Membrane Structure and Function
The extracellular side of a transmembrane helix of an integral membrane protein does NOT contain __.
mostly hydrophobic amino acids
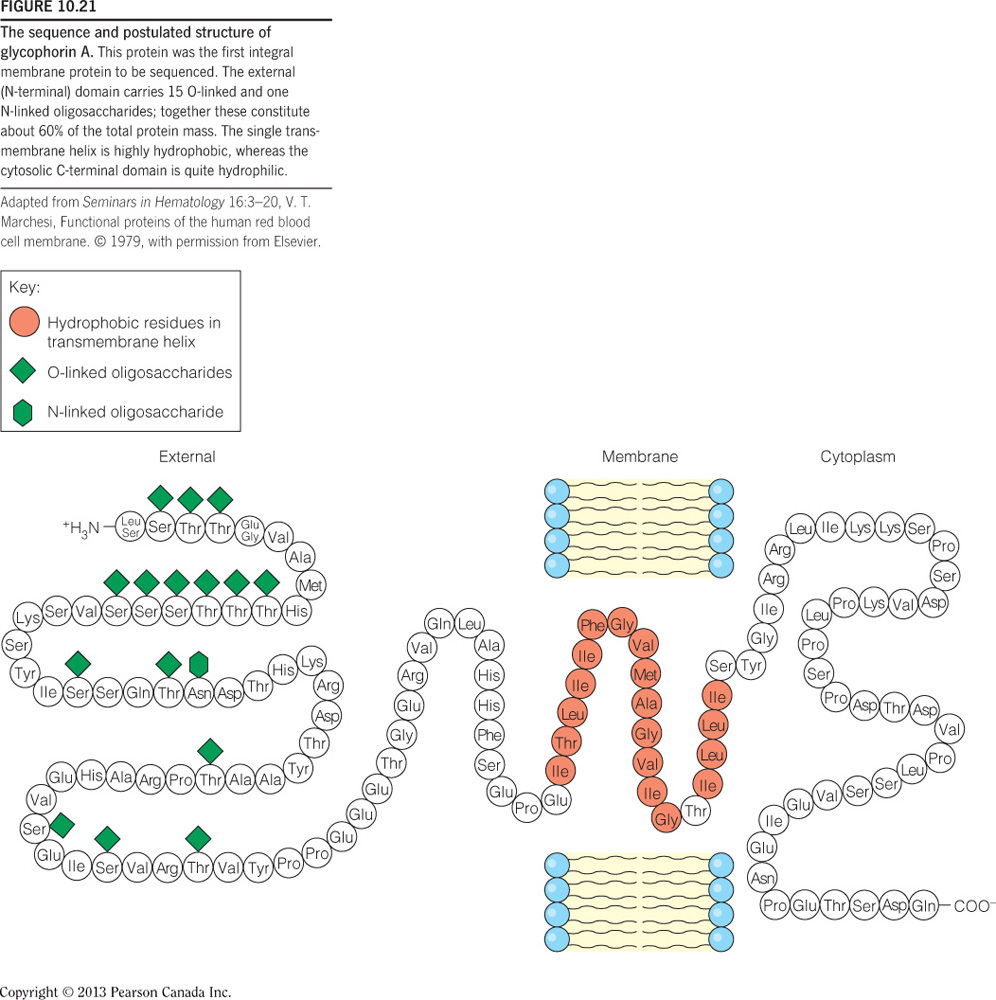
- As the figure below illustrates, the extracellular side of a transmembrane protein contains mostly hydrophilic, not hydrophobic, amino acids, as these are exposed to water and can make many favorable intermolecular interactions.
- This side of the membrane is also glycosylated, as mono/polysaccharides are important for cell-cell recognition, antigen-antibody interactions, etc.
- These saccharides are covalently bound to glycoproteins via the amino acids Ser, Thr, and Asn, and therefore the extracellular side of the transmembrane protein contains many of these specific residues.
Bacteria that thrive in cooler growth temperatures compensate by __ as compared to bacteria growing at warmer temperatures.
putting more unsaturated fatty acids into their membranes
- Bacteria growing at cold temperatures need a membrane composition that facilitates increased membrane fluidity, because the cooler temperatures decrease molecular motion.
- Therefore, bacteria thriving in cool temperatures have increased populations of unsaturated fatty acids on phospholipids because the cis double bonds cause kinks or separations within the membrane.
Consider the following structure:
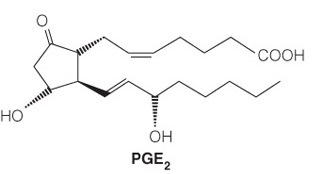
The molecule’s function is to __.
send out localized cellular signals
- The structure is a prostaglandin, which is a type of eicosanoid.
- These lipids are derived from a polyunsaturated fatty acid and have a distinctive five-membered ring that is sometimes oxidized.
- These are local signaling molecules (sending out localized cellular signals) that can control pain, inflammation, and local housekeeping. They are less polar than steroids and therefore less soluble in the bloodstream, so they do not stray far from the cell that synthesized them.
Consider the following structure:
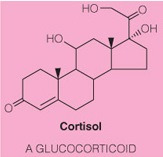
The molecule’s function is to __.
send out global cellular signals
- The structure is a steroid because it has a steroid nucleus and a higher level of oxidation than a sterol.
- These molecules are more hydrophilic than sterols and therefore travel throughout the bloodstream readily.
- They bind to receptor proteins and initiate signaling cascades.
- Thus it’s function is to send out global cellular signals.
- In contrast, eicosanoids are localized signaling molecules because they are less polar.




The active transport of chloride ions into the cytoplasm, from low to high concentration, sets up a(an) __.
electrochemical gradient
Movement of charged ions across a membrane, resulting in unequal concentrations, has a twofold effect:
- (1) the concentration difference sets up a concentration gradient, whereas if the chloride moved out of the cell (i.e., down its concentration gradient), it would release energy
- (2) the charge of the ion sets up membrane polarization, wherein the membrane is more negative on the side of the membrane with the highest chloride concentration.
Again, if chloride were to move out of the cell it would be moving toward the positive side of the membrane and this also releases energy.
The combined effect is the creation of an electrochemical gradient.
The term is derived as follows:
- electro for charge separation,
- chemical because a chemical/ion created this charge separation
- gradient due to the concentration difference between the sides of the membrane.
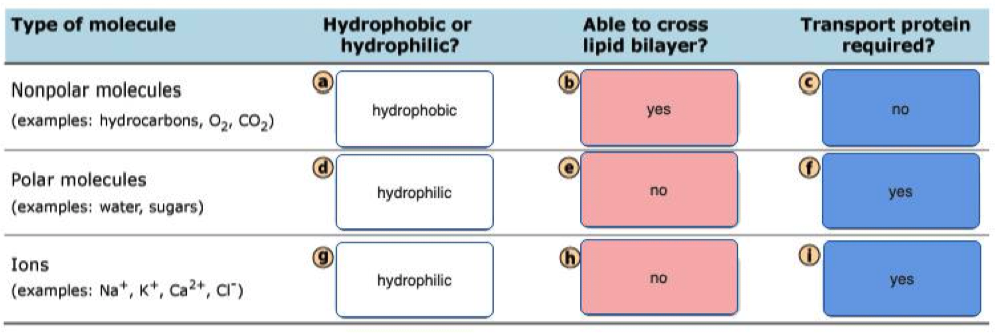
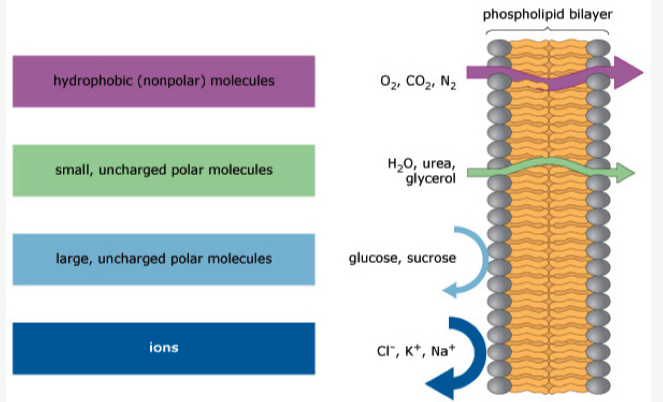
A lipid bilayer membrane without pores or transporters is freely permeable to which of the following?
- Lipid bilayers are permeable to small, uncharged, and nonpolar molecules.
- Of all the choices, only carbon dioxide () meets all three requirements.
- Na+ and K+ are small but they are charged, polar ions that require transporters.
- Glucose is uncharged but it is large and polar, so it also requires a transporter.
- Lys is a large, charged, and polar molecule that also requires a transporter at pH 7.
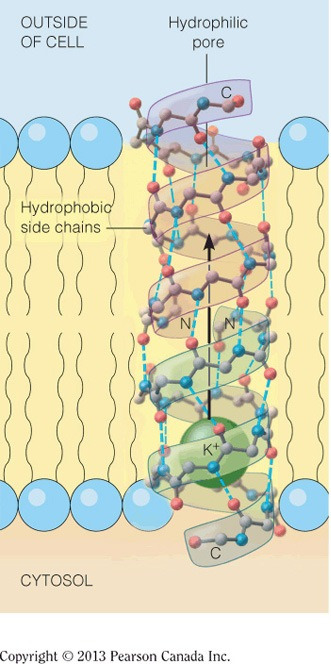
The inner core of a pore transport protein for potassium is most likely lined with __.
hydrophilic amino acids
- The inner core of the pore is responsible for binding potassium, which is a charged ion.
- The amino acids that will be attracted to this positive potassium ion through intermolecular interaction are the acidic and polar neutral amino acids, which belong to the hydrophilic group of amino acids.
- The surface of the pore protein will be coated in mostly hydrophobic amino acids, as these amino acids will have favorable intermolecular interactions with the hydrophobic lipids’ tails.
- The figure below shows a pore that transports potassium, illustrating the hydrophilic core and the hydrophobic surface.
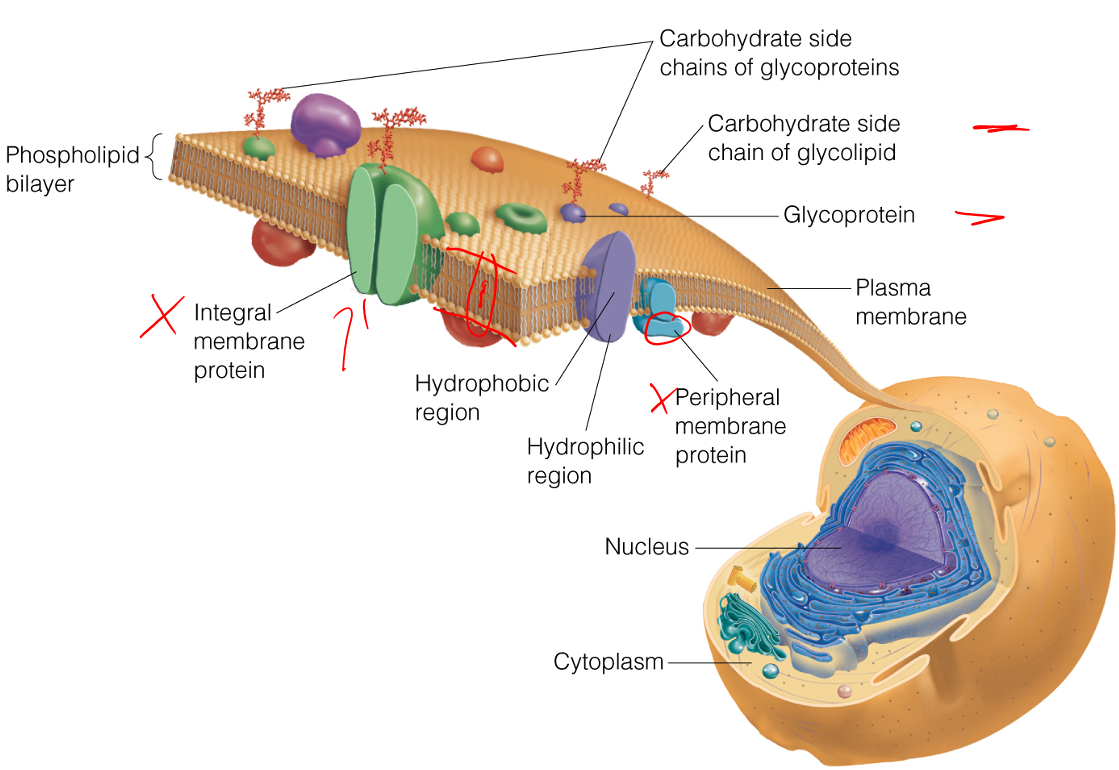
Define the terms peripheral membrane protein and integral membrane proteins.
Peripheral Membrane Proteins = exposed at only one membrane face or the other
- Held to the membrane by noncovalent interaction with lipid heads or integral membrane proteins
Integral Membrane Proteins = largely buried within the membrane but are usually exposed on both faces
- Frequently involved in transporting specific substances, or transducing chemical signals through the membrane
The relative orientation of polar and nonpolar amino acid side chains of integral membrane proteins is "inside-out" compared to the amino acid side chain orientation for globular water-soluble proteins. Explain why.
Membrane proteins are lipid soluble rather than water soluble
- We want the hydrophobic chains to be touching the lipid rather than pressed in
A typical globular protein is found in the cytosol, and because the cytosol is predominately water, what we typically find is:
- Any of the amino acid side chains that are hydrophilic are going to be going towards the water or towards the outside
- Any of the hydrophobic residues are going to be hiding on the inside of the protein and staying as far away from water as possible
The lipid bilayer we have nonpolar regions inside the polar heads.
- phospholipids, sphinolipids, ect
Because these proteins are embedded inside that region, they're actually in a nonpolar environment
Our hydrophilic amino acids are not going to like that nonpolar environment
So we're going flip everything inside out
- The hydrophobics are going to be on the outside protecting the hydrophilic which are now hiding on the inside
Information
- Integral membrane proteins often contain helical segments of appropriate length to span the lipid bilayer.
- In a protein that has a single segment that spans the membrane, the helix usually only contains hydrophobic residues and is called a single-span membrane protein.
- In transmembrane proteins with multiple segments that span the membrane, hydrophilic residues are often found in sequences of the helices.
Why are hydrophobic residues favored in single-span membrane proteins? Find an example of a single-span membrane protein in your book.
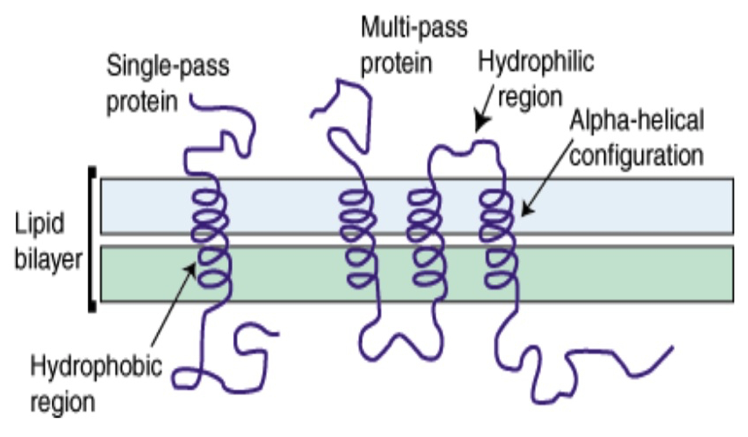
Hydrophobic residues are favored because the majority of the
- when we have a single span membrane protein its going to be using
Bacteriorhodopsin - Figure 10.14
Majority of exterior of protein will be interacting with hydrophobic region
When we have a single-span membrane protein, its typically going to have a helix with side chains sticking out
The majority of the single-span membrane protein is going to be in the hydrophobic region
Propose a structural orientation and a function for multiple hydrophilic residues in the helices of multi-span protein. Find an example of a multi-span membrane protein in your book
- We have all of these multispan proteins, some of which are involved in protein transporrt
- The helices end up forming a circle/pore with hydrophilic residues facing inward because they want to be away from the lipid hydrophobic region
- For example, sodium is going to come down and interact with those hydrophilic residues in order for the transport protein to push it through from one side of the membrane to the other
- Example = Retinal
From your understanding of protein structure and membrane structure, explain the fact that of the approximately 51,000 protein structures (3D) published and listed int he Protein Data Bank (in 2008) only about 930 of theses structures are transmembrane proteins. NOTE: 3D structures are typically obtained from X-ray crystallography data.
X-Ray Crystallography requires a lot of "salt" in order to crystallize the protein
In a nonpolar solvent, where we would normally expect to find our membrane proteins, it is going to be difficult to get a "salt" in there to make a crystal
So if we take the integral membrane protein and put it into a hydrophilic solution, water for example, and then crystallize it, the general structure is going to be completely changed
- Because all of the hydrophobic residues are going to want to hide on the inside
- So the structure observed will not be the "true" structure
Propose a reasonable mode of action by which these antihistamines exert their activity
- Anti-histimines could be a non-competitive inhibitor that makes a conformational change and prevents histimine from binding
- Claratin could latch onto histimine and prevent it from binding to H1 receptors
- Competitive antagonist
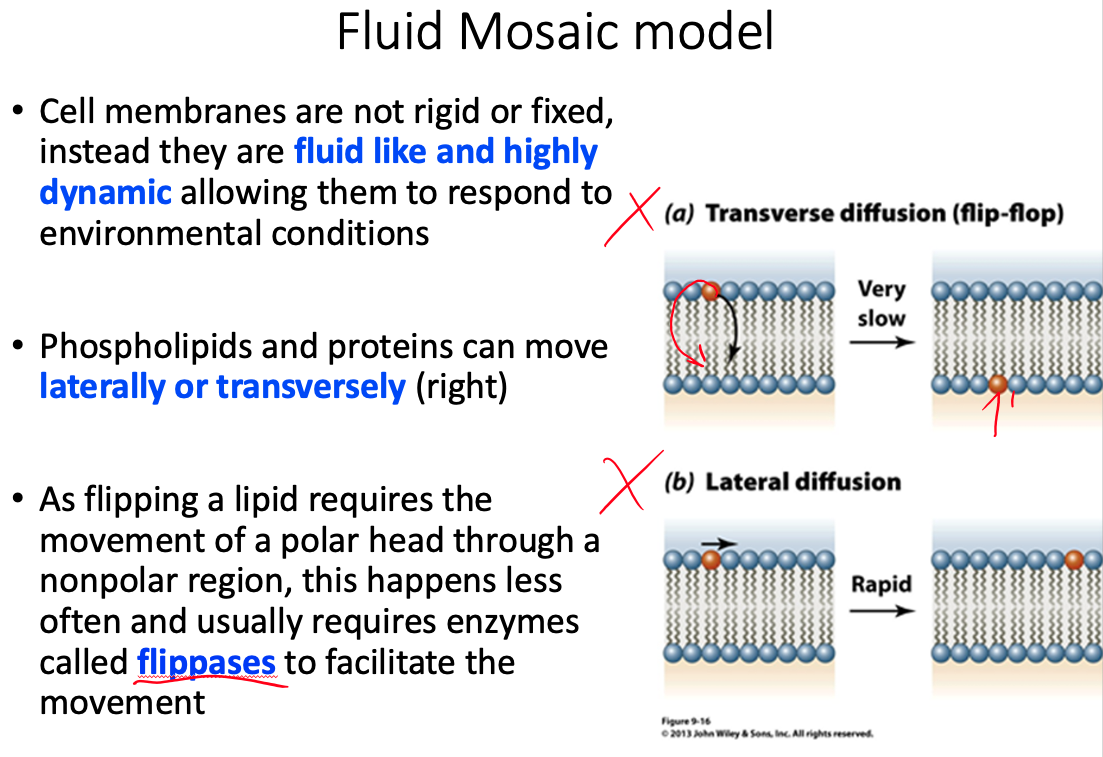
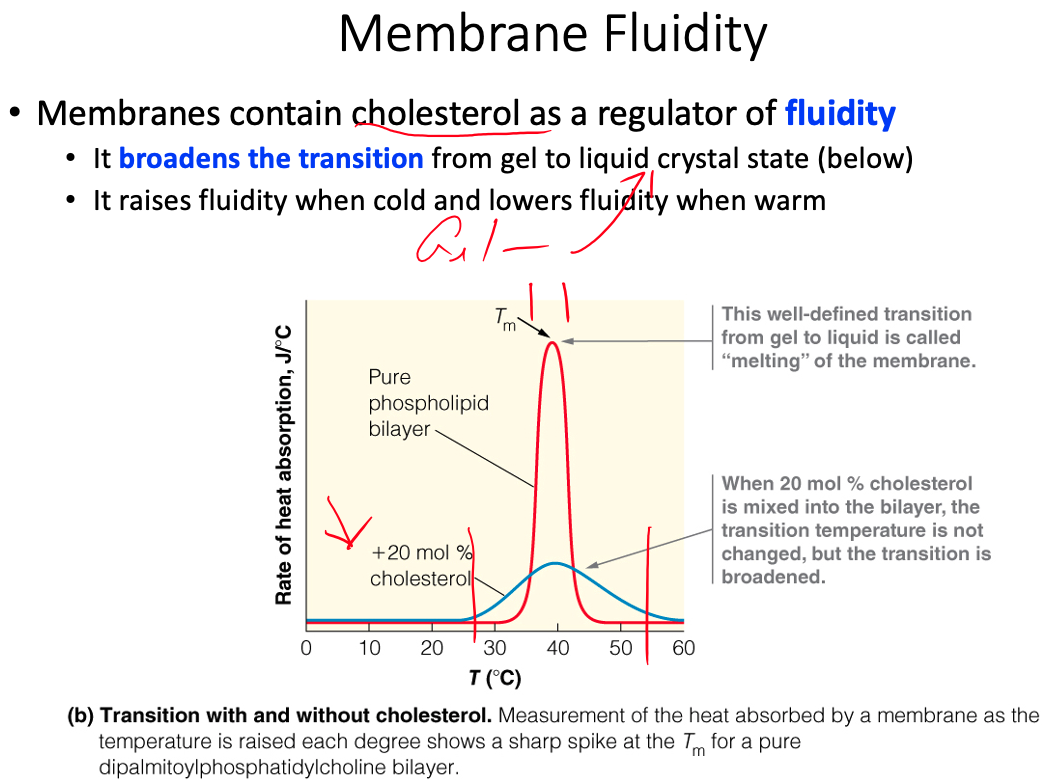
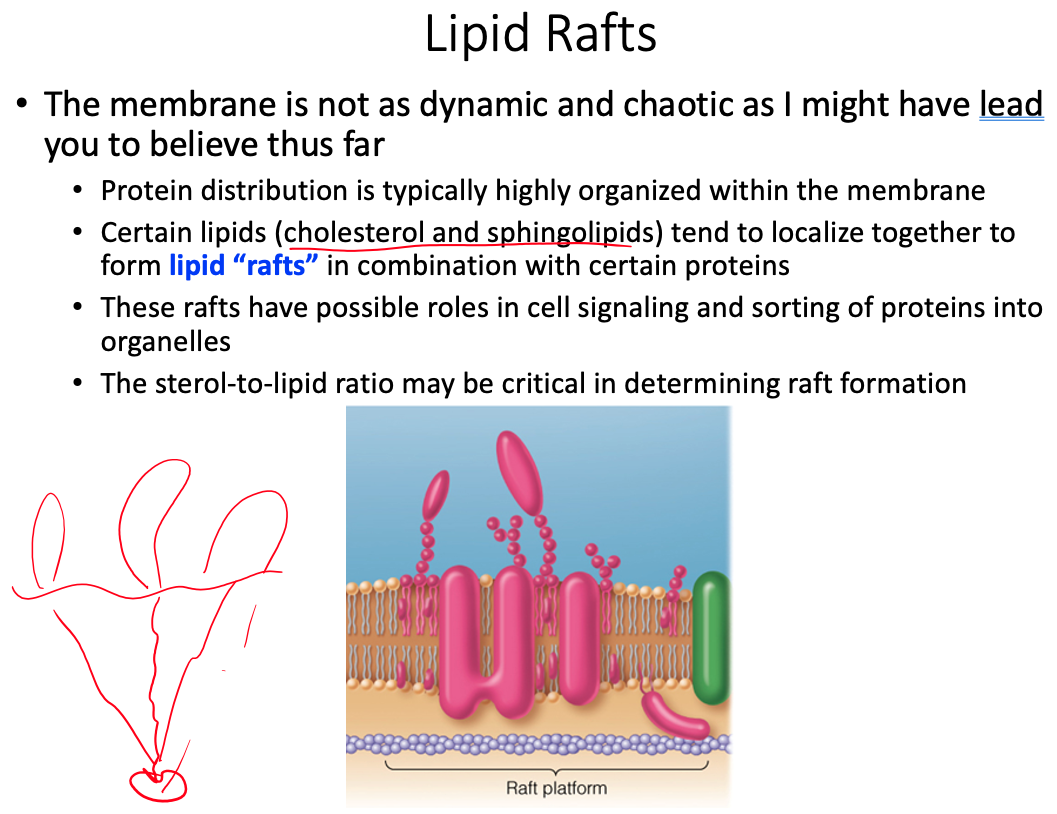
Cell membranes are described using the fluid mosaic model, yet they are also discussed as containing microdomains ( lipid rafts ). Define the fluid mosaic model. Does this model fully describe the reality of the cell membrane? Explain why or why not. Describe lipid rafts and suggest two possible functions.
Fluid = because individual phospholipids and proteins can move side-to-side within the layer
Mosaic = because of the pattern produced by the scattered protein molecules when the membrane is viewed from above
Doesn't fully describe because there is a lot more going on than what the model shows
- Current research suggests that membrane surface is even more crowded, and the organization is highly organized.
lipid rafts play significant roles in cell signaling and the sorting of proteins into specific organelles within a cell
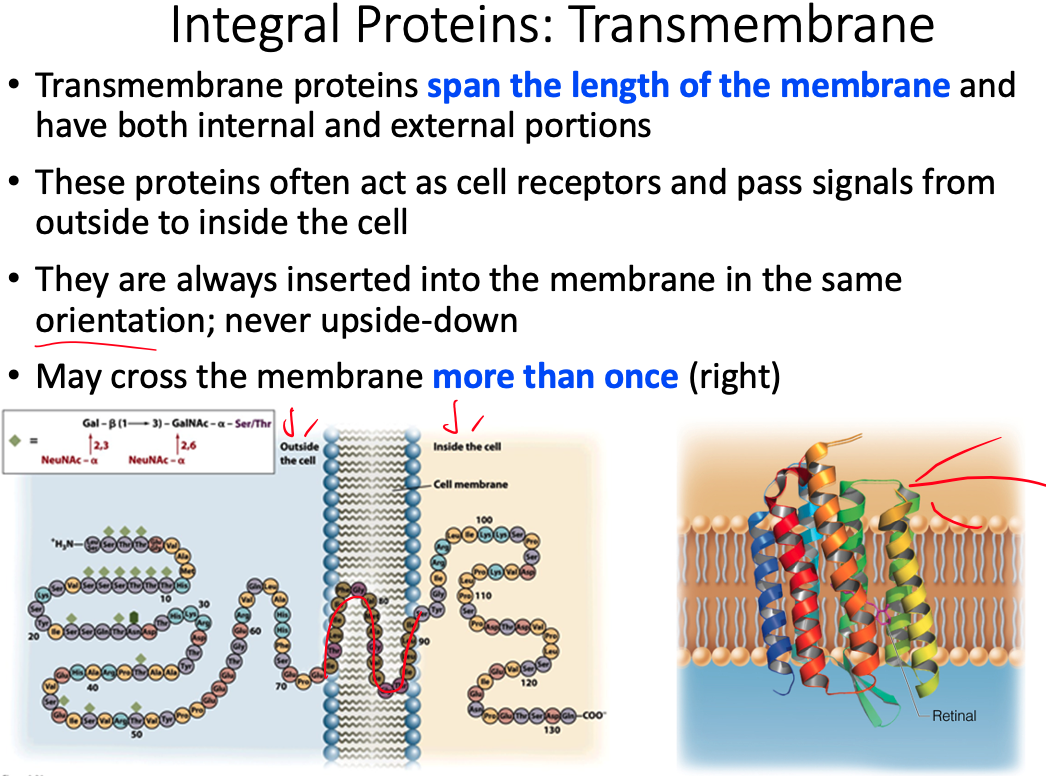
Why is it important for determining the function of a membrane protein to know if it spans the bilayer or appears only on one face of the membrane? As part of your answer give three example proteins and their functions.
- Structure and location determines function, knowing if it crosses the entire membrane or is just embedded can tell us.
- If it spans the entire membrane it could be a transporter or pore for diffusion
- if its bound to one side only, it would be a peripheral protein
- Valinomycin
- GLUT-1
- sodium–potassium pump
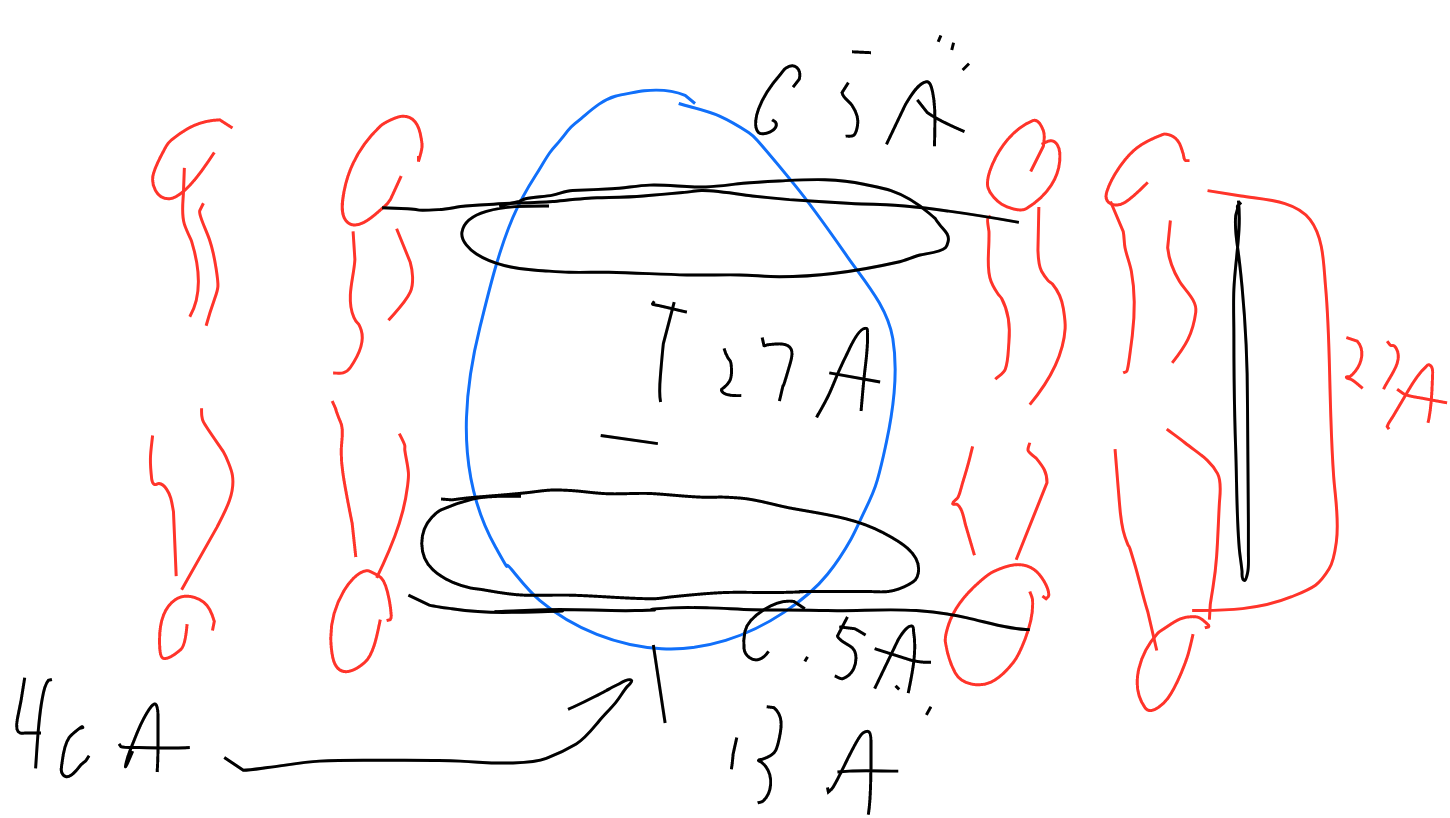
The protein OmpF porin is embedded in the cell membrane and serves as a pore through the membrane. Structural analysis of the protein shown that the outside of the protein has a band of hydrophobic residues that is 27 Å tall and interacts directly with the nonpolar membrane (Penel et al Biochime 1998; 80; 543-51). The upper and lower bounds of the band are defined by phenylalanine residues. The following shape represents a side view of the protein that is 40 Å tall. Draw the lipid bilayer on the shape below and indicate the location of the phenylalanine residues.
Phenylalanine is non-polar. Residues inside the protein, parallel to the phospholipids
In your group recall at least three instances of membrane transport that we have discussed so far in central and lipid metabolism. Identify the molecules being transported.
- NAD
- FADH
- Carnitine Translocase
- Glucose entering cell via glucose transporters for glycolysis
In your group recall at least three instances of membrane transport that we have discussed so far in central and lipid metabolism. Identify the molecules being transported.
- NAD
- FADH
- Carnitine Translocase
- Glucose entering cell via glucose transporters for glycolysis
What replaces Q in our free energy equation.
https://39363.org/NOTES/WSU/2021/Spring/BIO4230/Calculators/MetabolicFreeEnergyChange/
How do we distinguish between the two sides of the membrane?
- Ending/Final Concentration is on top
- Starting/Initial Concentration is on bottom
- https://uh.edu/dtu/05-Transport-1-07.htm
- https://www.biology-pages.info/M/MembraneTransport.html
What happens to the equation if we decide to move the molecule in the opposite direction across the membrane? What would this do to the value of ? Justify your answer with an example.
- would change signs
Would moving a molecule of sodium across a membrane into a vesicle which has a high positive charge on its interior have a positive or negative free energy?
http://oregonstate.edu/instruct/bb451/451material/lectures/highlightsmembtrans.html
Positive , and unfavorable
- You don't want to move positive ions into something that is already positive on the inside
Would moving a molecule of magnesium instead of the sodium above be more or less favored? Why?
- Less favored because more positive charge
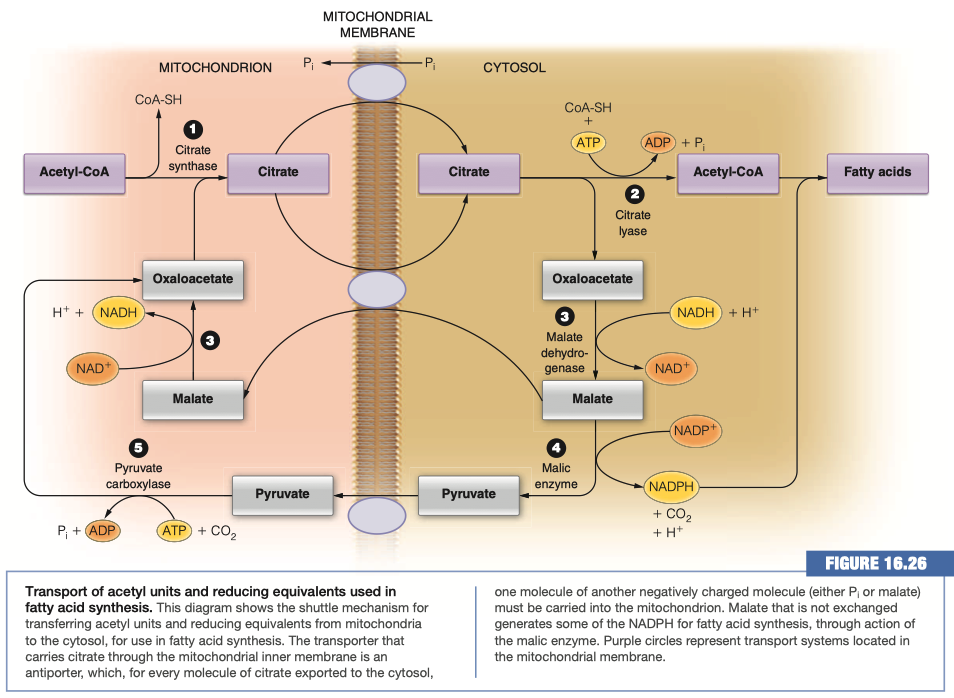
What molecule is actually moved across the membrane to generate Acetyl-CoA in the cytosol?
Citrate
There are two transport mechanisms for the above molecule indicated in the figure. For each determine whether the transport is symport or antiport and which molecules are moving down their concentration gradient and which up.
Citrate/Malate Antiporter
- Citrate is moving down its concentration gradient
- Malate is moving up its concentration gradient
Citrate/Phosphate Antiporter
- Citrate is moving down its concentration gradient
- Phosphate is moving up its concentration gradient
What is the adenine nucleotide translocase?
- Transports ADP into matrix
- Transports ATP out of the matrix
Does it work as an antiporter or symporter?
- Antiporter
Thermodynamically, why can this transport mechanism work?
- Proton gradient favors highly negative ATP to be transported toward the positive intermembrane space
- Net decrease in negativity allows us to pull ADP into the matrix
Why does this transport make logical/evolutionary sense based on the needs of the cell?
we want to get ATP out of the mitochondria to be used by the cell
we want to get ADP into the mitochondria to regenerate ATP
We are worried about ATP concentration
- This transport mechanism moves ATP outside of the matrix into the cytosol
- We need ADP in order to do that
- Le Chatelier's principle
The cell needs ATP for energy
- We need to be able to get ATP outside of the matrix and into the cytosol
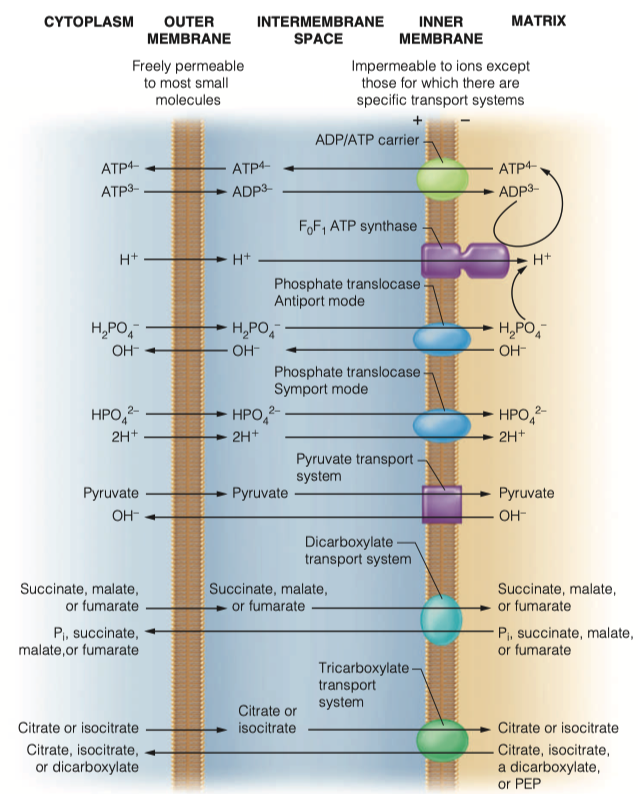
How does the mitochondria obtain its phosphate groups to generate ATP?
- phosphate translocase
- Both sym and antiporter
Transport of those phosphate groups can be symport or antiport. What molecules are moving in which directions in this transport
As an antiporter
- Transports dihydrogen phosphate into the matrix along with two protons
- Hydroxide leaves the mitochondria
As symporter
- transports monohydrogen phosphate into matrix with two protons
Both modes of transport maintain electrical neutrality and are driven by the proton concentration difference component of the proton motive force generated by electron transport
For the above phosphate transporters, thermodynamically, why can these transport mechanisms work?
Both are driven by proton concentration difference component of proton motive force generated by electron transport
maintaining electric neutrality
Antiporter:
- OH is moving down its gradient
- is also moving down its gradient
Symporter:
- is moving down its concentration gradient
- is moving down concentration gradient
For the above phosphate transporters, why do these transports make logical/ evolutionary sense based on the needs of the cell?
couples ADP and Pi inside and drives ATP outside
- In order to keep producing ATP inside the matrix
Symporter:
You need hydrogen ions inside the matrix so you can pump them back out
- This helps generate energy in moving FADH2
Antiporter:
- movement of helps regulate pH in the cytosol and in the matrix
What electron carrier is generated during glycolysis?
NADH
Do you suppose the above molecule can easily enter the mitochondria to generate ATP?
- No , it is too large
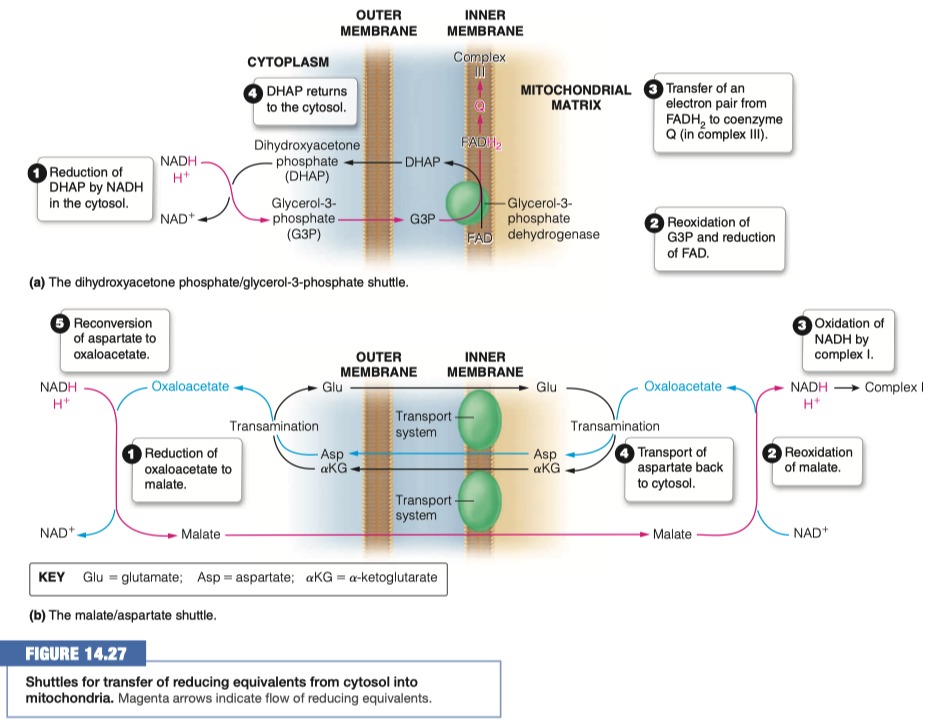
Identify two strategies that are used to transport the electrons into the mitochondria
- dihydroxyacetone phosphate - glycerol-3-phosphate shuttle
- malate-aspartate shuttle
In the malate/aspartate shuttle what molecule is shuttled into the mitochondria?
Malate
What happens to that molecule and what is generated?
- Reoxidized to oxaloacetate
- NADH and H+ is generated
What other transport mechanism is this similar to? How is it different?
Fatty acid synthesis
Glycolysis
- moving NADH inside
Gluconeogenesis
- moves malate out, in order to generate NADH
In the dihydroxyacetone phosphate what molecule is shuttled into the mitochondria?
Glycerol-3-Phosphate (G3P)
What then happens to the above molecule? How many ATP are eventually made?
- G3P is reoxidized and FAD is reduced to FADH2
- We are turning things into FADH2 this time instead of NADH
- FADH2 transfers electrons to coenzyme Q in complex III
- It makes FADH2 (1.5 ATP ) instead of NADH (2.5 ATP)
- 1.5 Moles
Why would a cell transfer the electrons to FADH2 instead of NADH at a loss of 1ATP?
We already have a high concentration of NADH
If we have lots of NADH inside the cell already, we are not going to be able to turn malate into oxaloacetate, generating another NADH
- This is kind of a shunt that we can use to turn it into FADH2 even at a loss of 1 ATP
High concentration of NADH in mitochondrial matrix occurs from Glycolysis and Anaerobic Respiration
Glycolysis in the cytosol is pumping NADH into the mitochondrial matrix via the malate/aspartate shuttle
Anytime we are in high stress energy, we need lots and lots of ATP
We are going to be shuttling through glycolysis, through the Citric Acid Cycle, building NADH concentrations up
It can only move "so fast" through the electron transport chain
- So we are going to use dihydroxyacetone phosphate - glycerol-3-phosphate shuttle to give us different molecules to work through FADH2
Different complexes are going to facilitate to give not as much energy, but some energy
- And allow glycolysis to continue on, because we "freed up" NADH into NAD+
We will be building up molecules of FADH2 and it will still be slow, but we freed NAD+ so we can do more anaerobic glycolysis
dihydroxyacetone phosphate - glycerol-3-phosphate shuttle is mainly in the brain and in the skeletal muscle
- high areas of activity
Things To Know
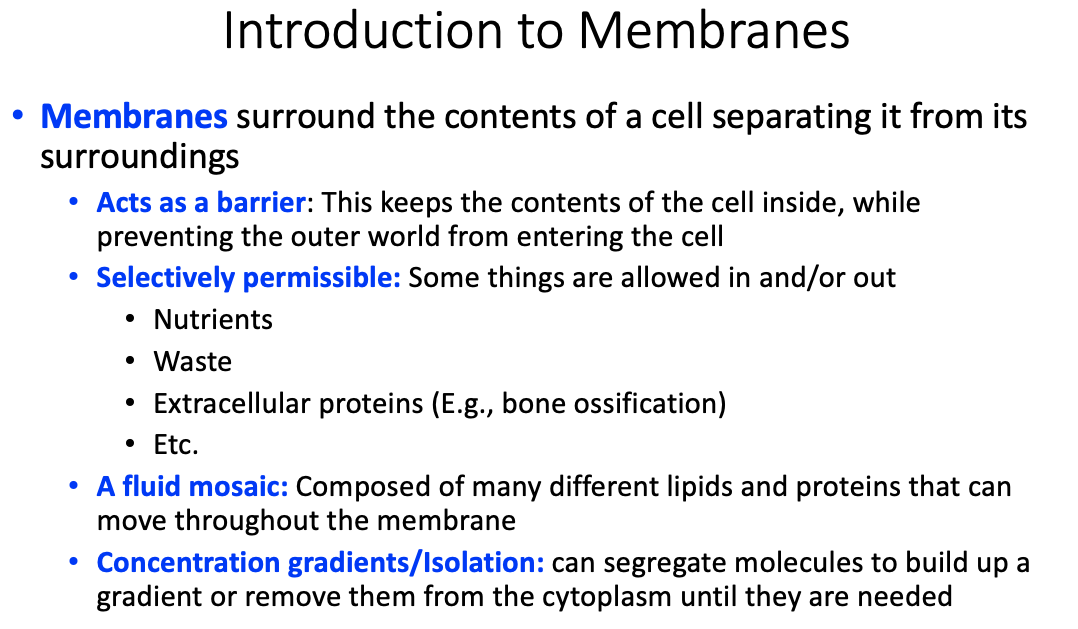
Properties and functions of membranes
- Acts as a barrier
- Selectively permissible
- A fluid mosaic
- Concentration gradients / isolation
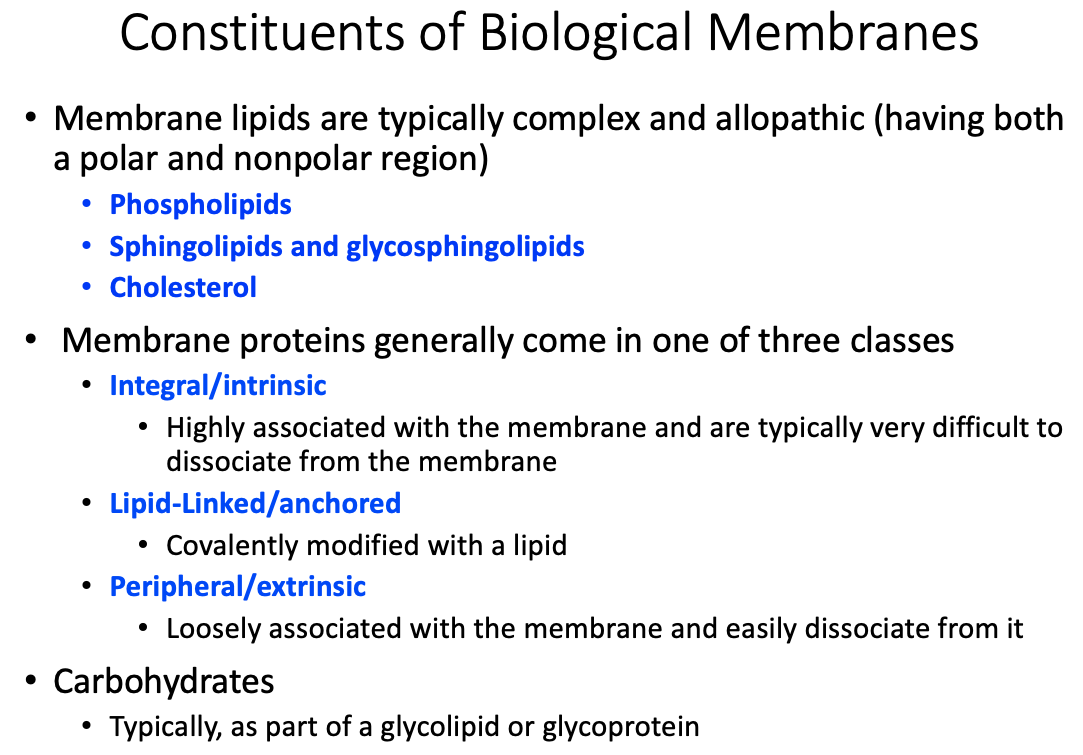
General membrane constituents and their organization
Fluid mosaic model and fluidity
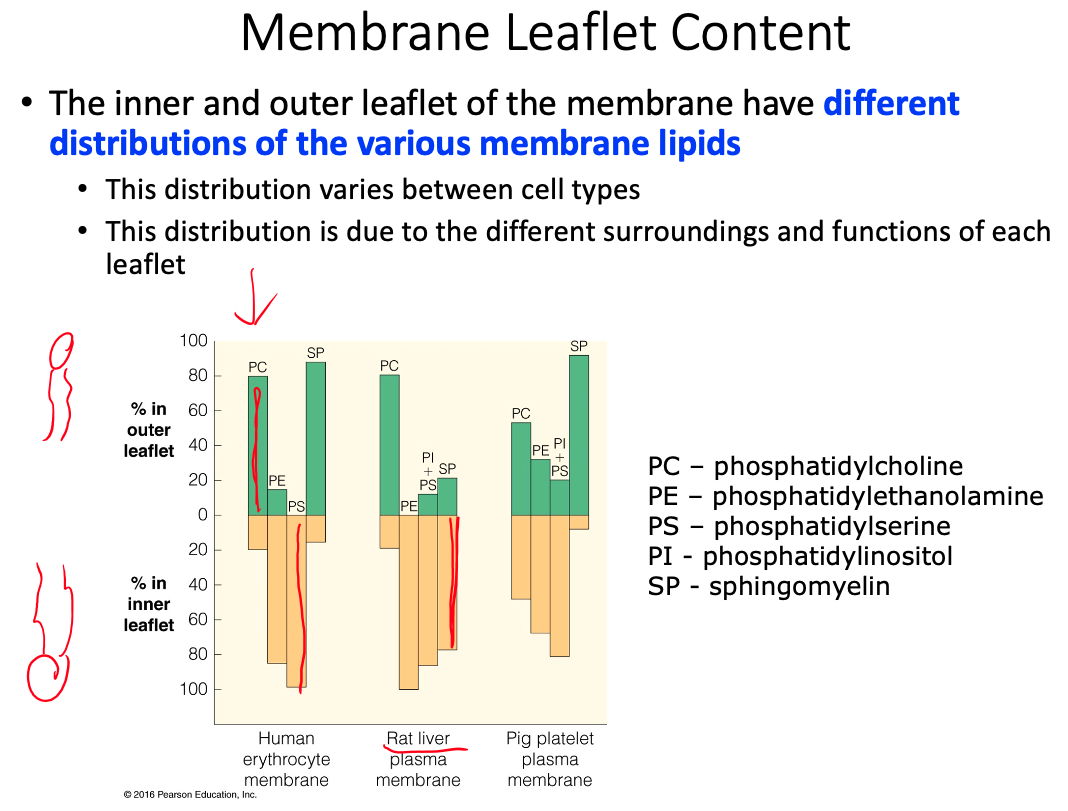
Differential content of proteins in membranes
Similarities and differences between membrane protein types
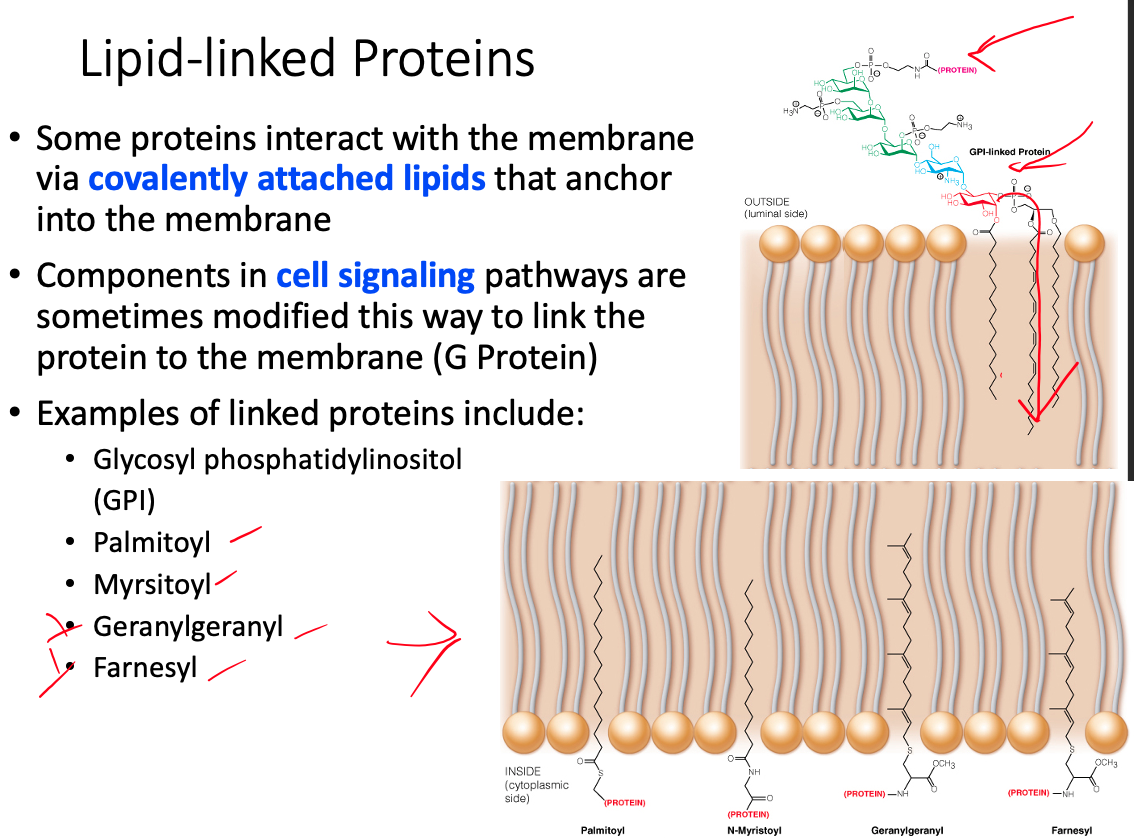
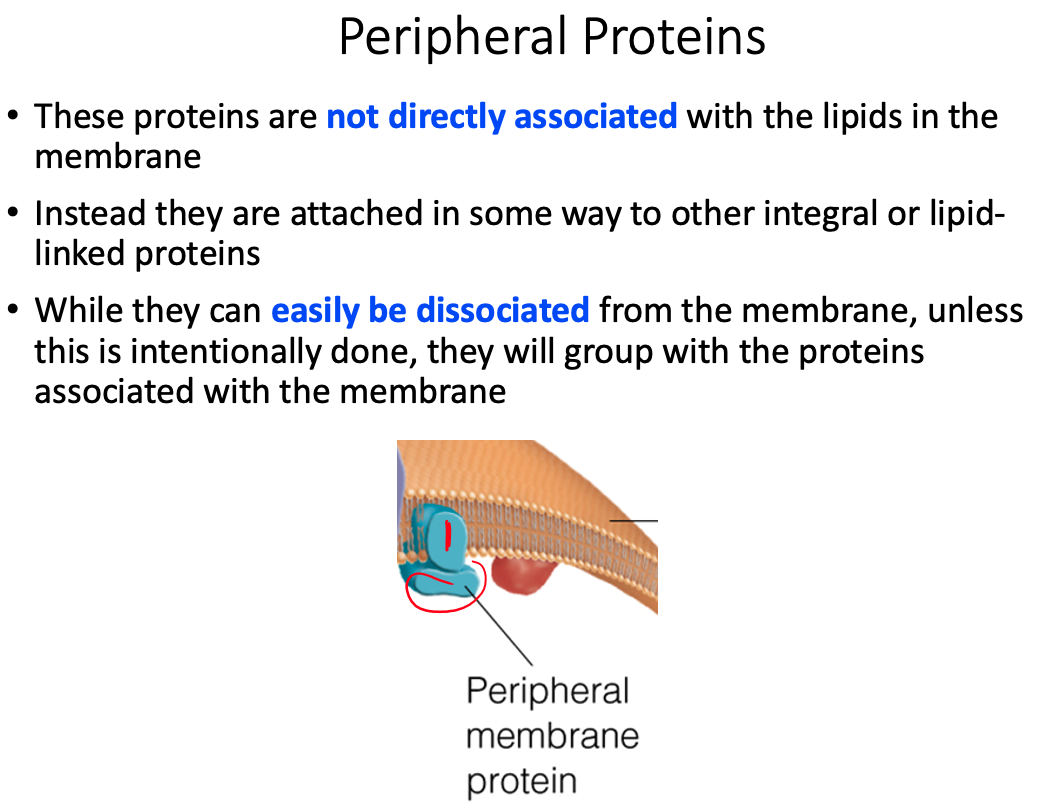
Differences between transmembrane, lipid-linked, and peripheral proteins
Transmembrane / Integral / Intrinsic
- Highly associated with the membrane and are typically very difficult to dissociate from the membrane
Lipid- Linked / Anchored
- Covalently modified with a lipid
Peripheral / Extrinsic
- Loosely associated with the membrane and easily dissociate from it