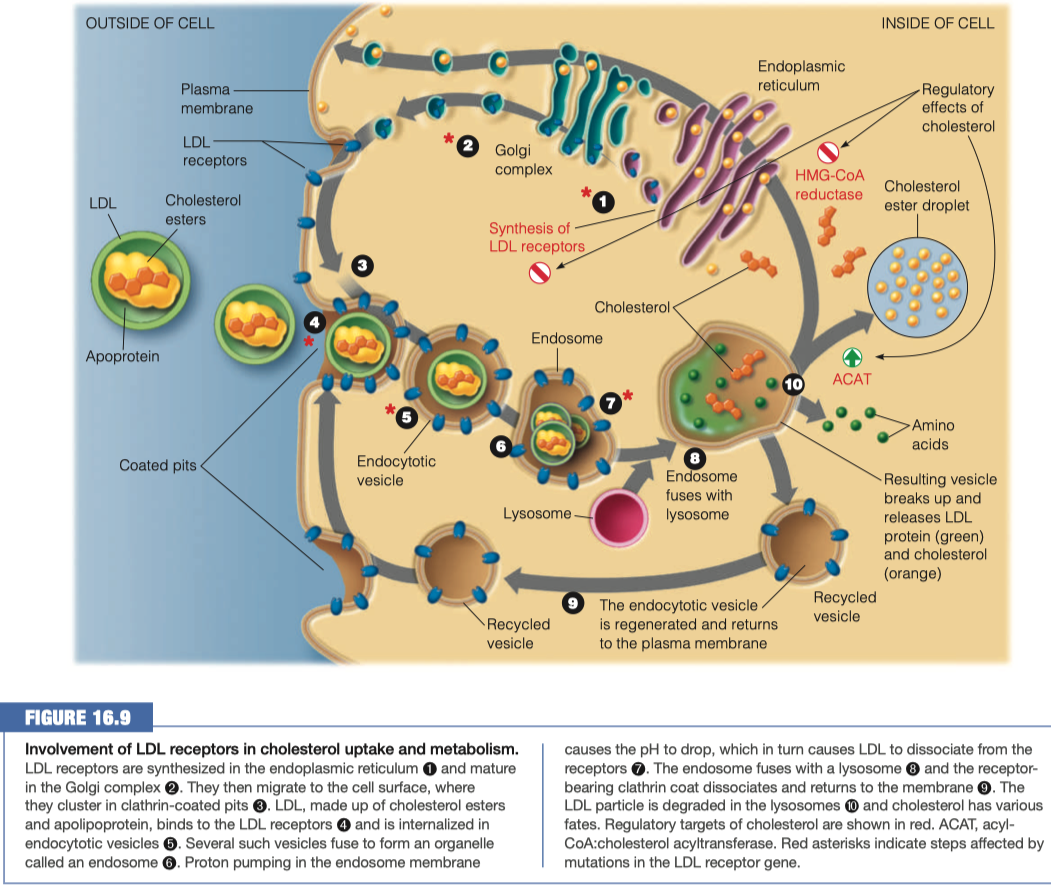
Activates LDL receptor Apo B-100
Triggers receptor-mediated endocytosis
- Receptors interact all the way around the vesicle and then pull it inside the cell to pass the plasma membrane making a vesicle which can then be broken down
Upon the above interaction what happens to the LDL lipoproteins to bring them into the cell?
- When LDL binds to its receptor, the entire LDL receptor complex is engulfed and taken into the cell (endocytosed)
- Binding to the LDL receptor occurs through recognition of the B-100 apolipoprotein, the primary protein component of the LDL particle.
- The plasma membrane fuses in the vicinity of the LDL–receptor complex, and the coated pit becomes an endocytic vesicle.
- Several of these clathrin-lined vesicles fuse to form an endosome.
Once inside the cell what happens to the lipoproteins?
The endosome then fuses with a lysosome, putting the LDL–receptor complex in contact with the hydrolytic enzymes of the lysosome.
The LDL apolipoprotein is hydrolyzed to amino acids, and the cholesterol esters are hydrolyzed to give free cholesterol.
The receptor itself is recycled, moving back to the plasma membrane to pick up more LDL
Lysosome fuses with endosome
- Breaks down all of the lipids and proteins
- Releases amino acids and cholesterol
Anything that was not broken down gets returned back to the plasma membrane so we can use it again
If there is excess cholesterol in a cell, what happens to it?
Internalized cholesterol:
Reduces endogenous cholesterol synthesis, by inhibiting HMG-CoA reductase and also by repressing transcription of the gene for this enzyme and accelerating degradation of the enzyme protein.
Activates acyl-CoA:cholesterol acyltransferase (ACAT), an intracellular enzyme that synthesizes cholesterol and esters from cholesterol and a long-chain acyl-CoA.
- This promotes the storage of excess cholesterol in the form of droplets of cholesterol esters
Regulates the synthesis of the LDL receptor itself by decreasing transcription of the receptor gene.
- Decreased synthesis of the receptor ensures that cholesterol will not be taken into the cell in excess of the cell’s needs, even when extracellular levels are very high.
This regulatory mechanism explains why excessive dietary cholesterol leads directly to elevations of blood cholesterol levels.
- With intracellular cholesterol levels so well regulated, the extracellular cholesterol accumulates because it has nowhere else to go.
Cholesterol esters are being stored
Inhibition of cholesterol synthesis
HMG-CoA reductase is a predominate enzyme in cholesterol synthesis
- It takes HMG-CoA and turns it into mevalonate
- we are stopping this reaction from happening here, so it stays as HMG-CoA
Cholesterol is going to be packaged into HDL
- HDL can be sent outside the cell to the liver to be excreted
- HDL can be sent to endocrine glands for steroid hormone synthesis
What lipids are the majority composition of HDL? Where does the HDL go? Why?
- Mainly cholesterol, some triclycerides
- Regulate levels of cholesterol in blood
- Goes back to liver
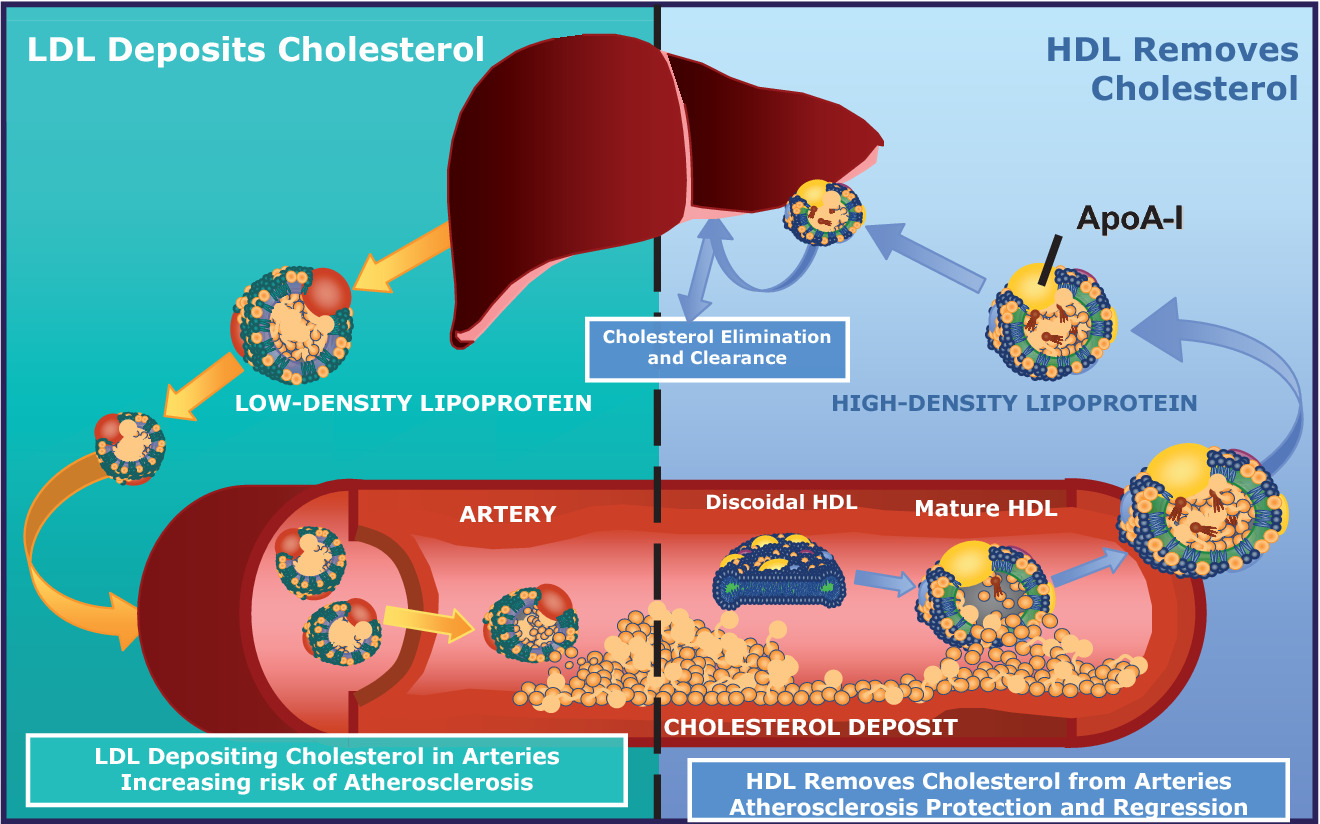
When people refer to LDL as “bad cholesterol” and HDL as “good cholesterol”, why is this a misunderstanding? Provide at least two reasons.
- Cholesterol itself is a natural metabolite, an essential component of all membranes, and the precursor to all steroid hormones and bile acids.
- Cholesterol present in LDL is considered “bad” because prolonged elevation of LDL levels is what leads to atherosclerosis.
- Cholesterol in HDL is called “good” because high levels of HDL counteract atherogenesis.
- Cholesterol cannot be metabolically degraded, and excess cholesterol is returned from peripheral cells to the liver, for passage through the bile to the intestine, for ultimate excretion (Figure 16.6).
- As the agent for this transport back to the liver, HDL plays a role in lowering total serum cholesterol levels, which is “good.”
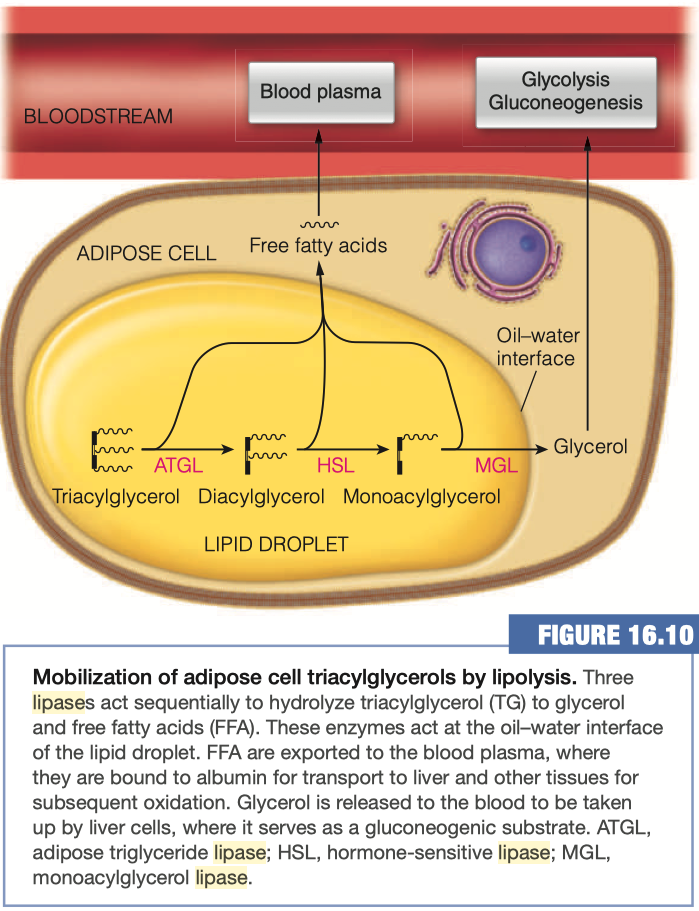
Mobilization of Triaclyglycerols from Adipose Tissue
As you already know, the adipocytes store our excess energy as fats, predominantly as triacylglycerols
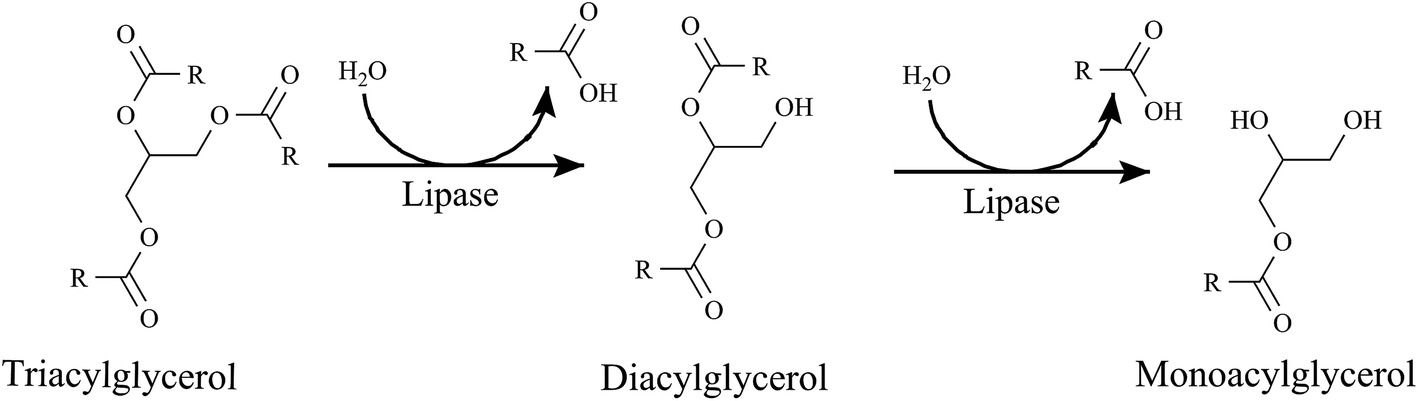
- Triacylglycerol lipase
- Hormone-sensitive lipase (HSL)
- Adipose triglyceride lipase (ATGL)
- Monoaclyglycerol Lipase (MGL)
Triacylglycerols (TAGs) are converted into diacylglycerols (DAGs) by what enzyme?
- Adipose triglyceride lipase (ATGL)
Diacylglycerols are converted into monoacylglycerols (MAGs) using which enzyme?
- Hormone-sensitive lipase (HSL)
What happens to the Free Fatty acids upon hydrolysis from glycerol?
- Goes back into blood plasma
- The free fatty acid hydrolysis products exit the adipocyte by passive diffusion and find their way to the blood plasma, where the fatty acids become bound to albumin.
- Each molecule of albumin can bind up to 10 molecules of free fatty acid, although the actual amount bound is usually far lower.
- Fatty acids are released from albumin and taken up by tissues largely by passive diffusion so that fatty acid uptake into cells is driven primarily by concentration.
How are lipids (a largely nonpolar molecule) transported in the blood (a polar solvent)?
Where are the majority of glycerol taken up after its release from the adipocytes? Why?
Most of the glycerol released to the bloodstream is taken up by liver cells, where it serves as a gluconeogenic substrate, leading to the production of glucose
Glycerol is being released into the bloodstream
When you are breaking down your fats, you are in a situation where you have low blood glucose
You have no supply of sugar around potentially
- you've even gone through all of your glycogen stores
And now we are starting to break down our fats in order to maintain our energy levels.
Well, in order to bring blood glucose back up, this glycerol is going off to the liver where it's going through gluconeogenesis.
And that gluconeogenesis will then provide some additional glucose for lots of other things around your body.
Now, this glycerol can be used in glycolysis.
- So it can go through that pathway either forward or backward, depending on what the needs of the body are, and what the needs of the individual cells are.
It's not always the case that your entire body is doing gluconeogenesis or your entire body is doing glycolysis.
- It just depends on what's working, what's not working, what needs more energy, what doesn't need more energy, etc.
It all comes down to what your cells need, and then how they indicate to your body whether or not they need those substrates.
05 - Lipid Transport - Things To Know
General statistics about heart disease
- Black men and women are more at risk than white
- Older individuals are more at risk
- Males are more at risk within their race
- two top leading causes of death in 2018 and 2019 the US were heart disease and cancer
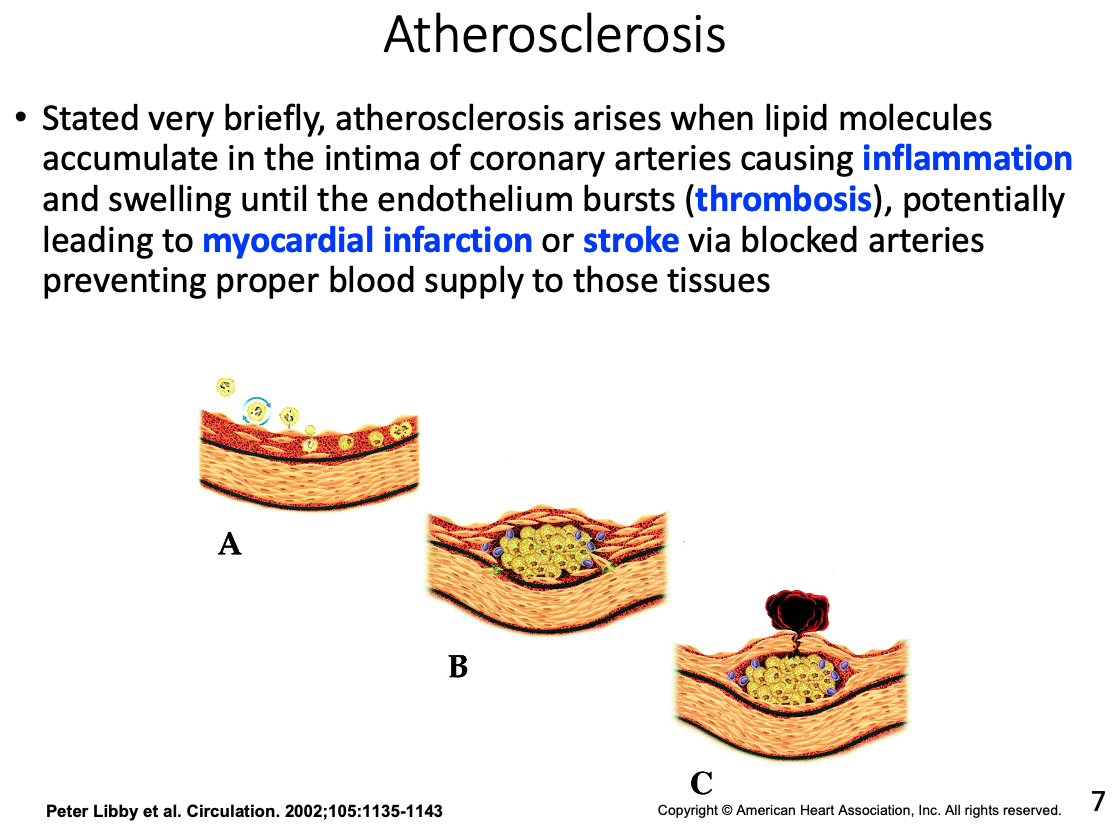
Atherosclerosis
Define it
hardening of the arteries over the course of several decades
- often leads to heart attack , stroke, or other peripheral vascular disease
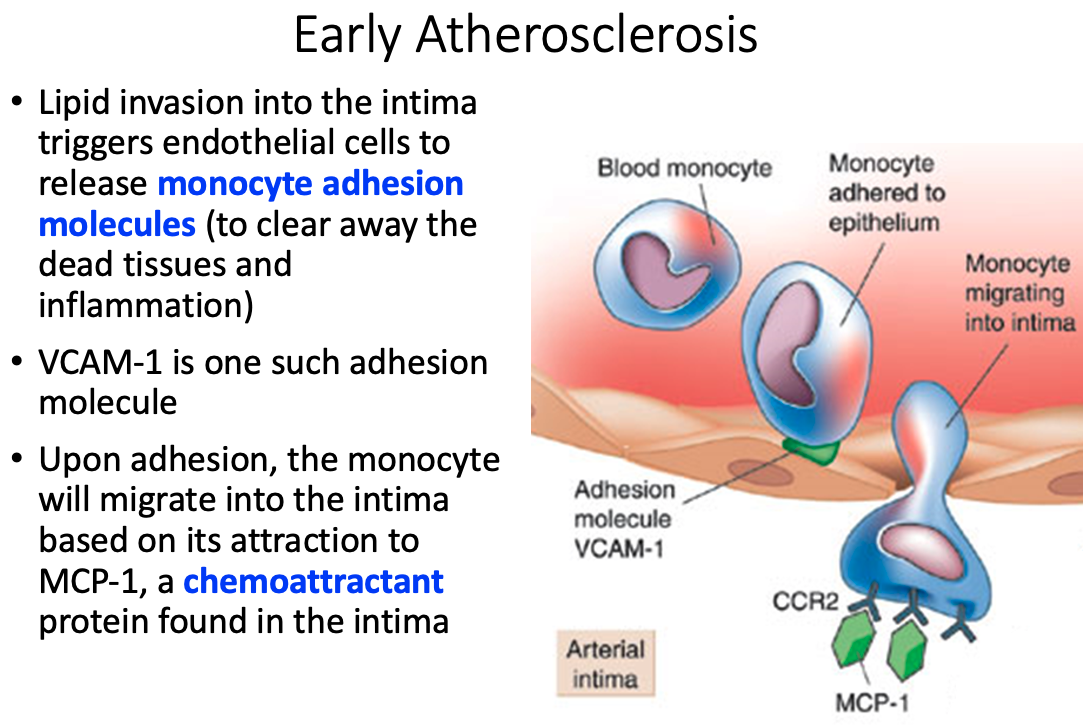
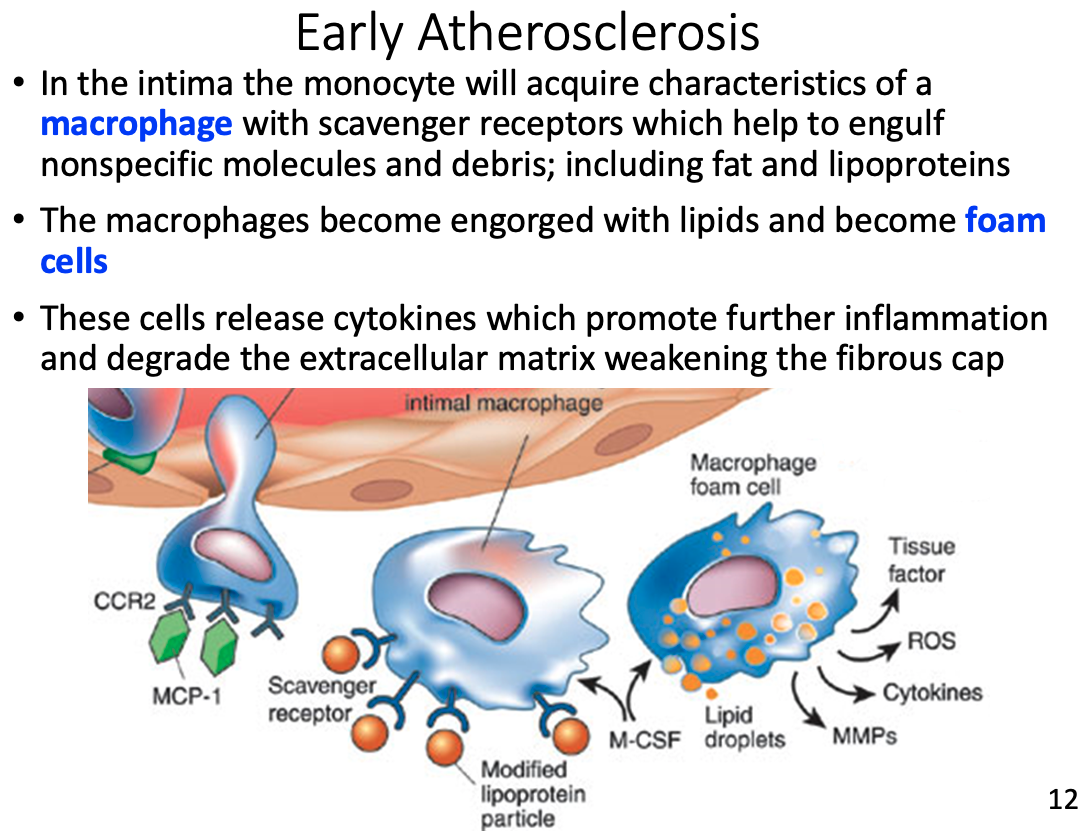
Role of macrophages and how they got there
- In the intima the monocyte will acquire characteristics of a macrophage with scavenger receptors which help to engulf nonspecific molecules and debris; including fat and lipoproteins
Foam cells
- engorged macrophages with lipids
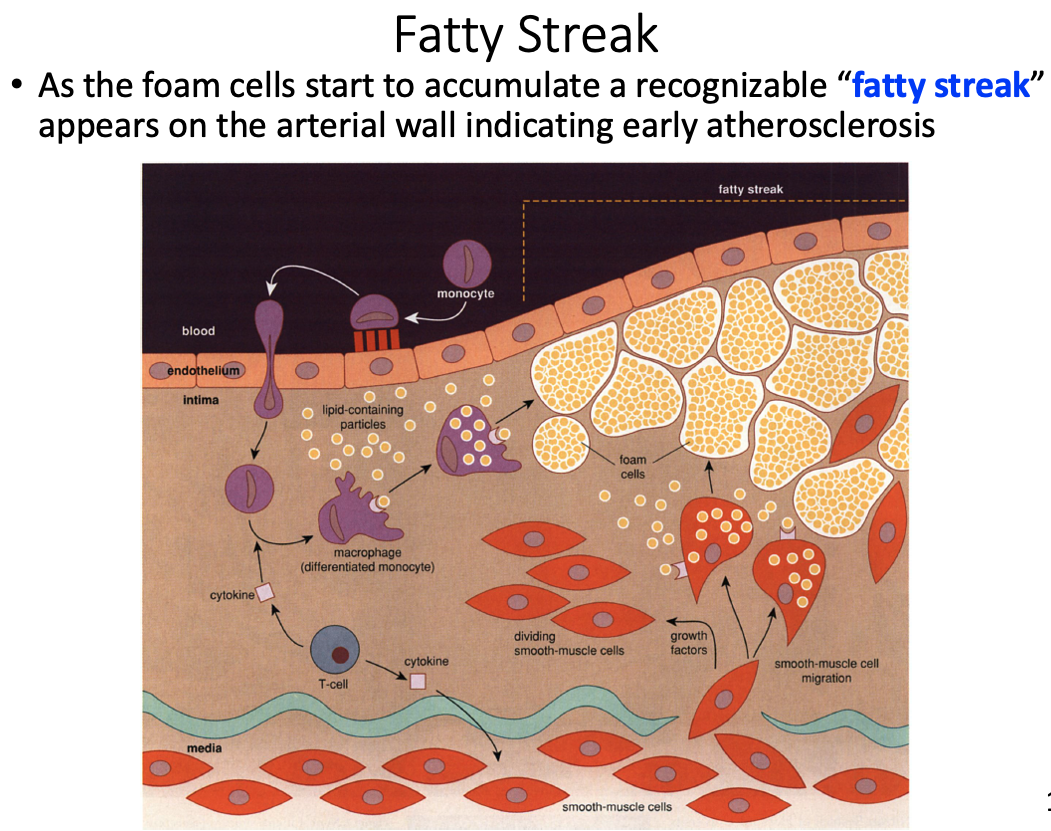
Fatty streak
- accumulation of foam cells that appear on arterial wall indicating early atherosclerosis
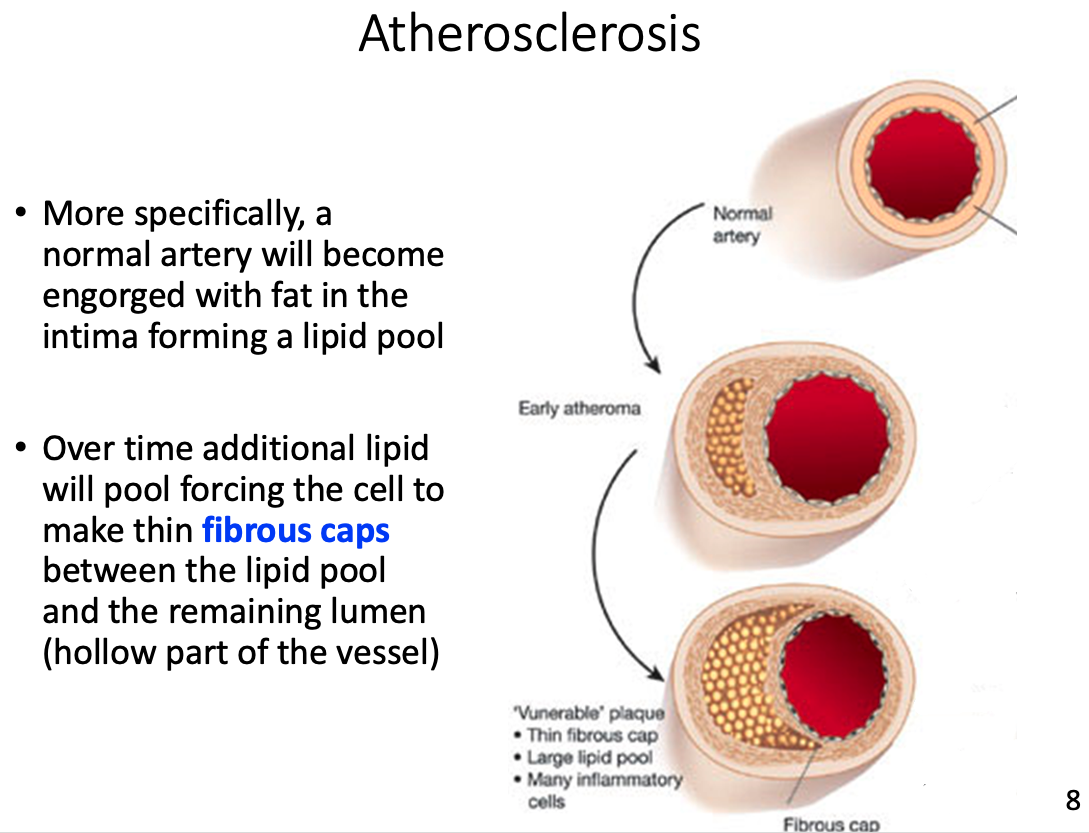
Fibrous cap
- Over time additional lipid will pool forcing the cell to make thin fibrous caps between the lipid pool and the remaining lumen (hollow part of the vessel)
Possible result of thrombosis
Why cholesterol is such a concern
If no tissues require cholesterol the LDL will continue to circulate, eventually depositing the excess fat on the arterial walls
- leads to Atherosclerosis
Medical diagnosis of LDL and HDL
- LDL: high cholesterol lipoprotein responsible for transporting cholesterol to peripheral tissues
- HDL: removes excess cholesterol for is removal from the body
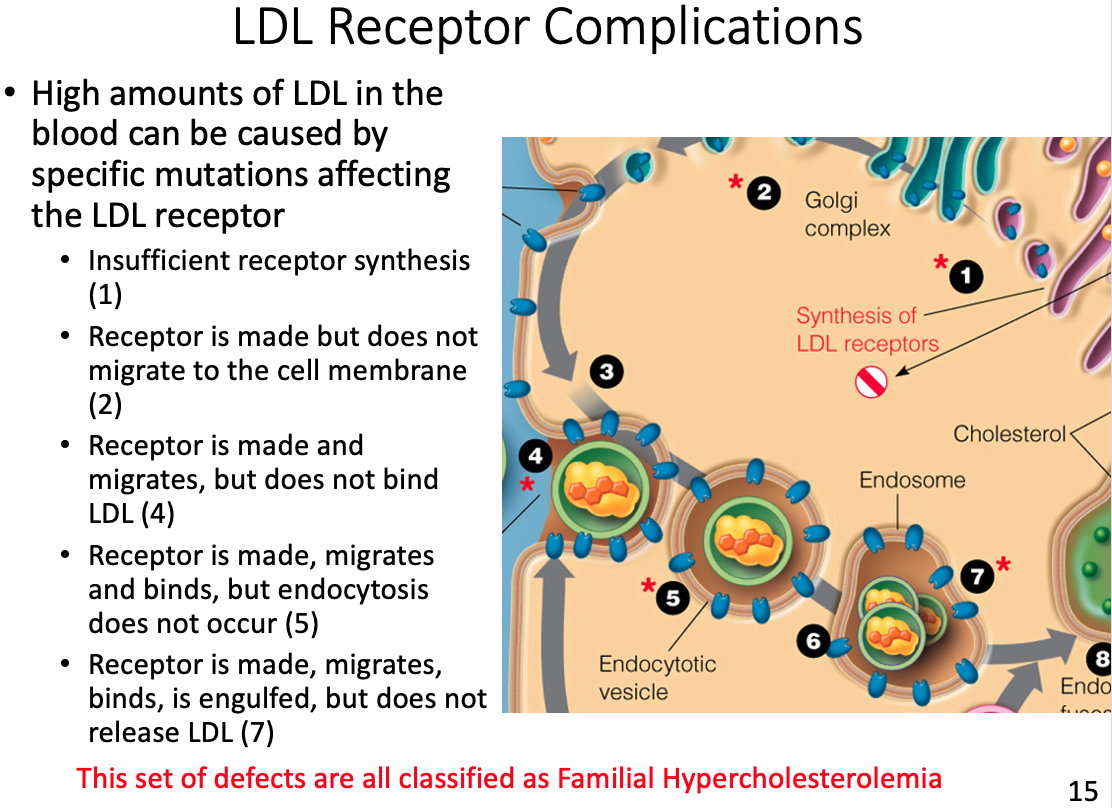
LDL receptor complications and results
- If no tissues require cholesterol the LDL will continue to circulate, eventually depositing the excess fat on the arterial walls
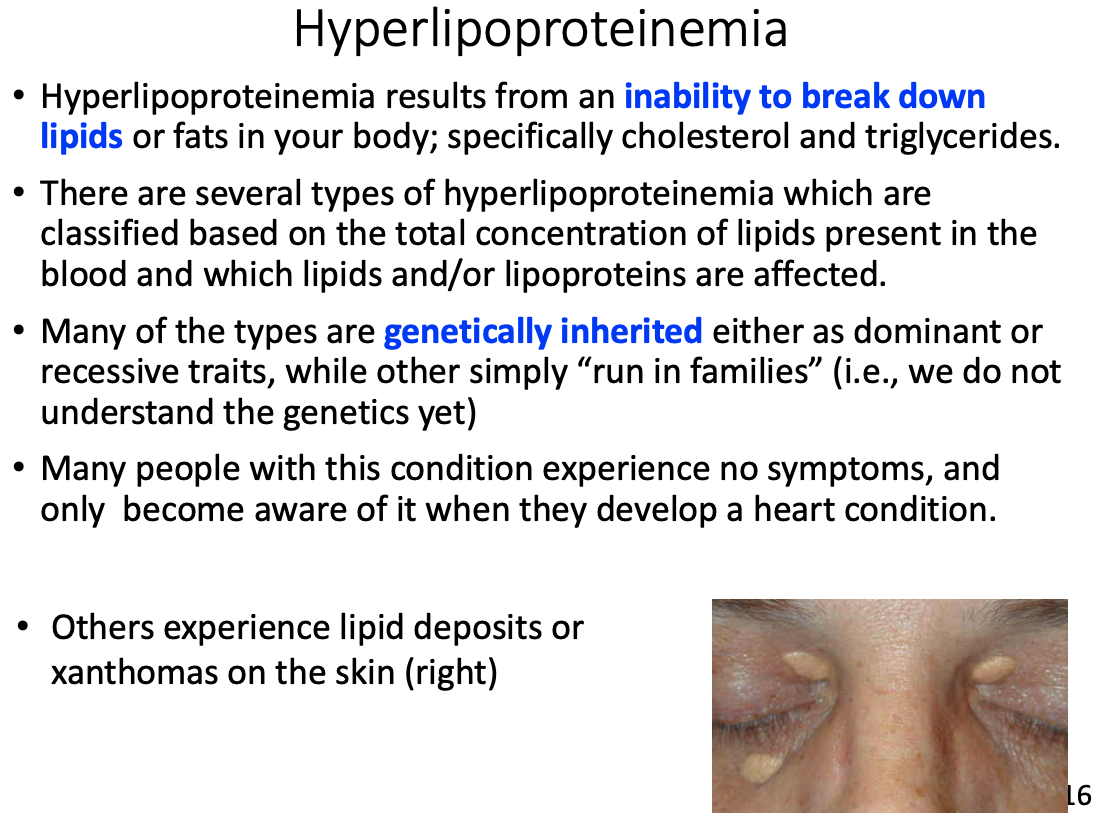
Hyperlipoproteinemia
General characteristics
- Inability to break down lipids or fats in your body; specifically cholesterol and triglycerides.
- Many of the types are genetically inherited either as dominant or recessive traits, while other simply “run in families”
Symptoms
- Many people with this condition experience no symptoms, and only become aware of it when they develop a heart condition
- Others experience lipid deposits or xanthomas on the skin
06 - Membrane Stucture and Function
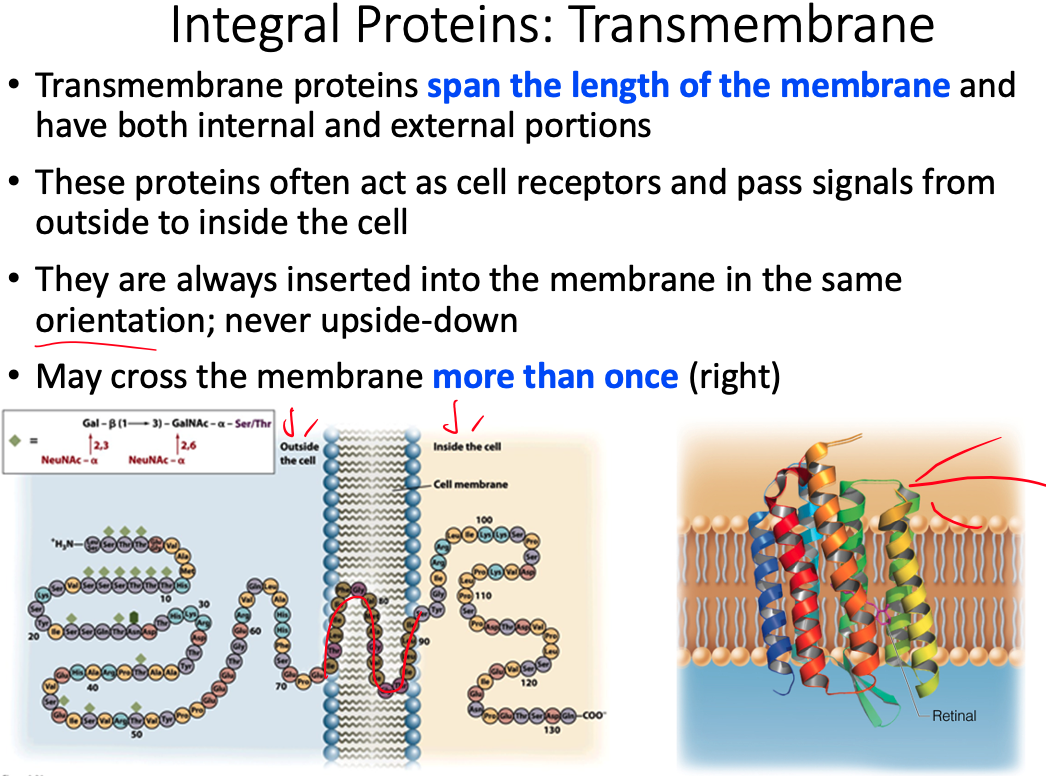
The extracellular side of a transmembrane helix of an integral membrane protein does NOT contain __.
mostly hydrophobic amino acids
- As the figure below illustrates, the extracellular side of a transmembrane protein contains mostly hydrophilic, not hydrophobic, amino acids, as these are exposed to water and can make many favorable intermolecular interactions.
- This side of the membrane is also glycosylated, as mono/polysaccharides are important for cell-cell recognition, antigen-antibody interactions, etc.
- These saccharides are covalently bound to glycoproteins via the amino acids Ser, Thr, and Asn, and therefore the extracellular side of the transmembrane protein contains many of these specific residues.
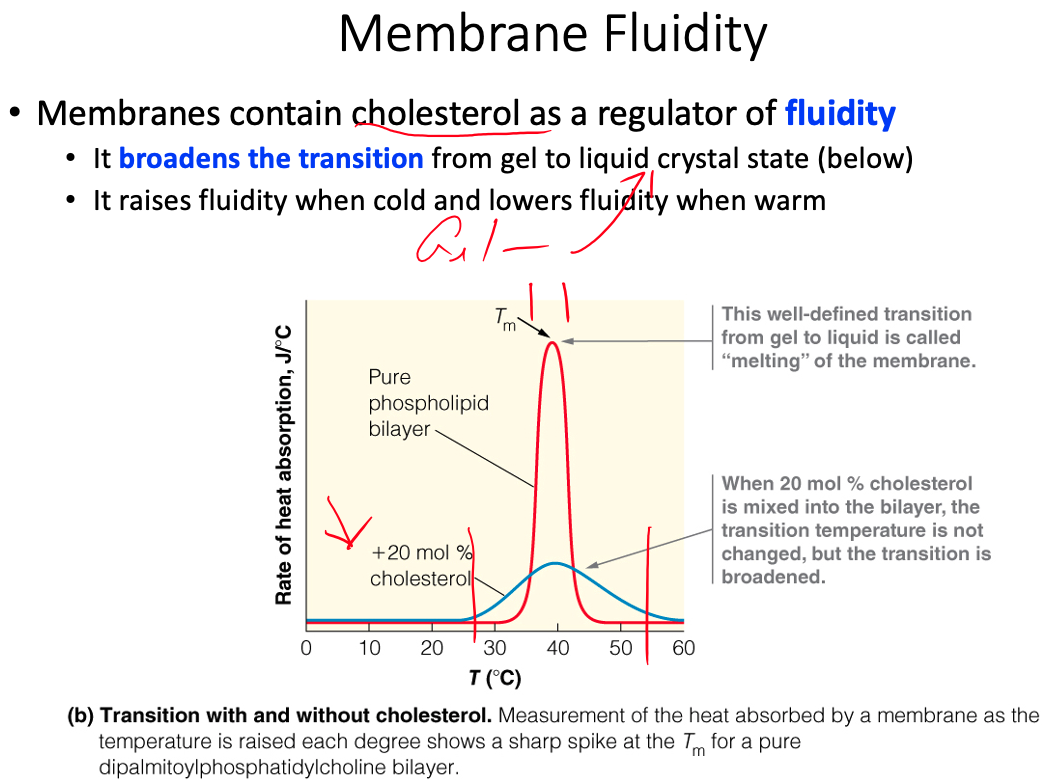
Bacteria that thrive in cooler growth temperatures compensate by __ as compared to bacteria growing at warmer temperatures.
putting more unsaturated fatty acids into their membranes
- Bacteria growing at cold temperatures need a membrane composition that facilitates increased membrane fluidity, because the cooler temperatures decrease molecular motion.
- Therefore, bacteria thriving in cool temperatures have increased populations of unsaturated fatty acids on phospholipids because the cis double bonds cause kinks or separations within the membrane.
Consider the following structure:
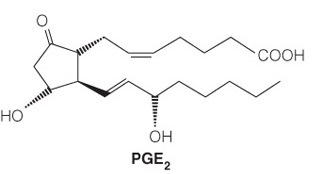
The molecule’s function is to __.
send out localized cellular signals
- The structure is a prostaglandin, which is a type of eicosanoid.
- These lipids are derived from a polyunsaturated fatty acid and have a distinctive five-membered ring that is sometimes oxidized.
- These are local signaling molecules (sending out localized cellular signals) that can control pain, inflammation, and local housekeeping. They are less polar than steroids and therefore less soluble in the bloodstream, so they do not stray far from the cell that synthesized them.
Consider the following structure:
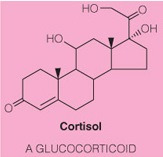
The molecule’s function is to __.
send out global cellular signals
- The structure is a steroid because it has a steroid nucleus and a higher level of oxidation than a sterol.
- These molecules are more hydrophilic than sterols and therefore travel throughout the bloodstream readily.
- They bind to receptor proteins and initiate signaling cascades.
- Thus it’s function is to send out global cellular signals.
- In contrast, eicosanoids are localized signaling molecules because they are less polar.




The active transport of chloride ions into the cytoplasm, from low to high concentration, sets up a(an) __.
electrochemical gradient
Movement of charged ions across a membrane, resulting in unequal concentrations, has a twofold effect:
- (1) the concentration difference sets up a concentration gradient, whereas if the chloride moved out of the cell (i.e., down its concentration gradient), it would release energy
- (2) the charge of the ion sets up membrane polarization, wherein the membrane is more negative on the side of the membrane with the highest chloride concentration.
Again, if chloride were to move out of the cell it would be moving toward the positive side of the membrane and this also releases energy.
The combined effect is the creation of an electrochemical gradient.
The term is derived as follows:
- electro for charge separation,
- chemical because a chemical/ion created this charge separation
- gradient due to the concentration difference between the sides of the membrane.
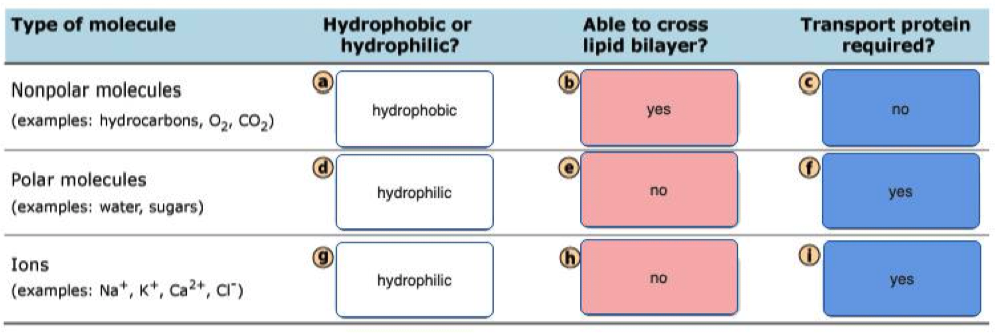
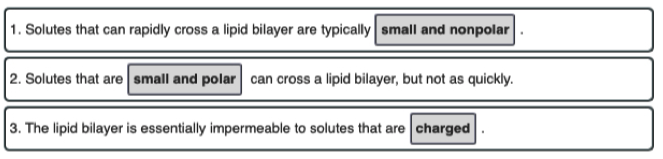
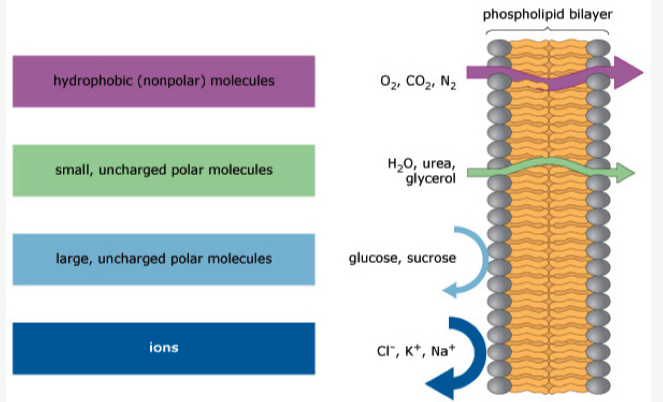
A lipid bilayer membrane without pores or transporters is freely permeable to which of the following?
- Lipid bilayers are permeable to small, uncharged, and nonpolar molecules.
- Of all the choices, only carbon dioxide () meets all three requirements.
- Na+ and K+ are small but they are charged, polar ions that require transporters.
- Glucose is uncharged but it is large and polar, so it also requires a transporter.
- Lys is a large, charged, and polar molecule that also requires a transporter at pH 7.
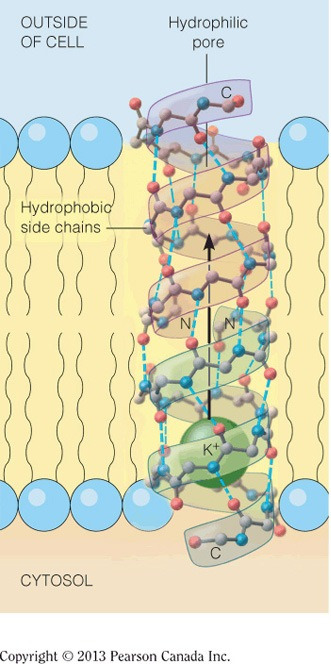
The inner core of a pore transport protein for potassium is most likely lined with __.
hydrophilic amino acids
- The inner core of the pore is responsible for binding potassium, which is a charged ion.
- The amino acids that will be attracted to this positive potassium ion through intermolecular interaction are the acidic and polar neutral amino acids, which belong to the hydrophilic group of amino acids.
- The surface of the pore protein will be coated in mostly hydrophobic amino acids, as these amino acids will have favorable intermolecular interactions with the hydrophobic lipids’ tails.
- The figure below shows a pore that transports potassium, illustrating the hydrophilic core and the hydrophobic surface.
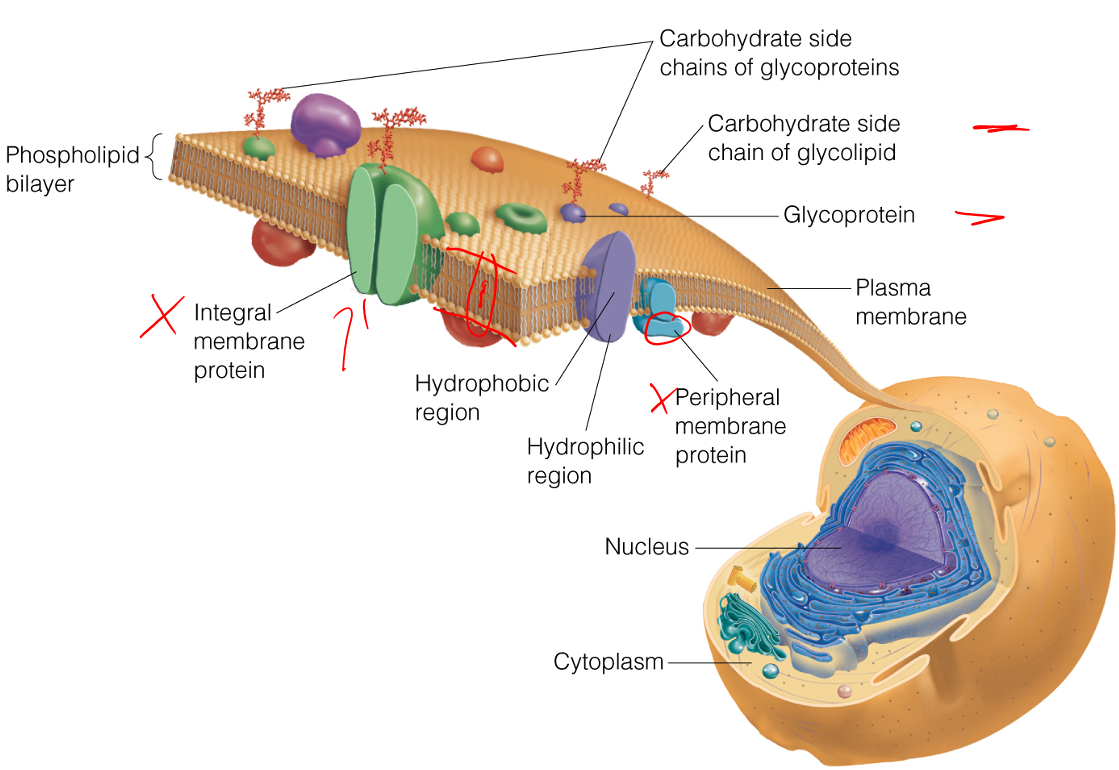
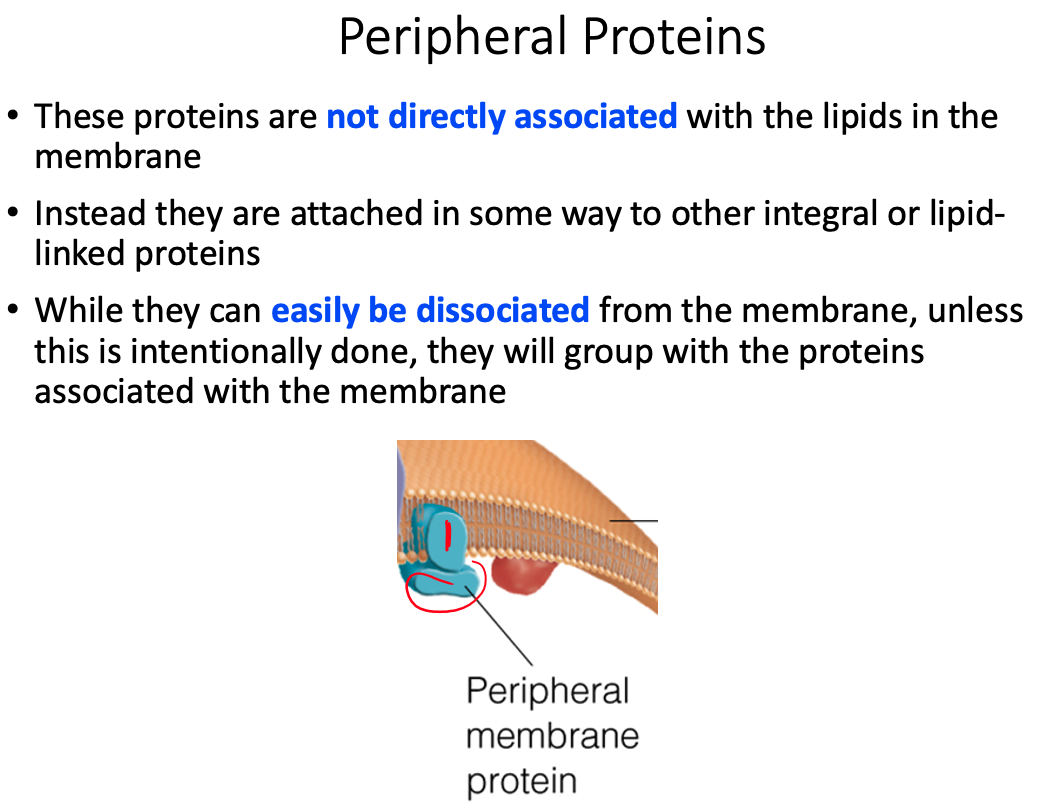
Define the terms peripheral membrane protein and integral membrane proteins.
Peripheral Membrane Proteins = exposed at only one membrane face or the other
- Held to the membrane by noncovalent interaction with lipid heads or integral membrane proteins
Integral Membrane Proteins = largely buried within the membrane but are usually exposed on both faces
- Frequently involved in transporting specific substances, or transducing chemical signals through the membrane
The relative orientation of polar and nonpolar amino acid side chains of integral membrane proteins is "inside-out" compared to the amino acid side chain orientation for globular water-soluble proteins. Explain why.
Membrane proteins are lipid soluble rather than water soluble
- We want the hydrophobic chains to be touching the lipid rather than pressed in
A typical globular protein is found in the cytosol, and because the cytosol is predominately water, what we typically find is:
- Any of the amino acid side chains that are hydrophilic are going to be going towards the water or towards the outside
- Any of the hydrophobic residues are going to be hiding on the inside of the protein and staying as far away from water as possible
The lipid bilayer we have nonpolar regions inside the polar heads.
- phospholipids, sphinolipids, ect
Because these proteins are embedded inside that region, they're actually in a nonpolar environment
Our hydrophilic amino acids are not going to like that nonpolar environment
So we're going flip everything inside out
- The hydrophobics are going to be on the outside protecting the hydrophilic which are now hiding on the inside
Information
- Integral membrane proteins often contain helical segments of appropriate length to span the lipid bilayer.
- In a protein that has a single segment that spans the membrane, the helix usually only contains hydrophobic residues and is called a single-span membrane protein.
- In transmembrane proteins with multiple segments that span the membrane, hydrophilic residues are often found in sequences of the helices.
Why are hydrophobic residues favored in single-span membrane proteins? Find an example of a single-span membrane protein in your book.
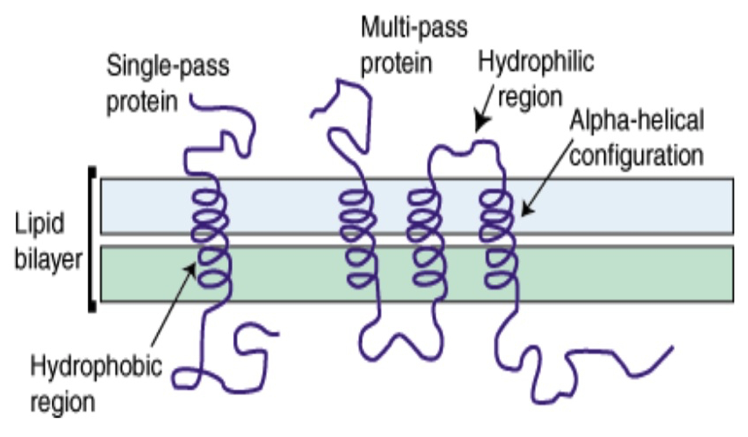
Hydrophobic residues are favored because the majority of the
- when we have a single span membrane protein its going to be using
Bacteriorhodopsin - Figure 10.14
Majority of exterior of protein will be interacting with hydrophobic region
When we have a single-span membrane protein, its typically going to have a helix with side chains sticking out
The majority of the single-span membrane protein is going to be in the hydrophobic region
Propose a structural orientation and a function for multiple hydrophilic residues in the helices of multi-span protein. Find an example of a multi-span membrane protein in your book
- We have all of these multispan proteins, some of which are involved in protein transporrt
- The helices end up forming a circle/pore with hydrophilic residues facing inward because they want to be away from the lipid hydrophobic region
- For example, sodium is going to come down and interact with those hydrophilic residues in order for the transport protein to push it through from one side of the membrane to the other
- Example = Retinal
From your understanding of protein structure and membrane structure, explain the fact that of the approximately 51,000 protein structures (3D) published and listed int he Protein Data Bank (in 2008) only about 930 of theses structures are transmembrane proteins. NOTE: 3D structures are typically obtained from X-ray crystallography data.
X-Ray Crystallography requires a lot of "salt" in order to crystallize the protein
In a nonpolar solvent, where we would normally expect to find our membrane proteins, it is going to be difficult to get a "salt" in there to make a crystal
So if we take the integral membrane protein and put it into a hydrophilic solution, water for example, and then crystallize it, the general structure is going to be completely changed
- Because all of the hydrophobic residues are going to want to hide on the inside
- So the structure observed will not be the "true" structure
Propose a reasonable mode of action by which these antihistamines exert their activity
- Anti-histimines could be a non-competitive inhibitor that makes a conformational change and prevents histimine from binding
- Claratin could latch onto histimine and prevent it from binding to H1 receptors
- Competitive antagonist
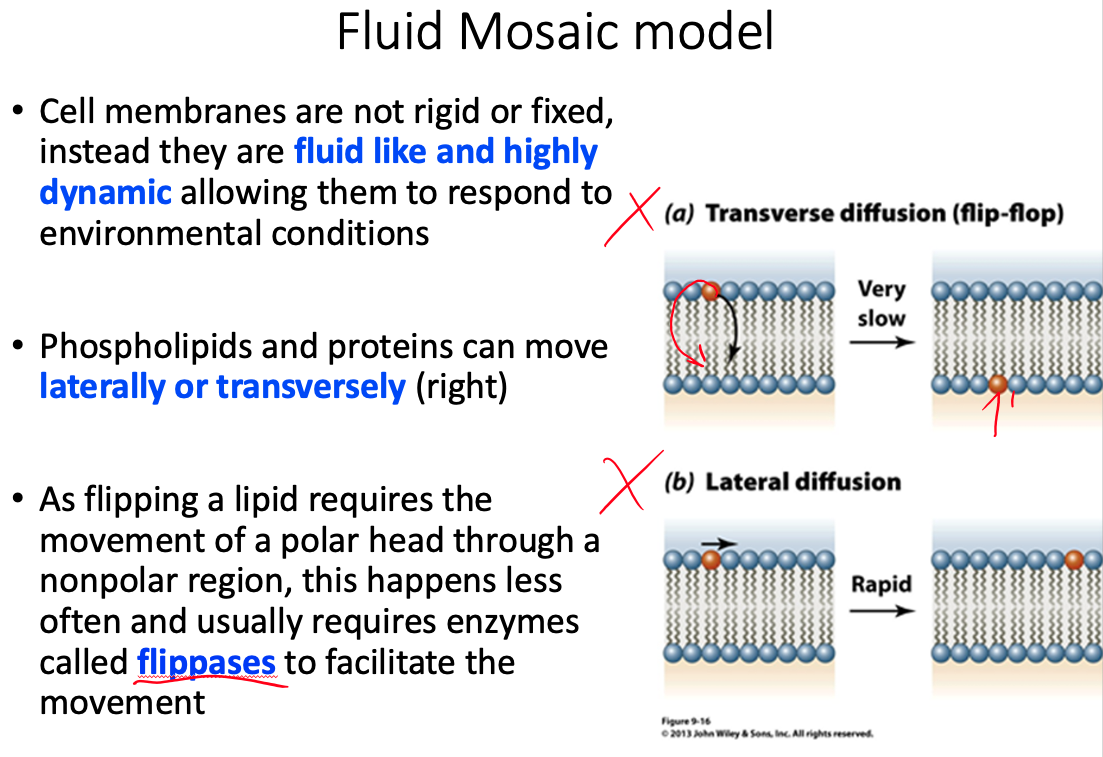
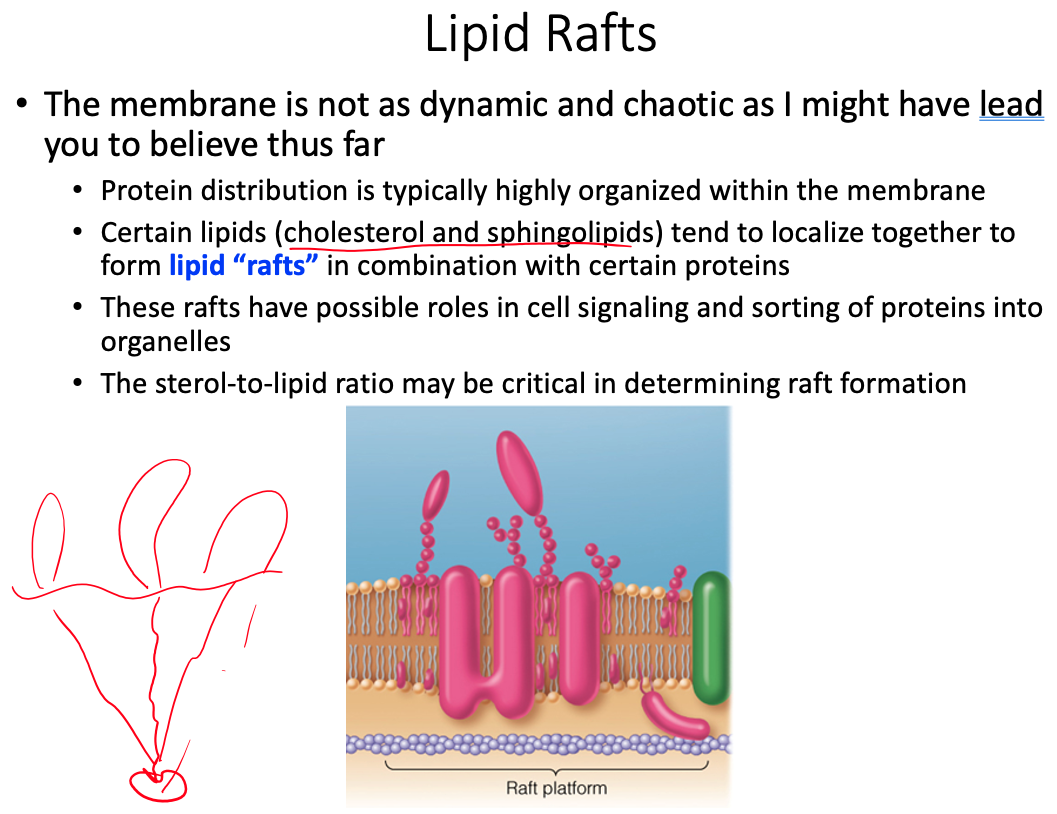
Cell membranes are described using the fluid mosaic model, yet they are also discussed as containing microdomains ( lipid rafts ). Define the fluid mosaic model. Does this model fully describe the reality of the cell membrane? Explain why or why not. Describe lipid rafts and suggest two possible functions.
Fluid = because individual phospholipids and proteins can move side-to-side within the layer
Mosaic = because of the pattern produced by the scattered protein molecules when the membrane is viewed from above
Doesn't fully describe because there is a lot more going on than what the model shows
- Current research suggests that membrane surface is even more crowded, and the organization is highly organized.
lipid rafts play significant roles in cell signaling and the sorting of proteins into specific organelles within a cell
Why is it important for determining the function of a membrane protein to know if it spans the bilayer or appears only on one face of the membrane? As part of your answer give three example proteins and their functions.
- Structure and location determines function, knowing if it crosses the entire membrane or is just embedded can tell us.
- If it spans the entire membrane it could be a transporter or pore for diffusion
- if its bound to one side only, it would be a peripheral protein
- Valinomycin
- GLUT-1
- sodium–potassium pump
The protein OmpF porin is embedded in the cell membrane and serves as a pore through the membrane. Structural analysis of the protein shown that the outside of the protein has a band of hydrophobic residues that is 27 Å tall and interacts directly with the nonpolar membrane (Penel et al Biochime 1998; 80; 543-51). The upper and lower bounds of the band are defined by phenylalanine residues. The following shape represents a side view of the protein that is 40 Å tall. Draw the lipid bilayer on the shape below and indicate the location of the phenylalanine residues.
Phenylalanine is non-polar. Residues inside the protein, parallel to the phospholipids
In your group recall at least three instances of membrane transport that we have discussed so far in central and lipid metabolism. Identify the molecules being transported.
- NAD
- FADH
- Carnitine Translocase
- Glucose entering cell via glucose transporters for glycolysis
In your group recall at least three instances of membrane transport that we have discussed so far in central and lipid metabolism. Identify the molecules being transported.
- NAD
- FADH
- Carnitine Translocase
- Glucose entering cell via glucose transporters for glycolysis
What replaces Q in our free energy equation.
https://39363.org/NOTES/WSU/2021/Spring/BIO4230/Calculators/MetabolicFreeEnergyChange/
How do we distinguish between the two sides of the membrane?
- Ending/Final Concentration is on top
- Starting/Initial Concentration is on bottom
- https://uh.edu/dtu/05-Transport-1-07.htm
- https://www.biology-pages.info/M/MembraneTransport.html
What happens to the equation if we decide to move the molecule in the opposite direction across the membrane? What would this do to the value of ? Justify your answer with an example.
- would change signs
Would moving a molecule of sodium across a membrane into a vesicle which has a high positive charge on its interior have a positive or negative free energy?
http://oregonstate.edu/instruct/bb451/451material/lectures/highlightsmembtrans.html
Positive , and unfavorable
- You don't want to move positive ions into something that is already positive on the inside
Would moving a molecule of magnesium instead of the sodium above be more or less favored? Why?
- Less favored because more positive charge
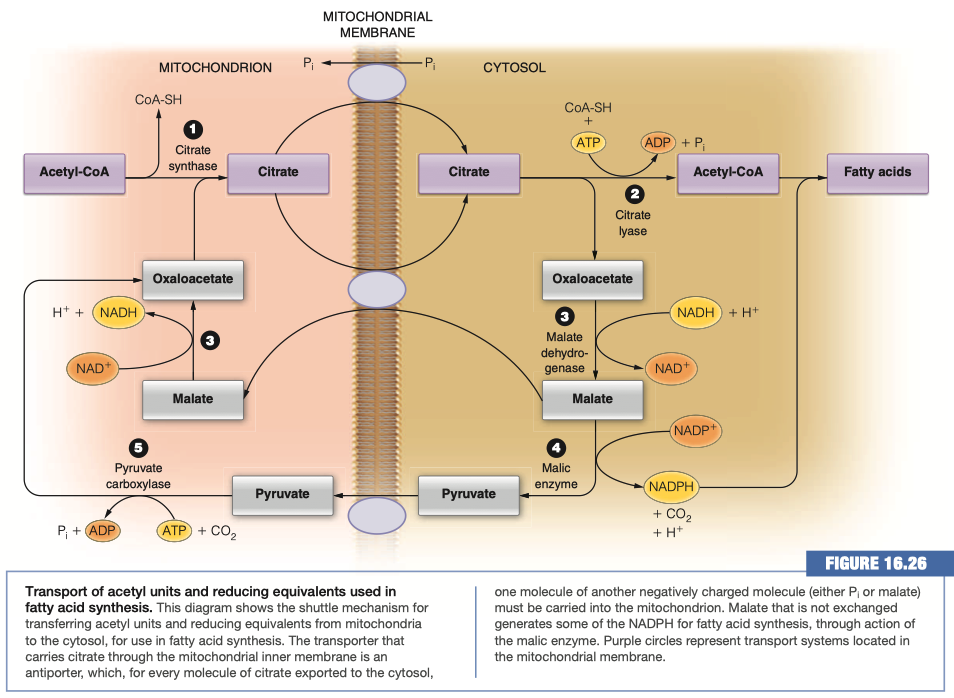
What molecule is actually moved across the membrane to generate Acetyl-CoA in the cytosol?
Citrate
There are two transport mechanisms for the above molecule indicated in the figure. For each determine whether the transport is symport or antiport and which molecules are moving down their concentration gradient and which up.
Citrate/Malate Antiporter
- Citrate is moving down its concentration gradient
- Malate is moving up its concentration gradient
Citrate/Phosphate Antiporter
- Citrate is moving down its concentration gradient
- Phosphate is moving up its concentration gradient
What is the adenine nucleotide translocase?
- Transports ADP into matrix
- Transports ATP out of the matrix
Does it work as an antiporter or symporter?
- Antiporter
Thermodynamically, why can this transport mechanism work?
- Proton gradient favors highly negative ATP to be transported toward the positive intermembrane space
- Net decrease in negativity allows us to pull ADP into the matrix
Why does this transport make logical/evolutionary sense based on the needs of the cell?
- we want to get ATP out of the mitochondria to be used by the cell
- we want to get ADP into the mitochondria to regenerate ATP
We are worried about ATP concentration
- This transport mechanism moves ATP outside of the matrix into the cytosol
- We need ADP in order to do that
- Le Chatelier's principle
The cell needs ATP for energy
- We need to be able to get ATP outside of the matrix and into the cytosol
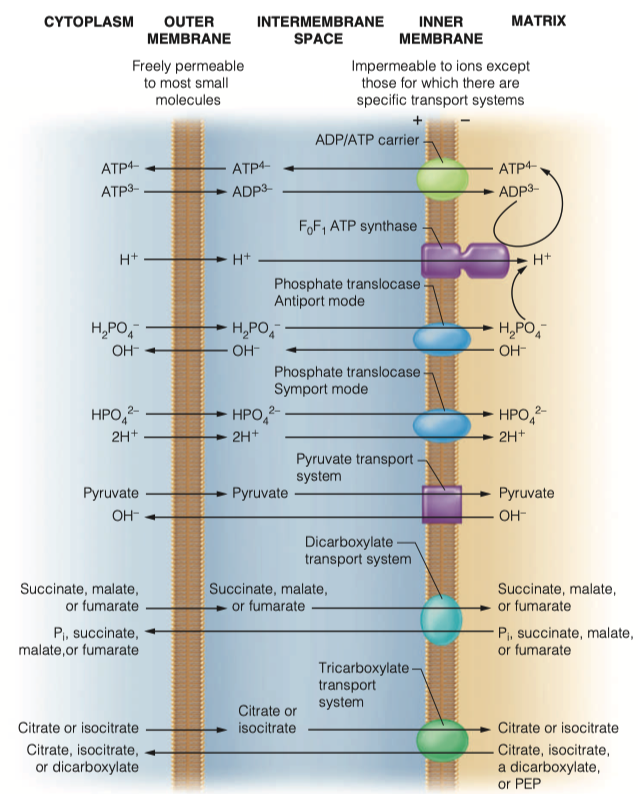
How does the mitochondria obtain its phosphate groups to generate ATP?
- phosphate translocase
- Both sym and antiporter
Transport of those phosphate groups can be symport or antiport. What molecules are moving in which directions in this transport
As an antiporter
- Transports dihydrogen phosphate into the matrix along with two protons
- Hydroxide leaves the mitochondria
As symporter
- transports monohydrogen phosphate into matrix with two protons
Both modes of transport maintain electrical neutrality and are driven by the proton concentration difference component of the proton motive force generated by electron transport
For the above phosphate transporters, thermodynamically, why can these transport mechanisms work?
Both are driven by proton concentration difference component of proton motive force generated by electron transport
maintaining electric neutrality
Antiporter:
- OH is moving down its gradient
- is also moving down its gradient
Symporter:
- is moving down its concentration gradient
- is moving down concentration gradient
For the above phosphate transporters, why do these transports make logical/ evolutionary sense based on the needs of the cell?
couples ADP and Pi inside and drives ATP outside
- In order to keep producing ATP inside the matrix
Symporter:
You need hydrogen ions inside the matrix so you can pump them back out
- This helps generate energy in moving FADH2
Antiporter:
- movement of helps regulate pH in the cytosol and in the matrix
What electron carrier is generated during glycolysis?
NADH
Do you suppose the above molecule can easily enter the mitochondria to generate ATP?
- No , it is too large
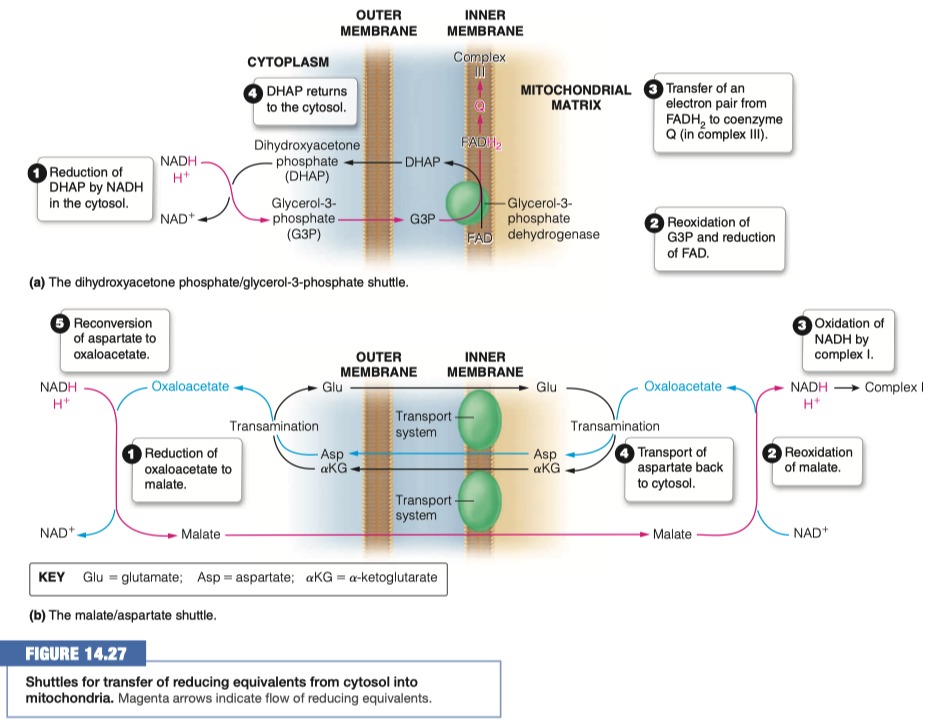
Identify two strategies that are used to transport the electrons into the mitochondria
- dihydroxyacetone phosphate - glycerol-3-phosphate shuttle
- malate-aspartate shuttle
In the malate/aspartate shuttle what molecule is shuttled into the mitochondria?
Malate
What happens to that molecule and what is generated?
- Reoxidized to oxaloacetate
- NADH and H+ is generated
What other transport mechanism is this similar to? How is it different?
Fatty acid synthesis
Glycolysis
- moving NADH inside
Gluconeogenesis
- moves malate out, in order to generate NADH
In the dihydroxyacetone phosphate what molecule is shuttled into the mitochondria?
Glycerol-3-Phosphate (G3P)
What then happens to the above molecule? How many ATP are eventually made?
- G3P is reoxidized and FAD is reduced to FADH2
- We are turning things into FADH2 this time instead of NADH
- FADH2 transfers electrons to coenzyme Q in complex III
- It makes FADH2 (1.5 ATP ) instead of NADH (2.5 ATP)
- 1.5 Moles
Why would a cell transfer the electrons to FADH2 instead of NADH at a loss of 1ATP?
We already have a high concentration of NADH
If we have lots of NADH inside the cell already, we are not going to be able to turn malate into oxaloacetate, generating another NADH
- This is kind of a shunt that we can use to turn it into FADH2 even at a loss of 1 ATP
High concentration of NADH in mitochondrial matrix occurs from Glycolysis and Anaerobic Respiration
Glycolysis in the cytosol is pumping NADH into the mitochondrial matrix via the malate/aspartate shuttle
Anytime we are in high stress energy, we need lots and lots of ATP
We are going to be shuttling through glycolysis, through the Citric Acid Cycle, building NADH concentrations up
It can only move "so fast" through the electron transport chain
- So we are going to use dihydroxyacetone phosphate - glycerol-3-phosphate shuttle to give us different molecules to work through FADH2
Different complexes are going to facilitate to give not as much energy, but some energy
- And allow glycolysis to continue on, because we "freed up" NADH into NAD+
We will be building up molecules of FADH2 and it will still be slow, but we freed NAD+ so we can do more anaerobic glycolysis
dihydroxyacetone phosphate - glycerol-3-phosphate shuttle is mainly in the brain and in the skeletal muscle
- high areas of activity
06 - Membrane Strucutre and Function - Things To Know
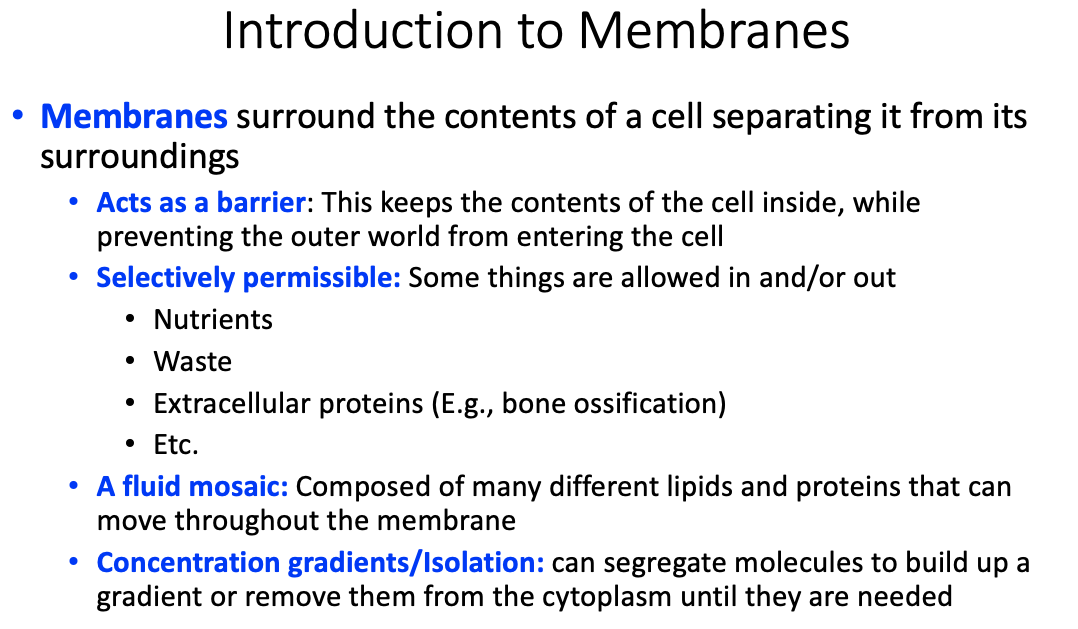
Properties and functions of membranes
- Acts as a barrier
- Selectively permissible
- A fluid mosaic
- Concentration gradients / isolation
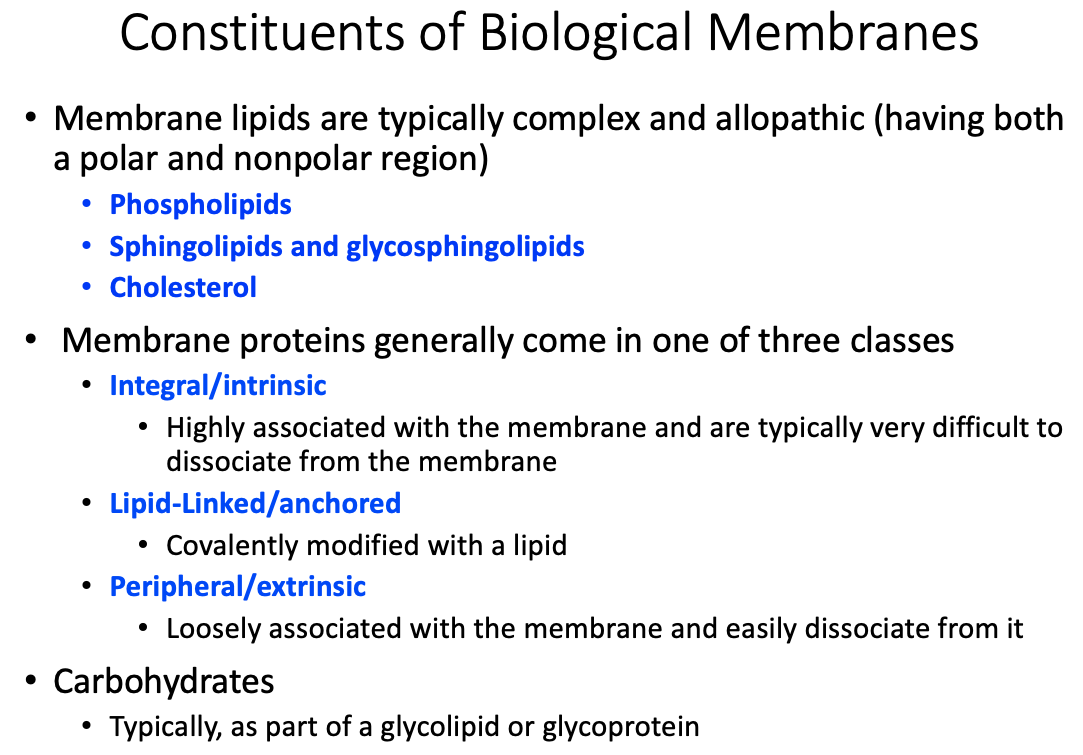
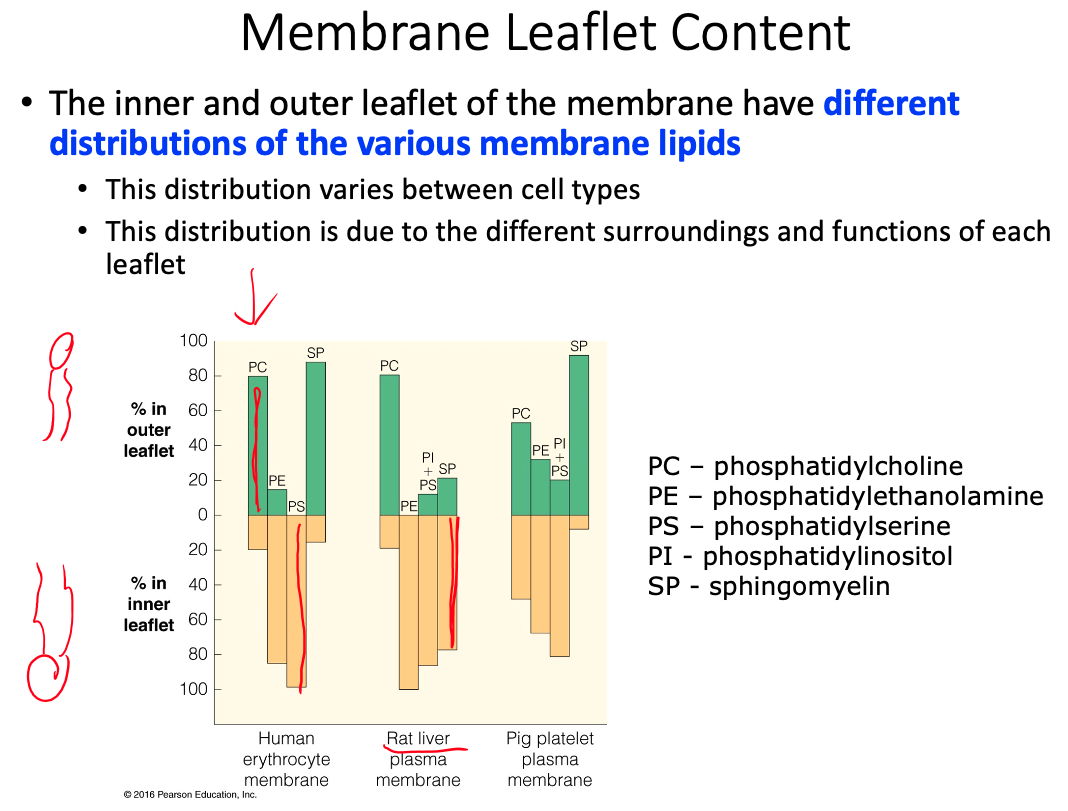
General membrane constituents and their organization
Fluid mosaic model and fluidity
Differential content of proteins in membranes
Similarities and differences between membrane protein types
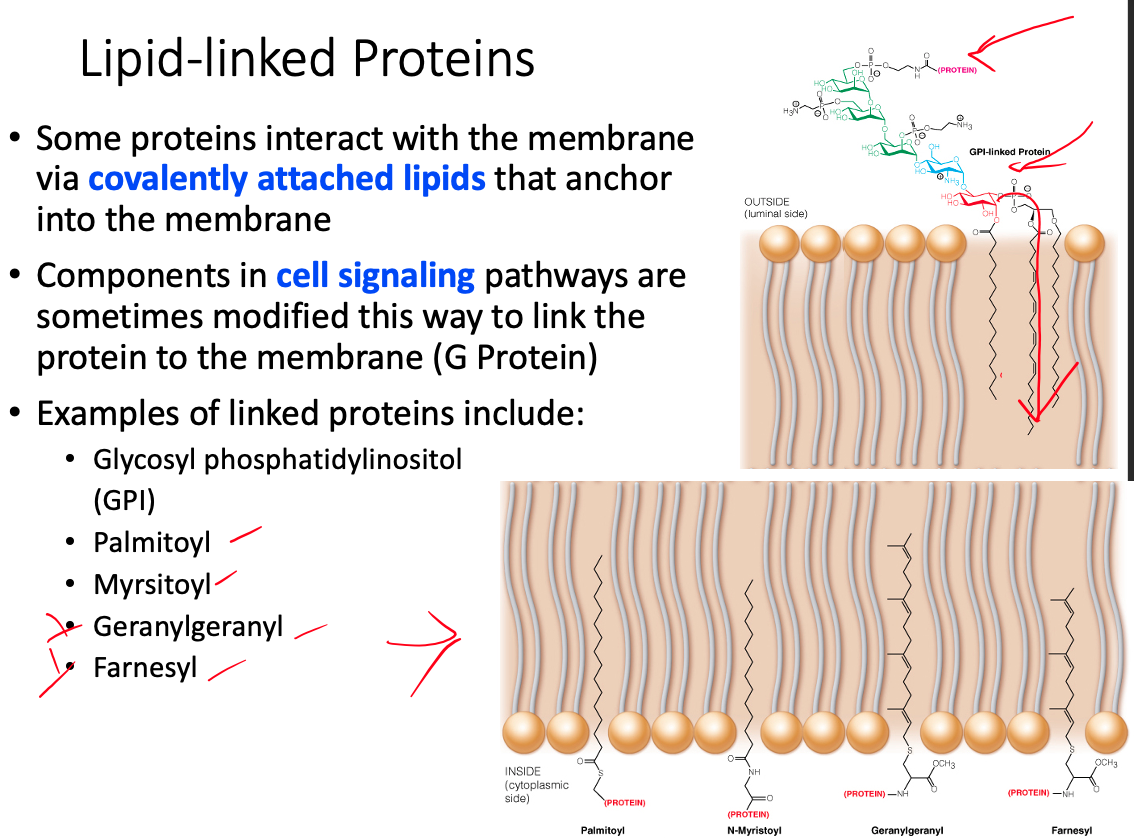
Differences between transmembrane, lipid-linked, and peripheral proteins
Transmembrane / Integral / Intrinsic
- Highly associated with the membrane and are typically very difficult to dissociate from the membrane
Lipid- Linked / Anchored
- Covalently modified with a lipid
Peripheral / Extrinsic
- Loosely associated with the membrane and easily dissociate from it