Lipid Transport


What are the three main sources of available fats (those that can be used in metabolism or serve as components of our membranes) for your body?
- diet
- synthesis
- storage from adipocytes
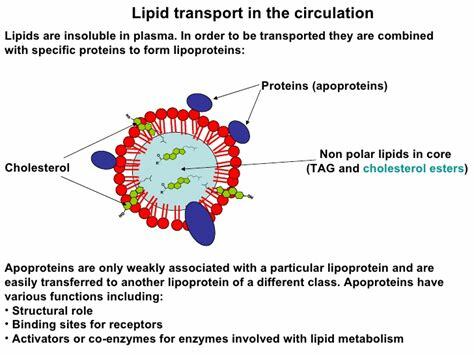
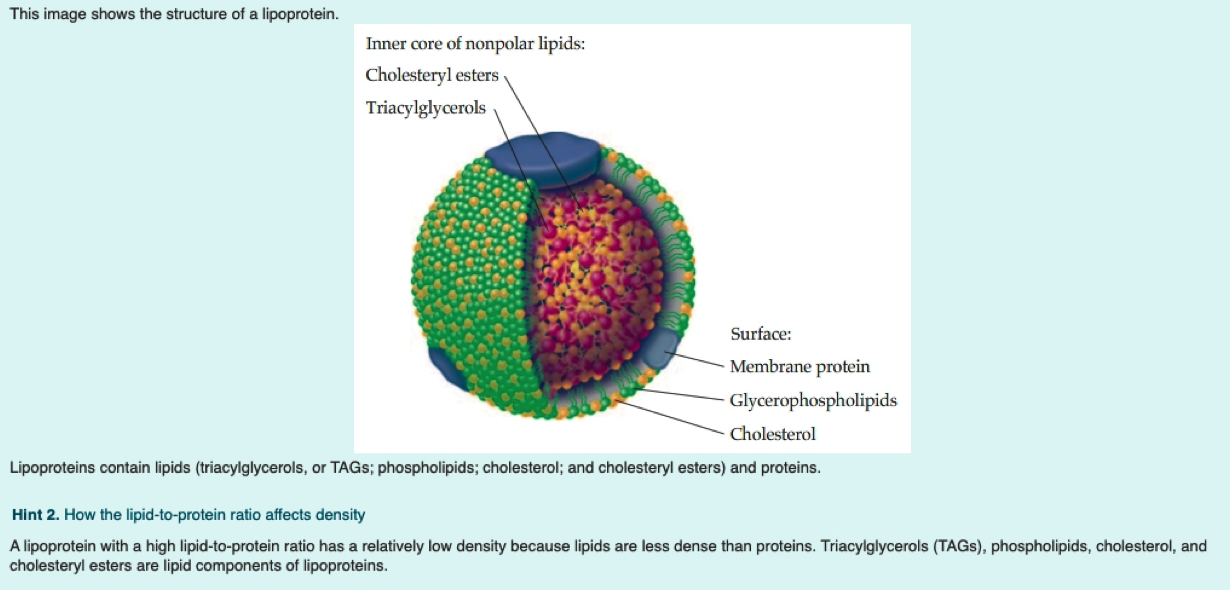
What is a lipoprotein (be general)?
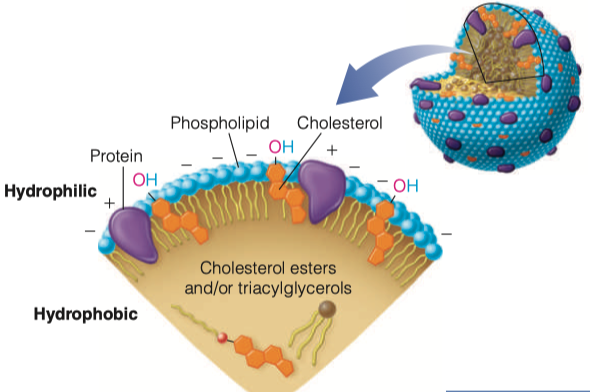
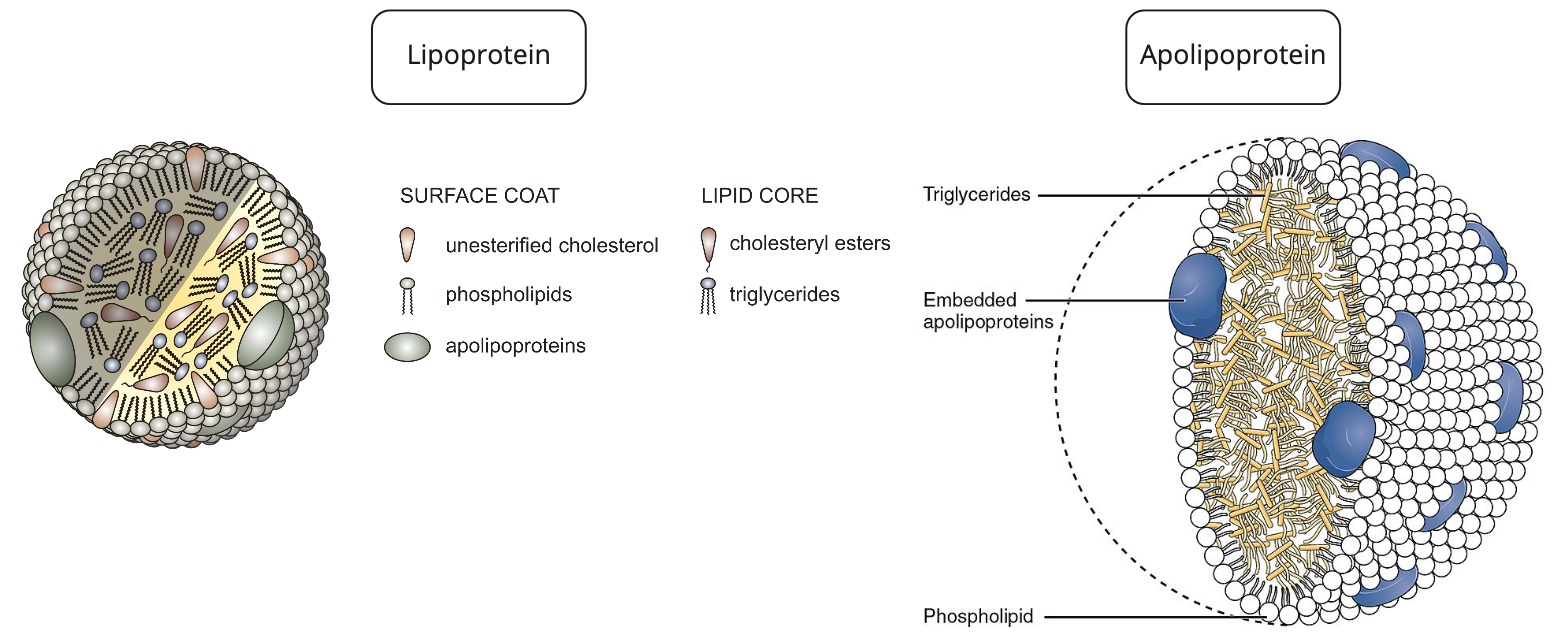
= Fats + Protein
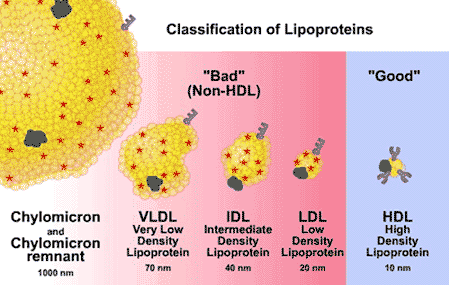
spherical particle
has a hydrophobic inner core (yellow) composed of cholesterol esters and triacylglycerols
- surrounded by a hydrophilic surface formed by the polar head groups of phospholipids and free cholesterol.
the apolipoproteins orient with their hydrophobic regions in the inner core and their hydrophilic regions at the surface.
How are apolipoproteins different from lipoproteins?
- apolipoprotein = the specific proteins that constitute the protein fraction of lipoproteins; they mediate the interactions of lipoproteins with tissues.
- lipoprotein = any lipid–protein conjugate. Specifically refers to lipid–protein associations that transport lipids in the circulation. Each consists of a core of hydrophobic lipids surrounded by a skin of amphipathic lipids with embedded apolipoproteins. Different kinds of lipoproteins play different roles in lipid transport.
- https://www.differencebetween.com/difference-between-lipoprotein-and-vs-apolipoprotein/
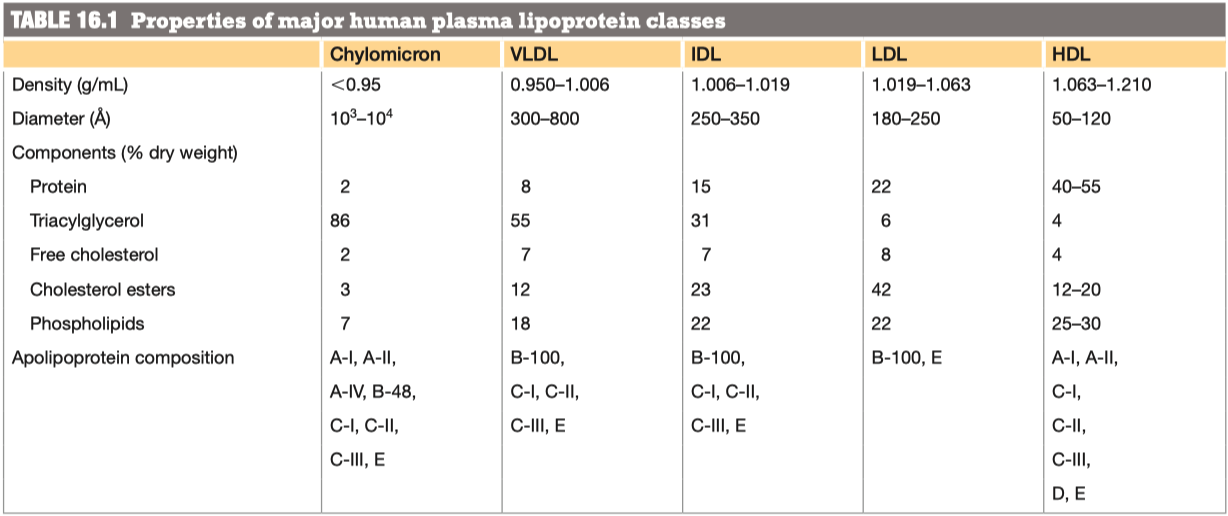
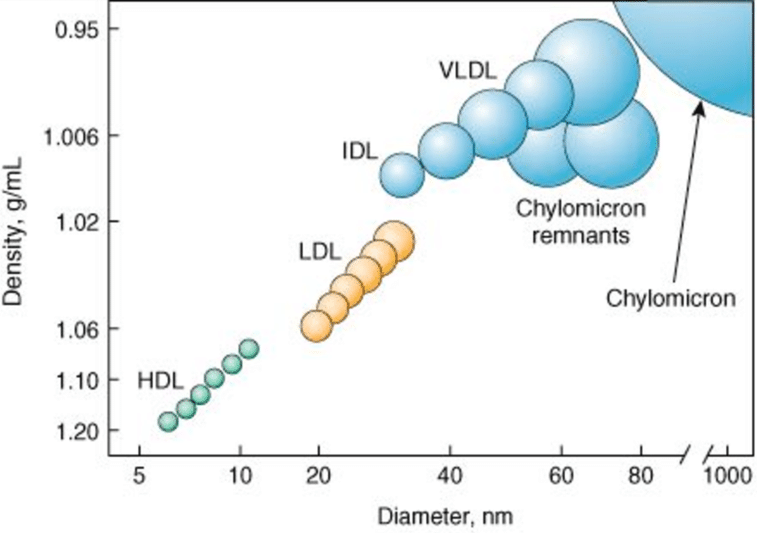
Why/How is density affected in these lipoproteins?
lipid content of a lipoprotein class is inversely related to its density
- the higher the lipid abundance, the lower the density
Digestion Absoption
We will start with identifying how fats are obtained from your diet. Lingual lipase and gastric lipase are enzymes that start the hydrolysis of short and medium chain fatty acids from their backbones, though this affect is minimal compared to the digestion that occurs in the small intestine
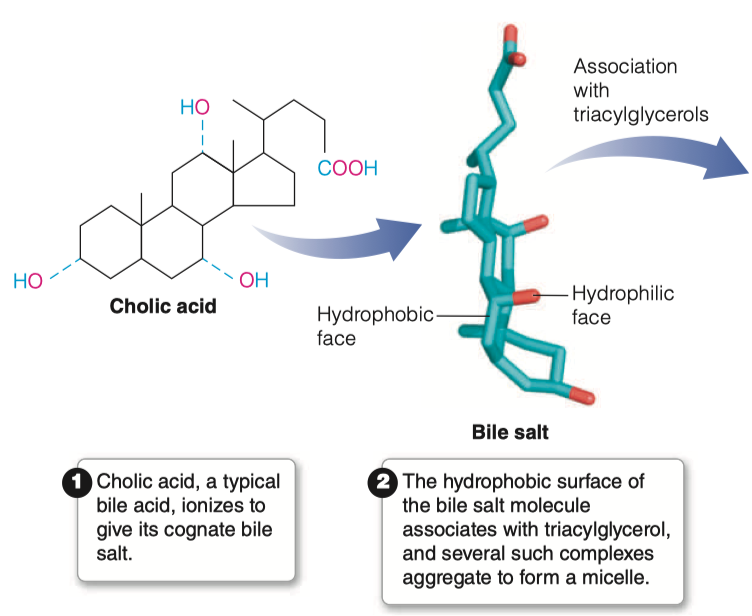
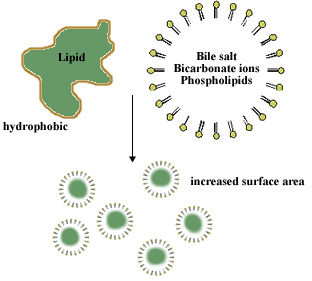
What are bile salts and where do they come from?
Bile Acids are derived from cholesterol
Bile acids are conjugated with taurine or glycine residues to give anions called bile salts
Bile Salts = detergent substances synthesized in liver and stored in gallbladder
- Essential to digestion of lipids and their absorption through intestinal mucosa
What characteristics of bile salts make them essential for lipid digestion?
A bile salt molecule has both hydrophobic and hydrophilic surfaces
This amphipathic character allows bile salts to orient at an oil–water interface, with the hydrophobic surface in contact with the apolar phase and the hydrophilic surface in contact with the aqueous phase.
- This detergent action emulsifies lipids and yields micelles, allowing digestive attack by water-soluble enzymes and facilitating the absorption of lipid through intestinal mucosal cells.
Bile salts emulsify fats, thereby promoting their hydrolysis in digestion
What does emulsify mean? How doe this relate to lipid digestion?
- Conjugated bile acids are almost always in their deprotonated (A-) form in the duodenum, which makes them much more water-soluble and much more able to fulfil their physiologic function of emulsifying fats
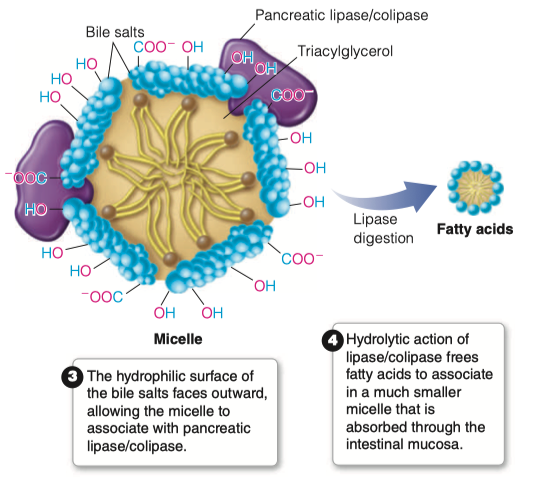
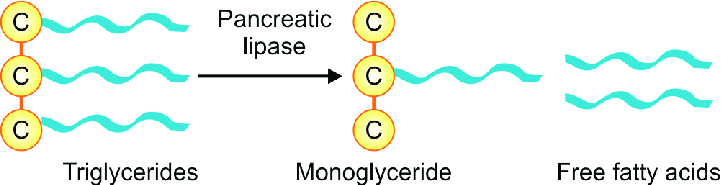
What is the role of pancreatic lipase?
An unusual calcium-requiring enzyme that catalyzes reactions at an oil–water interface.
The substrate being cleaved is in an apolar phase, and the other substrate, of course, is water.
Pancreatic lipase also functions in a 1:1 complex with colipase, a 90-amino acid protein that aids in the binding to the lipid surface.
Bile salts will come in contact with pancreatic lipase
Pancreatic lipase/colipase then catalyzes the digestion of lipids
Breaks down all of the triacylglycerols into individual fatty acids
- Individual fatty acids are small enough to be then absorbed by intestinal mucosa
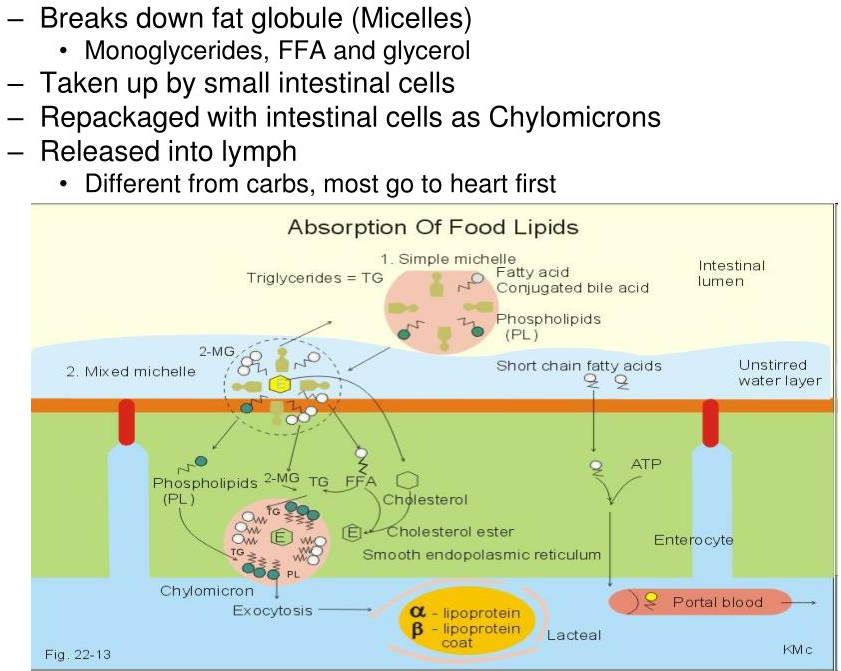
Where are lipids absorbed in the intestine?
- The products of fat digestion in the intestine comprise a mixture of glycerol, free fatty acids, monoacylglycerols, and diacylglycerols.
- During absorption through intestinal mucosal cells, much resynthesis of triacylglycerols occurs from the hydrolysis products.
- This resynthesis occurs in the endoplasmic reticulum and Golgi complex of mucosal cells
What happens to the lipids once they are absorbed?
Once fatty acids are absorbed into intestinal mucosa they are resynthesized back into triacylglycerols
- We break them down so we can get them into the mucosal cells
- Then the mucosal cells synthesize everything back into triacylglycerols
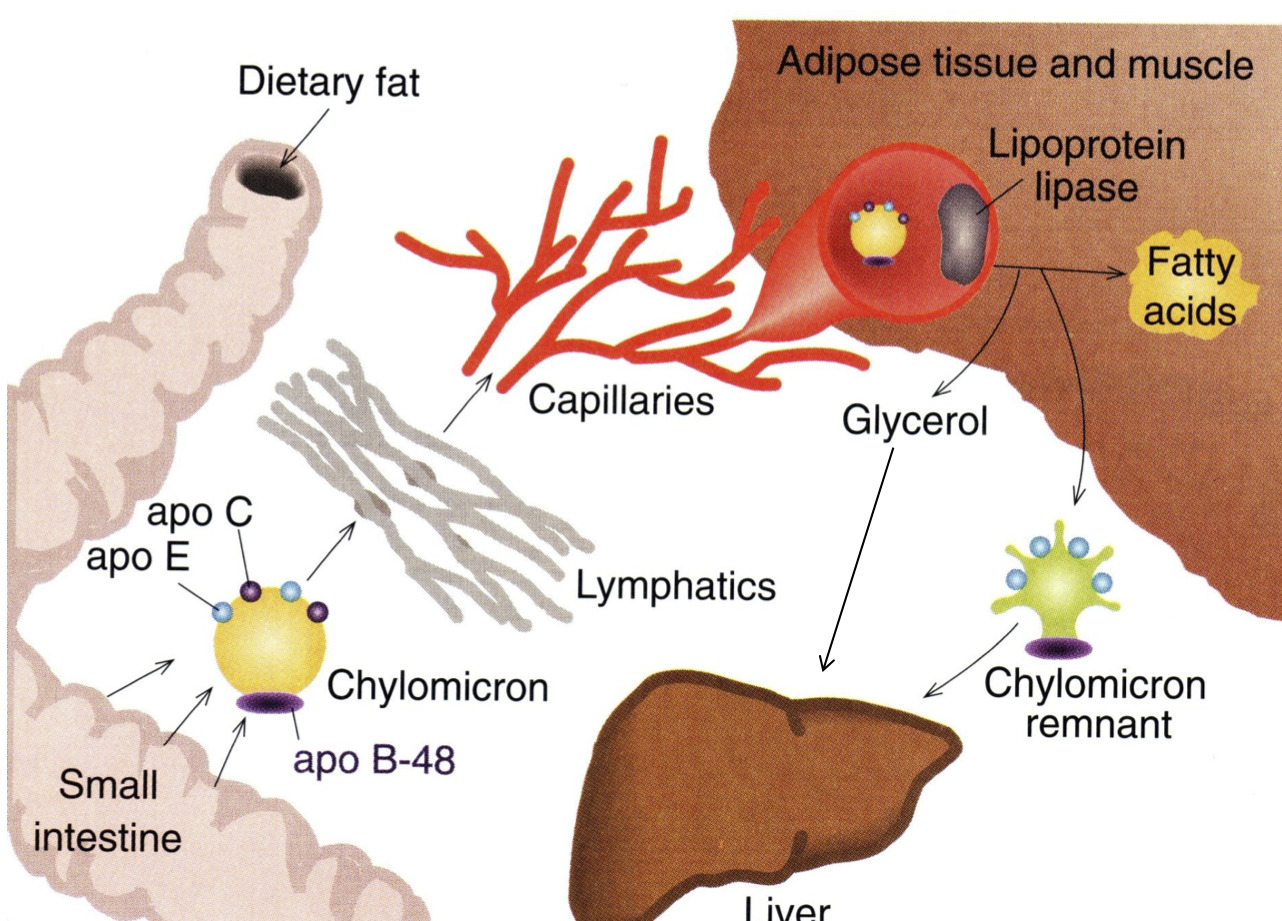
Once triacylglycerols have been resynthesized, they are assembled into chylomicrons
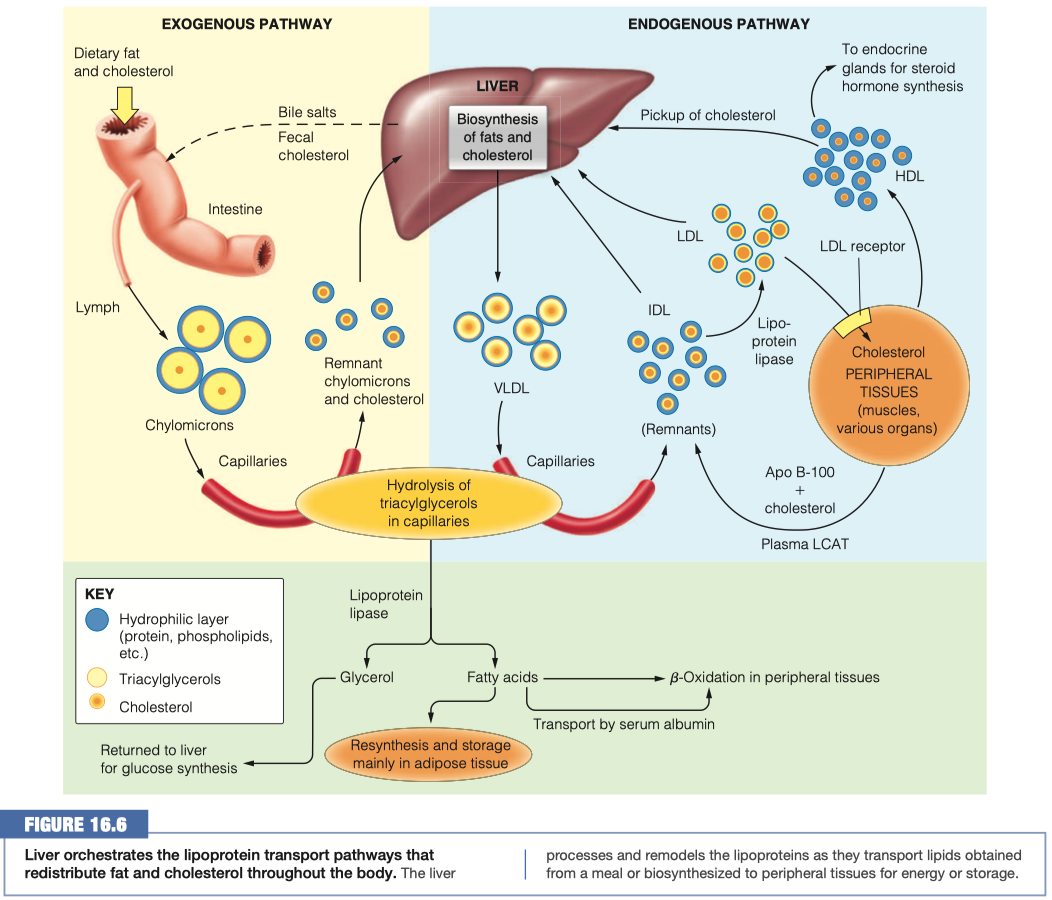
Now that the lipids are present in our cells, they must be transported to other areas of the body via the blood When the lipids leave the mucosal cells, which lipoprotein is formed in the lymph? How does this compare to the other lipoproteins?
Chylomicrons
- Least dense, massive amount of triacylglycerols
Chylomicrons are pushed into the lymphatic system and then into blood vessels / capillaries
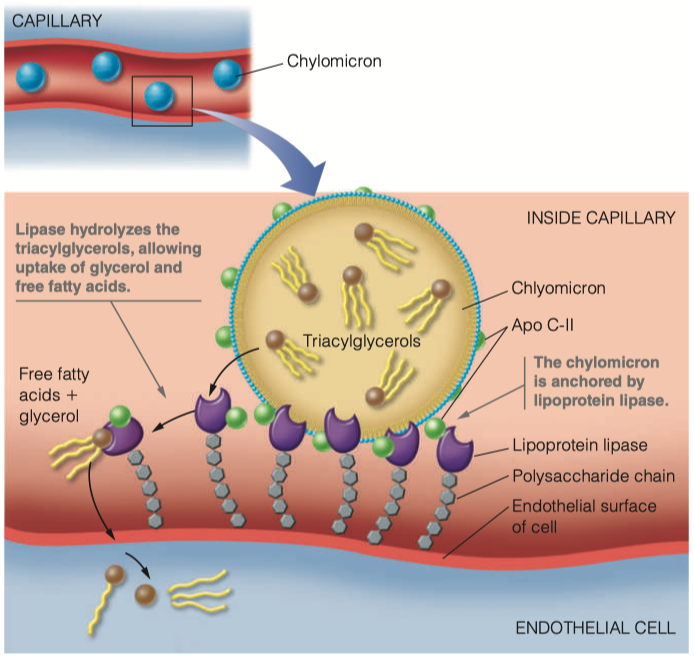
The lipoproteins are transferred form the lymph to the blood. Once they are in the blood, how are they recognized by lipoprotein lipase?
The chylomicron is anchored by lipoprotein lipase, which is linked by a polysaccharide chain to the lumenal surface of the endothelial cell.
When activated by apolipoprotein C-II (Apo C-II), the lipase hydrolyzes the triacylglycerols in the chylomicron, allowing uptake into the cell of the glycerol and the free fatty acids.
We have proteins ( Apo C-II ) on the outside of the chylomicron that functions as the ligand for lipoprotein lipase
Once lipoprotein lipase has connected with Apo C-II , it will grab the triacylglycerols from the chylomicron and bring them inside the endothelial cell
- Then triacylglycerols will begin to become hydrolyzed back into individual fatty acids
What is the function of lipoprotein lipase? Where do the products of the enzymatic reaction end up?
- The lipase hydrolyzes the triacylglycerols in the chylomicron, allowing uptake into the cell of the glycerol and the free fatty acids.
- We've gone from digestion, where we used pancreatic lipase to hydrolyze triaclyglycerols
- We brought them into the mucosal cells, resynthesized triaclyglycerols, and then assembled them into chylomicrons
- Chylomicrons then float around in capilaries
- The cells which need fat, will have a lipoprotein lipase receptors which then identify and attach to Apo C-II
- They then bring triaclyglycerols into the endothelial cell
- Once all the the triacylglycerols are gone, it becomes a "chylomicron remnant"
Once all of the triacylglycerols have been removed from the chylomicron, what is left? What name do we give this overall structure? Where does it ultimately go?
- Chylomicron Remnant
- Goes back to the Liver
Packaging of Existing Lipids
The remnant lipids and those synthesized in the liver must travel through the bloodstream to reach cells that need them. The liver packages these lipids into VLDL lipoproteins similar to how the intestinal mucosal cells make chylomicrons.
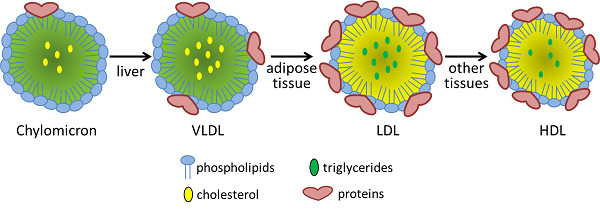
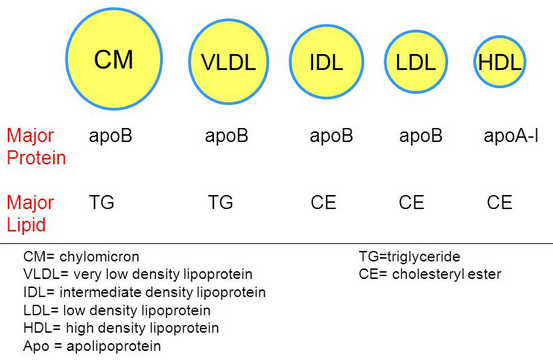
Compare and contrast the diameter, lipid components and apolipoprotein components of chylomicrons and VLDL lipoproteins
VLDL has more protein , and less triacylglycerol
Chylomicron Remnant has a lot less "fat"
Chylomicron vs VLDL Similarities:
- VLDL has more protein, and less triacylglycerol
Chylomicron vs VLDL Differences:
- Chylomicron has a much higher triacylglycerol level than VLDL
- VLDL has more cholesterol esters and phospholipids
How do you suppose triacylglycerols in VLDLs are hydrolyzed? Support your conjecture
Triacylglycerols in VLDLs are hydrolyzed to glycerol in the inner surface of capillaries in peripheral tissues
- Uses LPL and Apolipoprotein C-II on the VLDLs
- Uses the same system, now we are working with endogenous pathway
Instead of chylomicron remnants, what is left of the VLDL after triacylglycerol hydrolysis? Compare and contrast the diameter, lipid components and apolipoprotein components of VLDL and this lipoprotein.
- Instead of chylomicron remnants, VLDL turns into IDL
- Main thing thats different is we are missing a bunch of the triacylglycerols because they have been absorbed into the endothelial cells
- Cholesterol percentage is "up"
- All of the proteins are identical
- One just has already gone through the process of having its triacylglycerols removed
IDLs can be reabsorbed into the liver as were the chylomicron remnants, but they also have an alternate path here, conversion into LDL. Compare and contrast the diameter, lipid components and apolipoprotein components of IDL and LDL lipoproteins and summarize the changes you see.
We could send the cholesterol right back to the liver and say "nobody needs it"
- Or it could be used by other cells
IDL has more triacylglycerol per protein ( more dense )
LDL has more cholester esters "percentage wise"
Its not actually more, its just more percentage wise because we have gotten rid of everything else
Difference in some of the apolipid proteins
- We got rid of Apo A-I , Apo C-II, and Apo C-III
- We still have Apo B-100 and Apo E
LDL Uptake and HDL
How do LDLs interact with a cell that requires additional lipids?
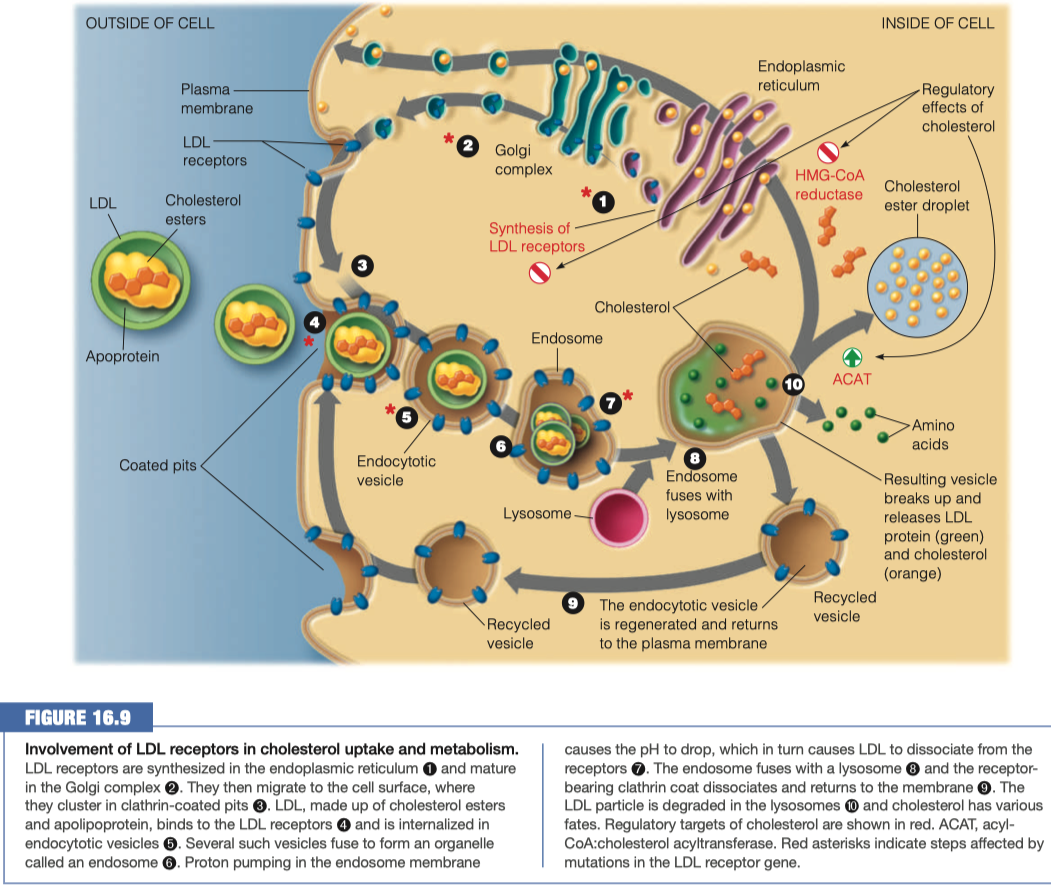
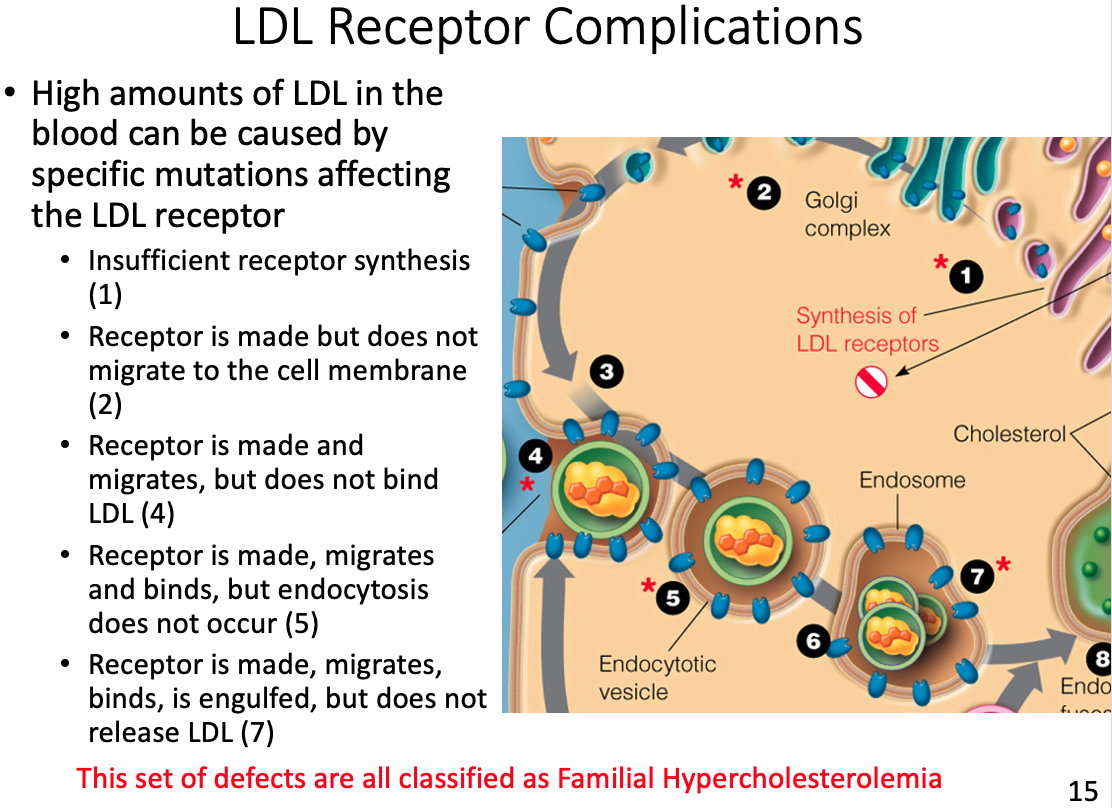
Activates LDL receptor Apo B-100
Triggers receptor-mediated endocytosis
- Receptors interact all the way around the vesicle and then pull it inside the cell to pass the plasma membrane making a vesicle which can then be broken down
Upon the above interaction what happens to the LDL lipoproteins to bring them into the cell?
- When LDL binds to its receptor, the entire LDL receptor complex is engulfed and taken into the cell (endocytosed)
- Binding to the LDL receptor occurs through recognition of the B-100 apolipoprotein, the primary protein component of the LDL particle.
- The plasma membrane fuses in the vicinity of the LDL–receptor complex, and the coated pit becomes an endocytic vesicle.
- Several of these clathrin-lined vesicles fuse to form an endosome.
Once inside the cell what happens to the lipoproteins?
The endosome then fuses with a lysosome, putting the LDL–receptor complex in contact with the hydrolytic enzymes of the lysosome.
The LDL apolipoprotein is hydrolyzed to amino acids, and the cholesterol esters are hydrolyzed to give free cholesterol.
The receptor itself is recycled, moving back to the plasma membrane to pick up more LDL
Lysosome fuses with endosome
- Breaks down all of the lipids and proteins
- Releases amino acids and cholesterol
Anything that was not broken down gets returned back to the plasma membrane so we can use it again
If there is excess cholesterol in a cell, what happens to it?
Internalized cholesterol:
Reduces endogenous cholesterol synthesis, by inhibiting HMG-CoA reductase and also by repressing transcription of the gene for this enzyme and accelerating degradation of the enzyme protein.
Activates acyl-CoA:cholesterol acyltransferase (ACAT), an intracellular enzyme that synthesizes cholesterol and esters from cholesterol and a long-chain acyl-CoA.
- This promotes the storage of excess cholesterol in the form of droplets of cholesterol esters
Regulates the synthesis of the LDL receptor itself by decreasing transcription of the receptor gene.
- Decreased synthesis of the receptor ensures that cholesterol will not be taken into the cell in excess of the cell’s needs, even when extracellular levels are very high.
This regulatory mechanism explains why excessive dietary cholesterol leads directly to elevations of blood cholesterol levels.
- With intracellular cholesterol levels so well regulated, the extracellular cholesterol accumulates because it has nowhere else to go.
Cholesterol esters are being stored
Inhibition of cholesterol synthesis
HMG-CoA reductase is a predominate enzyme in cholesterol synthesis
- It takes HMG-CoA and turns it into mevalonate
- we are stopping this reaction from happening here, so it stays as HMG-CoA
Cholesterol is going to be packaged into HDL
- HDL can be sent outside the cell to the liver to be excreted
- HDL can be sent to endocrine glands for steroid hormone synthesis
What lipids are the majority composition of HDL? Where does the HDL go? Why?
- Mainly cholesterol, some triclycerides
- Regulate levels of cholesterol in blood
- Goes back to liver
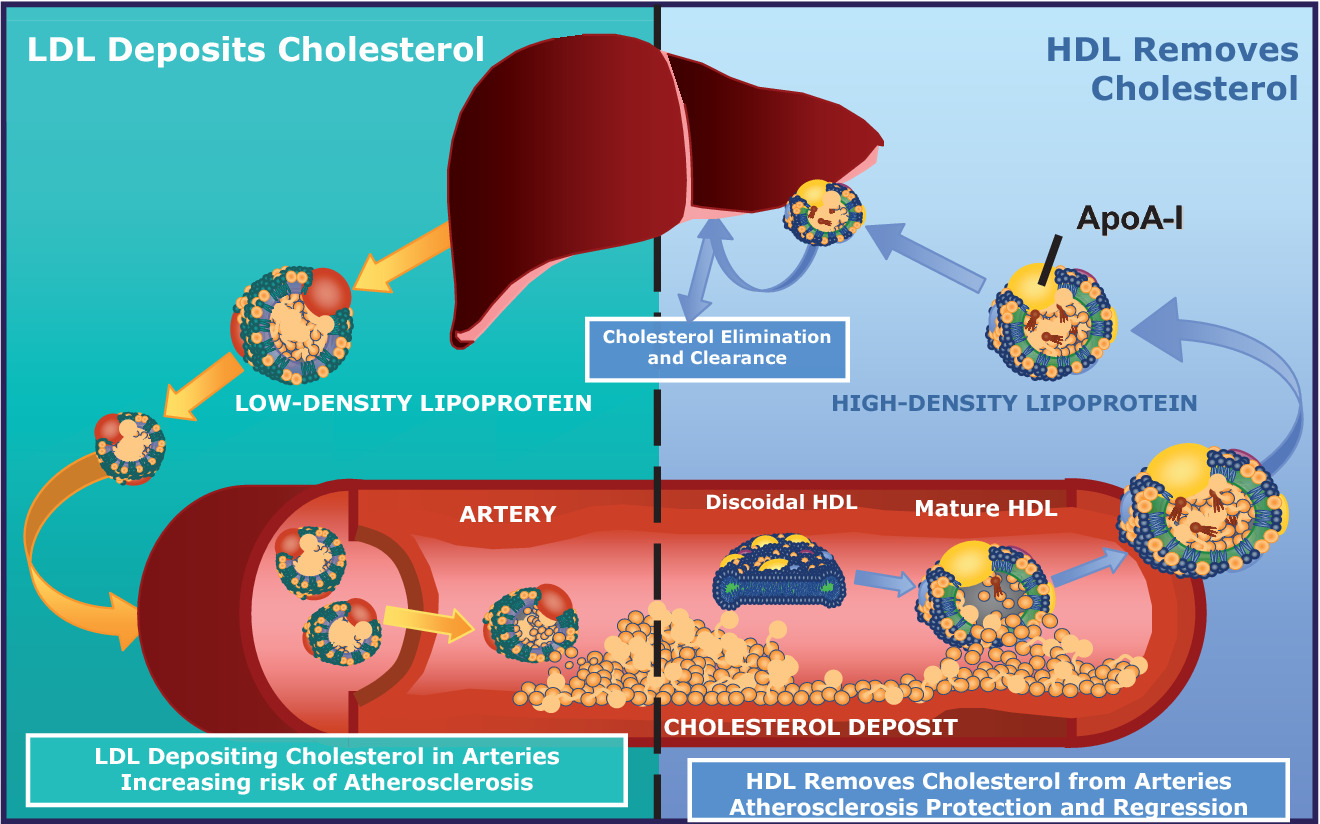
When people refer to LDL as “bad cholesterol” and HDL as “good cholesterol”, why is this a misunderstanding? Provide at least two reasons.
- Cholesterol itself is a natural metabolite, an essential component of all membranes, and the precursor to all steroid hormones and bile acids.
- Cholesterol present in LDL is considered “bad” because prolonged elevation of LDL levels is what leads to atherosclerosis.
- Cholesterol in HDL is called “good” because high levels of HDL counteract atherogenesis.
- Cholesterol cannot be metabolically degraded, and excess cholesterol is returned from peripheral cells to the liver, for passage through the bile to the intestine, for ultimate excretion (Figure 16.6).
- As the agent for this transport back to the liver, HDL plays a role in lowering total serum cholesterol levels, which is “good.”
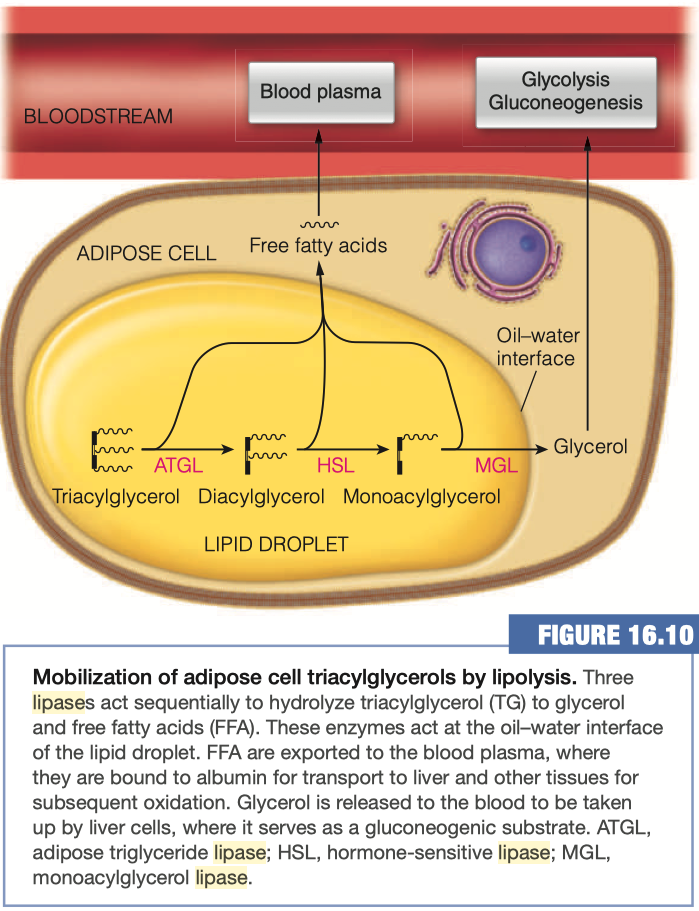
Mobilization of Triaclyglycerols from Adipose Tissue
As you already know, the adipocytes store our excess energy as fats, predominantly as triacylglycerols
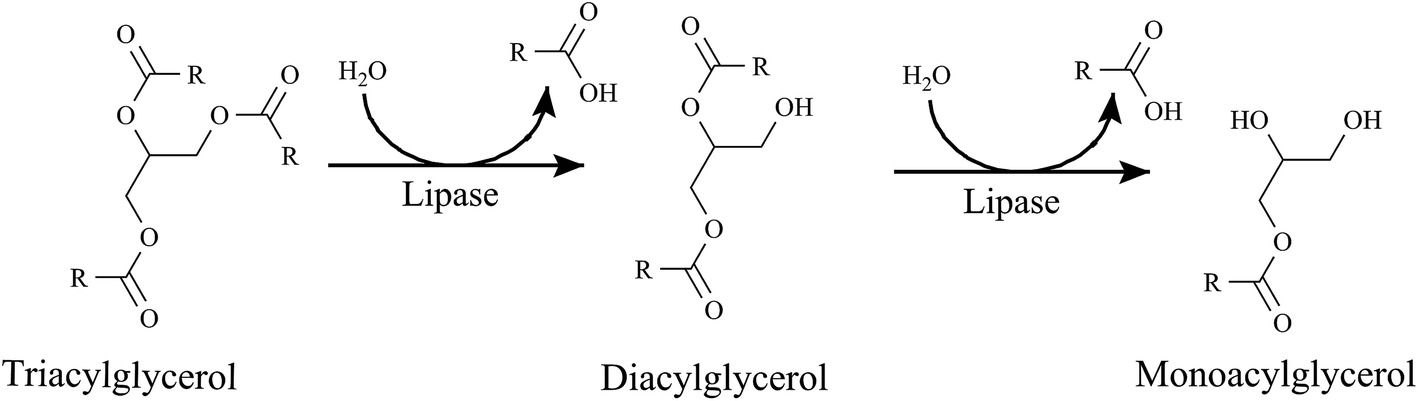
- Triacylglycerol lipase
- Hormone-sensitive lipase (HSL)
- Adipose triglyceride lipase (ATGL)
- Monoaclyglycerol Lipase (MGL)
Triacylglycerols (TAGs) are converted into diacylglycerols (DAGs) by what enzyme?
- Adipose triglyceride lipase (ATGL)
Diacylglycerols are converted into monoacylglycerols (MAGs) using which enzyme?
- Hormone-sensitive lipase (HSL)
What happens to the Free Fatty acids upon hydrolysis from glycerol?
- Goes back into blood plasma
- The free fatty acid hydrolysis products exit the adipocyte by passive diffusion and find their way to the blood plasma, where the fatty acids become bound to albumin.
- Each molecule of albumin can bind up to 10 molecules of free fatty acid, although the actual amount bound is usually far lower.
- Fatty acids are released from albumin and taken up by tissues largely by passive diffusion so that fatty acid uptake into cells is driven primarily by concentration.
How are lipids (a largely nonpolar molecule) transported in the blood (a polar solvent)?
Where are the majority of glycerol taken up after its release from the adipocytes? Why?
Most of the glycerol released to the bloodstream is taken up by liver cells, where it serves as a gluconeogenic substrate, leading to the production of glucose
Glycerol is being released into the bloodstream
When you are breaking down your fats, you are in a situation where you have low blood glucose
You have no supply of sugar around potentially
- you've even gone through all of your glycogen stores
And now we are starting to break down our fats in order to maintain our energy levels.
Well, in order to bring blood glucose back up, this glycerol is going off to the liver where it's going through gluconeogenesis.
And that gluconeogenesis will then provide some additional glucose for lots of other things around your body.
Now, this glycerol can be used in glycolysis.
- So it can go through that pathway either forward or backward, depending on what the needs of the body are, and what the needs of the individual cells are.
It's not always the case that your entire body is doing gluconeogenesis or your entire body is doing glycolysis.
- It just depends on what's working, what's not working, what needs more energy, what doesn't need more energy, etc.
It all comes down to what your cells need, and then how they indicate to your body whether or not they need those substrates.
Things To Know
General statistics about heart disease
- Black men and women are more at risk than white
- Older individuals are more at risk
- Males are more at risk within their race
- two top leading causes of death in 2018 and 2019 the US were heart disease and cancer
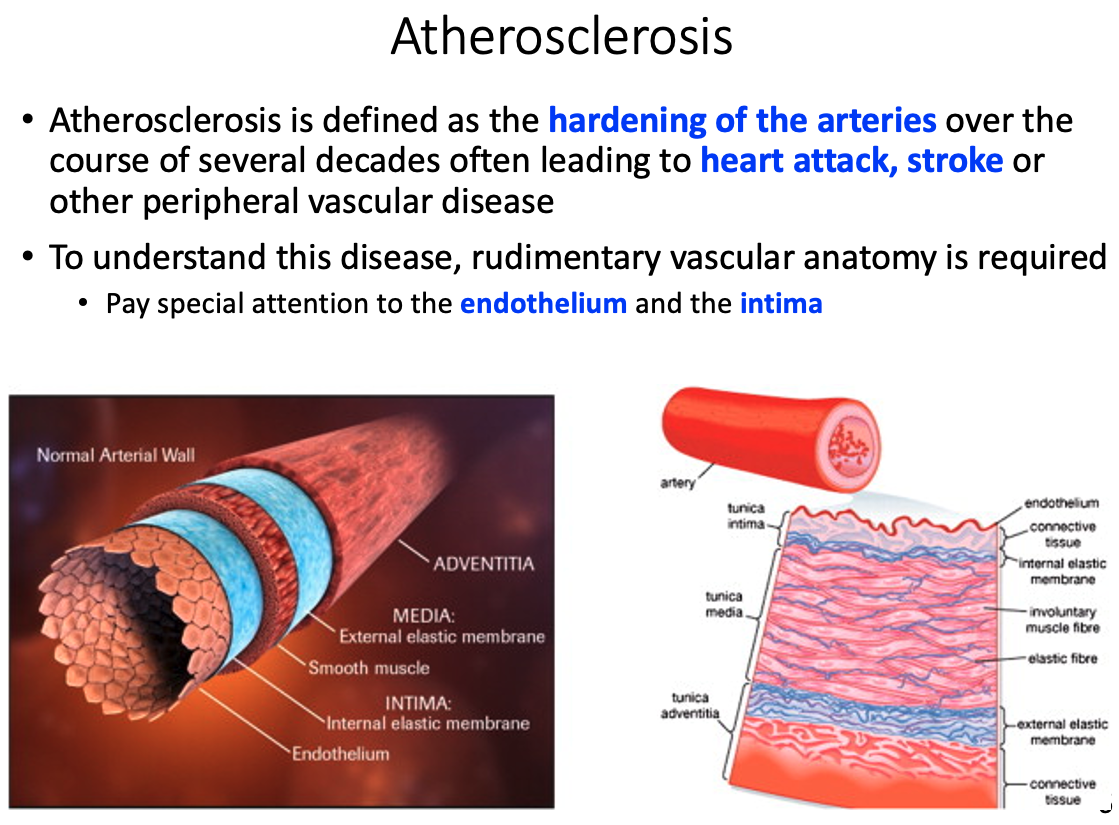
Atherosclerosis
Define it
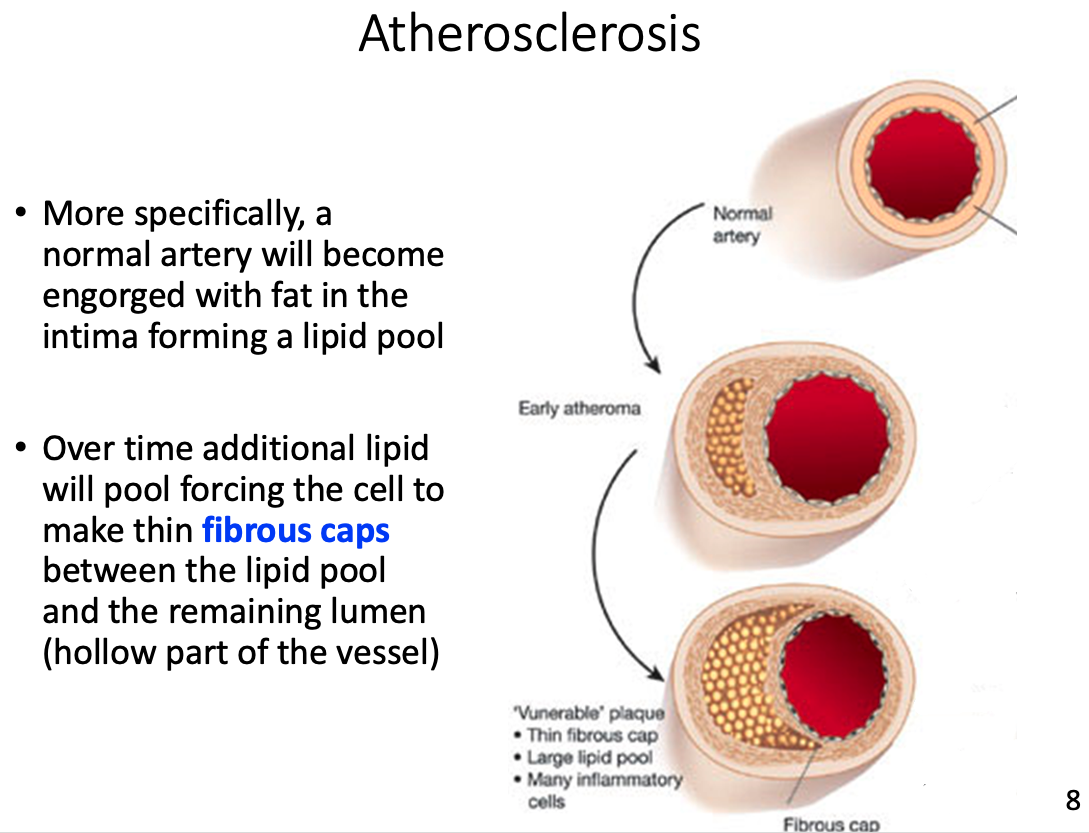
hardening of the arteries over the course of several decades
- often leads to heart attack , stroke, or other peripheral vascular disease
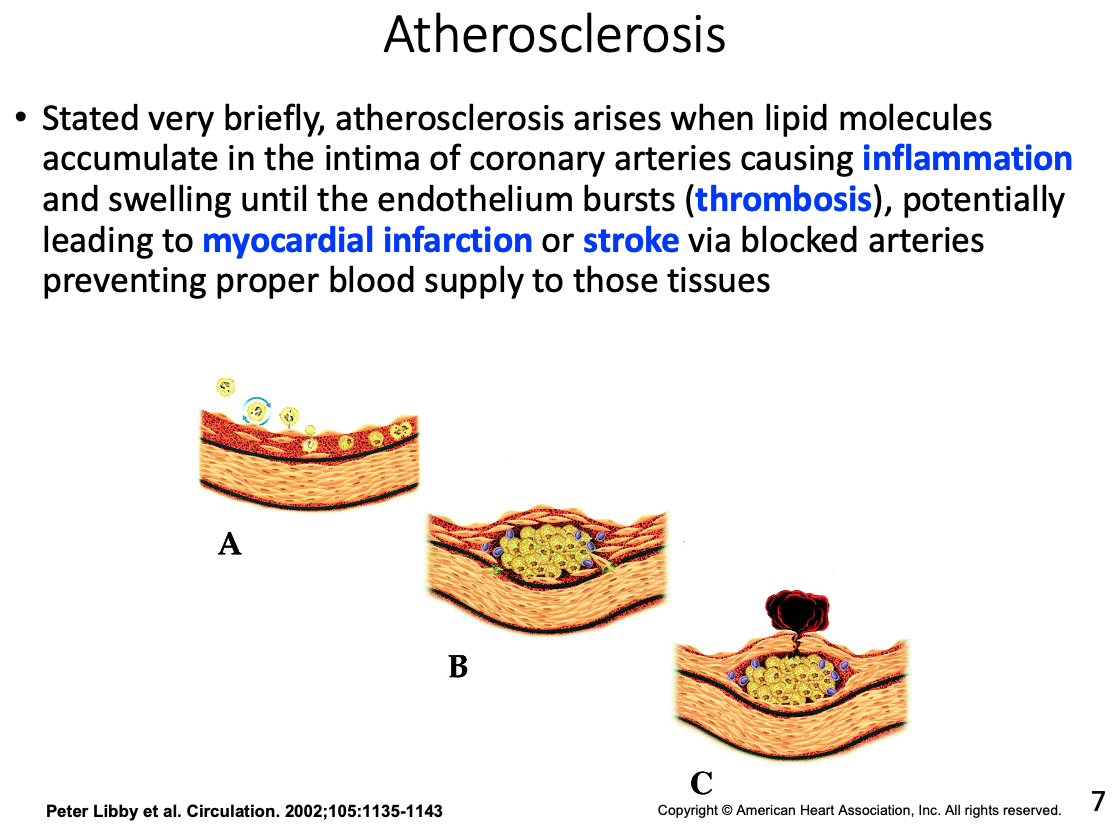
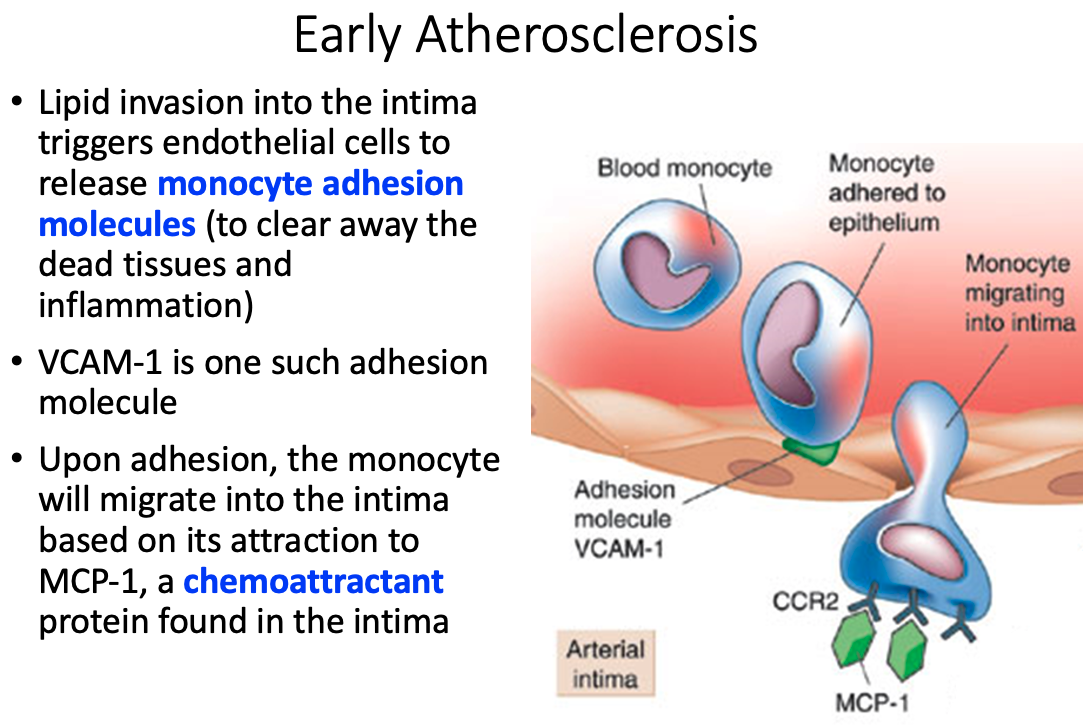
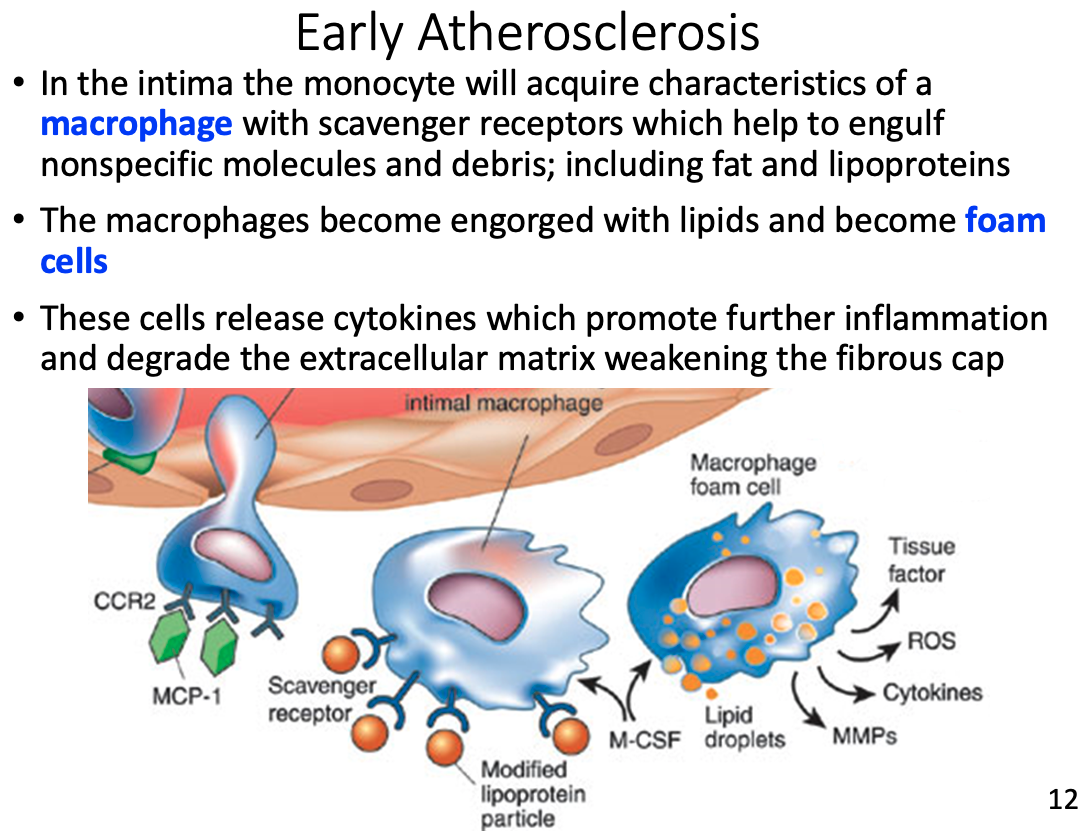
Role of macrophages and how they got there
- In the intima the monocyte will acquire characteristics of a macrophage with scavenger receptors which help to engulf nonspecific molecules and debris; including fat and lipoproteins
Foam cells
- engorged macrophages with lipids
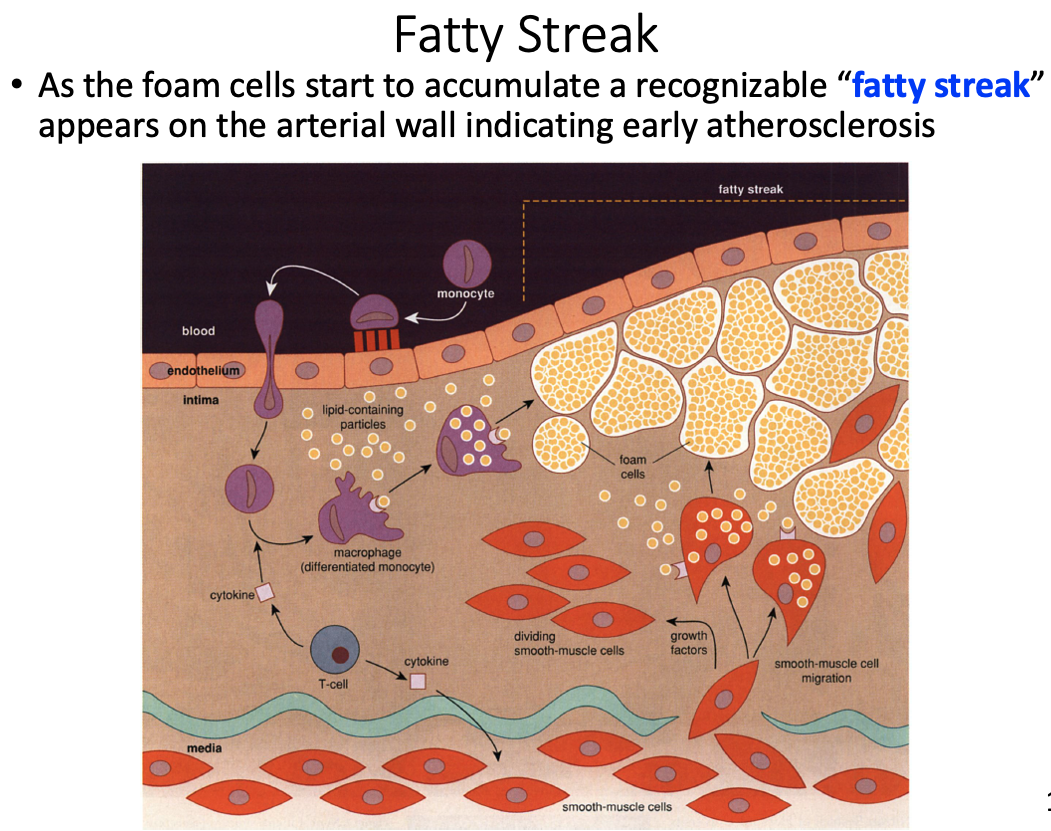
Fatty streak
- accumulation of foam cells that appear on arterial wall indicating early atherosclerosis
Fibrous cap
- Over time additional lipid will pool forcing the cell to make thin fibrous caps between the lipid pool and the remaining lumen (hollow part of the vessel)
Possible result of thrombosis
Why cholesterol is such a concern
If no tissues require cholesterol the LDL will continue to circulate, eventually depositing the excess fat on the arterial walls
- leads to Atherosclerosis
Medical diagnosis of LDL and HDL
- LDL: high cholesterol lipoprotein responsible for transporting cholesterol to peripheral tissues
- HDL: removes excess cholesterol for is removal from the body
LDL receptor complications and results
- If no tissues require cholesterol the LDL will continue to circulate, eventually depositing the excess fat on the arterial walls
Hyperlipoproteinemia
General characteristics
- Inability to break down lipids or fats in your body; specifically cholesterol and triglycerides.
- Many of the types are genetically inherited either as dominant or recessive traits, while other simply “run in families”
Symptoms
- Many people with this condition experience no symptoms, and only become aware of it when they develop a heart condition
- Others experience lipid deposits or xanthomas on the skin