Cholesterol Synthesis
Pre-Activity
- Find the structure of cholesterol and determine its properties
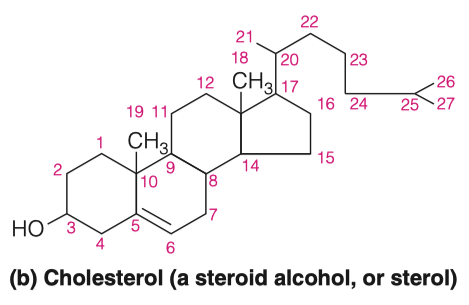
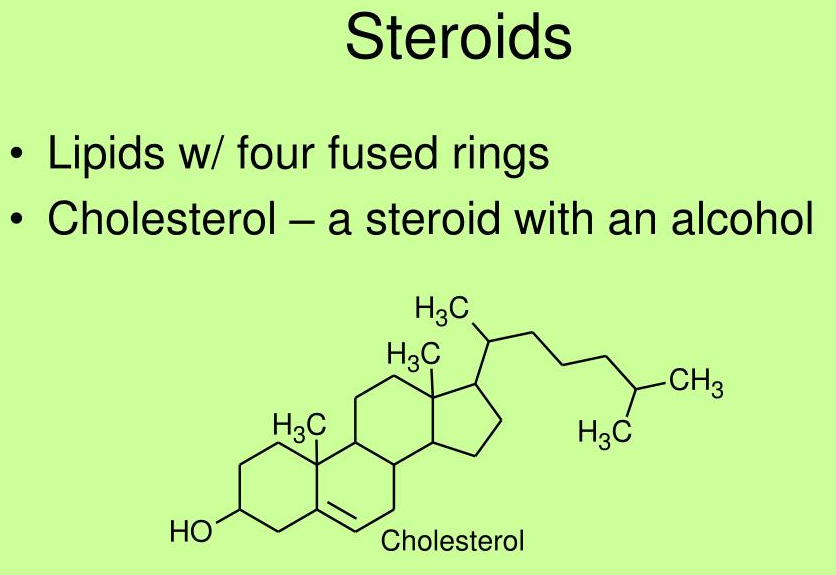
Cholesterol differs from the basic ring system in having:
- an aliphatic chain at C-17
- axial methyl groups at C-10 and C-13
- a double bond in ring B
- a hydroxyl group in ring A
The alcoholic functional group and the carbon chain at C-17 make cholesterol a sterol
- which is the generic term used to identify steroid alcohols
Much of the cholesterol in lipoproteins and intracellular storage droplets is esterified at this position with a long-chain fatty acid
- which makes the resultant cholesterol ester much more hydrophobic than cholesterol itself
- Also determine how many carbons are present in the structure.
27
Activity
Book sections 16.2 (Last section: Ketones) and 16.7 as well as the internet will be used for this activity.
Ketones
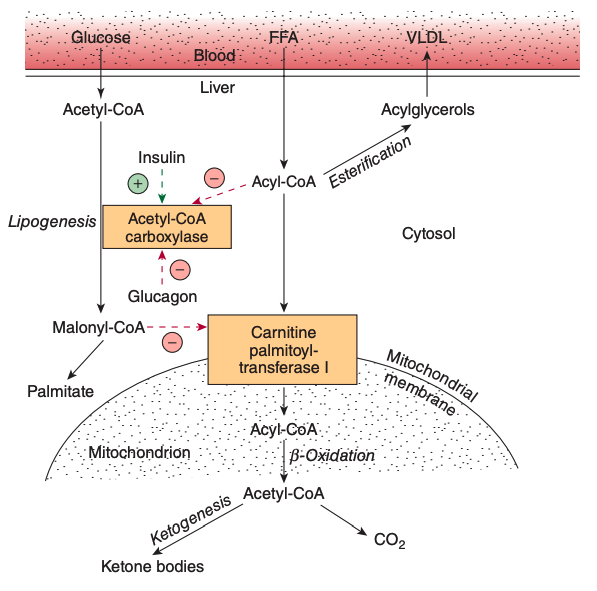
- Thus far, you have seen that Acetyl-CoA can be used to synthesize fatty acids or be used in the citric acid cycle to eventually make ATP.
1.A. What do you suppose would happen in a cell when -oxidation levels far exceed that of the limits of the citric acid cycle? Where would this occur?
- Massive buildup of acetyl-CoA
- Under conditions in which further oxidation of acetyl-CoA through the citric acid cycle is limited, acetyl-CoA is used to synthesize ketone bodies, which are excellent energy substrates for brain and heart.
- Occurs in the liver
1.B. How will this affect the rate of -oxidation?
http://edusanjalbiochemist.blogspot.com/2014/04/fatty-acid-metabolism-regulation-of.html
Thermodynamics is regulated by concentration levels of products and reactants
If we get a huge build up of acetyl-CoA, we are no longer able to generate more acetyl-CoA through -oxidation
Which means we start loosing our sources of NADH
- Without our sources of NADH, we can't produce ATP as readily
The body has created a system where it generates ketone bodies instead
High levels of acetyl-CoA = make ketones
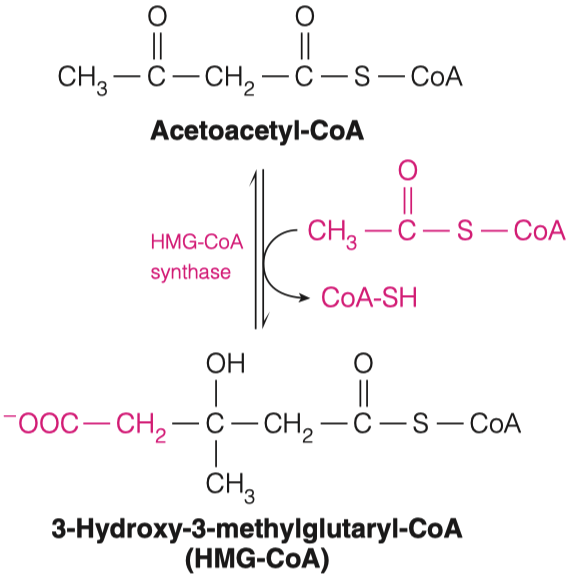
- To circumvent the issues from question 1, the body can use acetyl CoA to make ketone bodies (Figure 16.19)
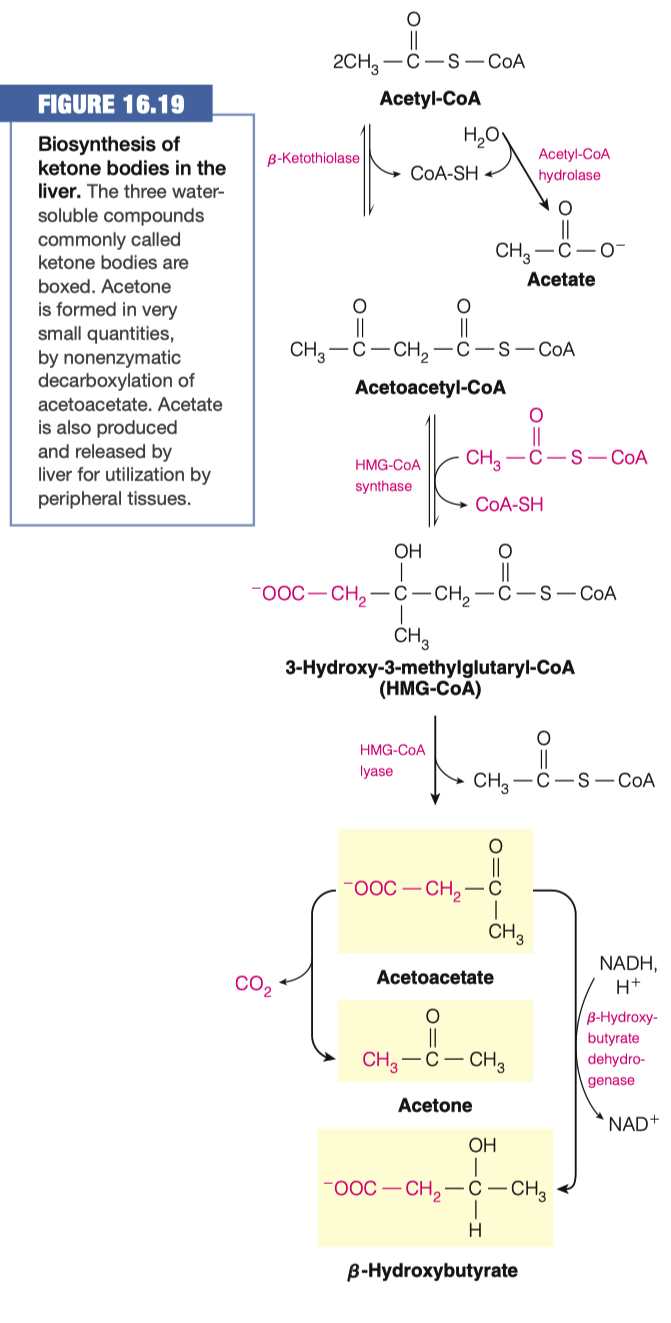
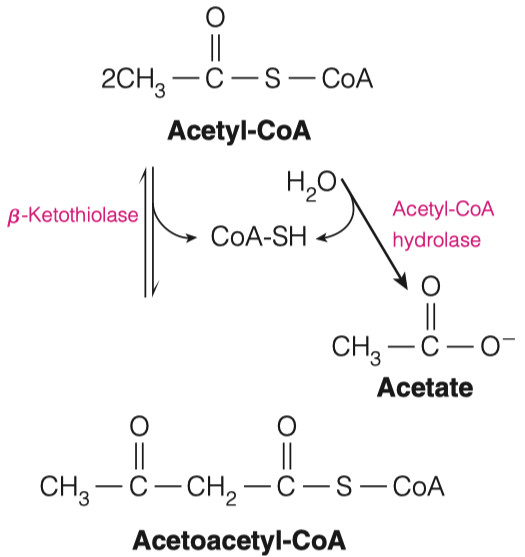
2.A. How many Acetyl-CoA are used to make Acetoacetyl-CoA?
- 2
2.B. What is generated when Acetoacetyl-CoA is joined with another molecule of Acetyl-CoA? What enzyme is responsible for this?
- 3-Hydroxy-3-methylglutaryl-CoA ( HMG-CoA )
- HMG-CoA Synthase
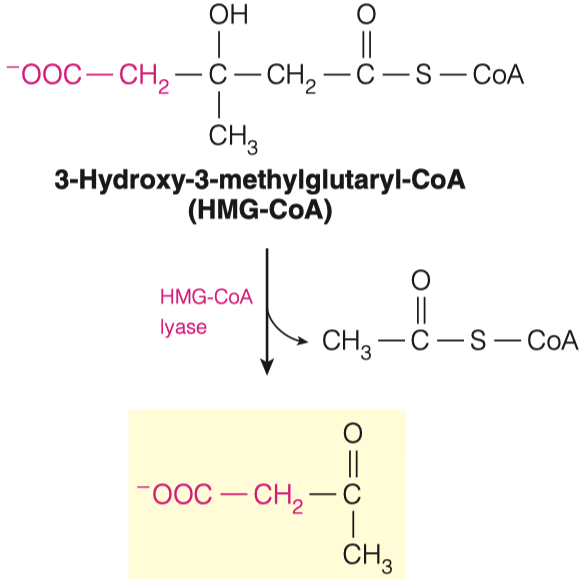
- HMG-CoA can be converted in to Acetoacetate.
3.A. What molecule is removed in this reaction?
- Acetyl-CoA
3.B. Hypothesize why we add an Acetyl-CoA to Acetoacetyl-CoA, to make HMG-CoA if we just then remove the Acetyl-CoA to make Acetoacetate
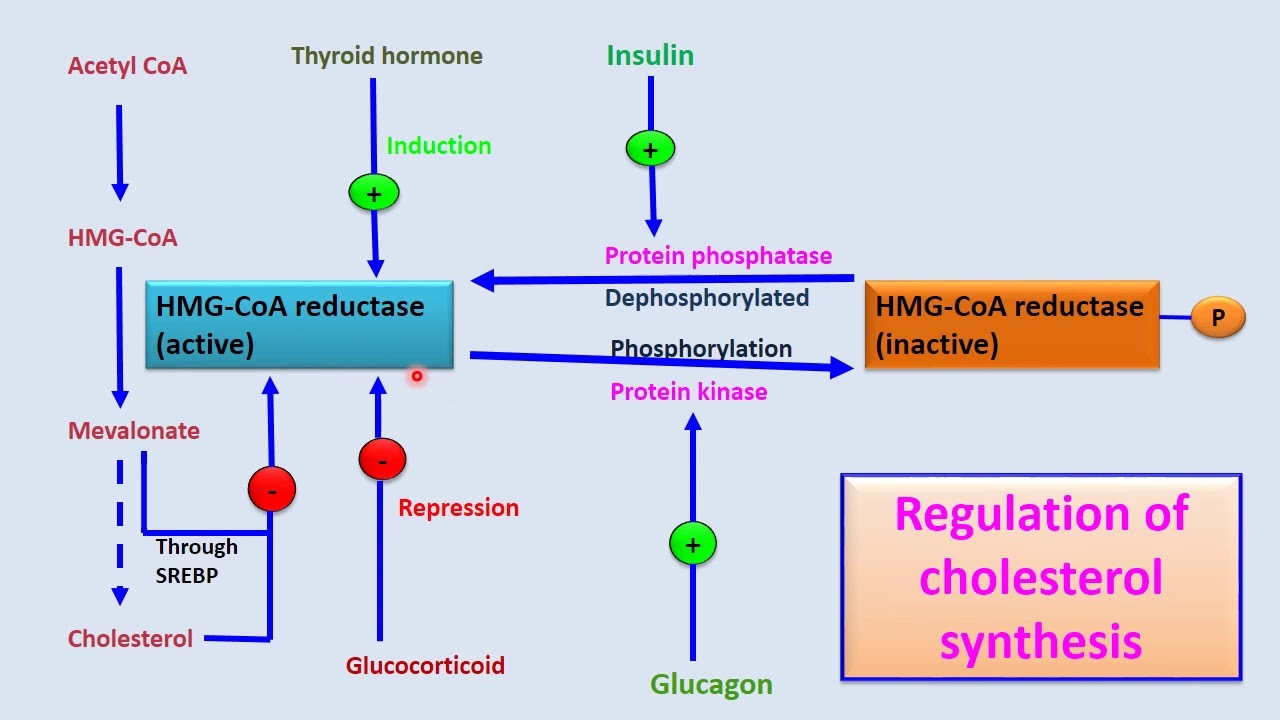
HMG-CoA synthase = regulated enzyme by glucagon (activates) and insulin (inactivates).
- It appears that this extra step was added so the pathway can be hormonally regulated.
We made HMG-CoA because its used in a lot of different things other than the production of ketone bodies
So we went through this side step to make HMG-CoA
- Only when we don't need any HMG-CoA will make ketone bodies
1st acetyl-CoA is attached "backwords"
- its a different directionality
- the second one is in the other direction (right hand side)
- "we are kind of shifting the molecule into a different structure in the way we are doing it"
- We need HMG-CoA for cholesterol synthesis
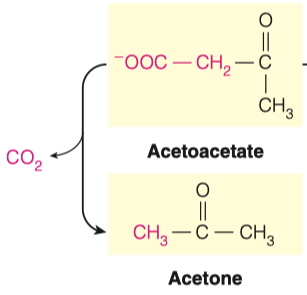
3.C. What reaction must take place to convert Acetoacetate to Acetone?
- Decarboxylation
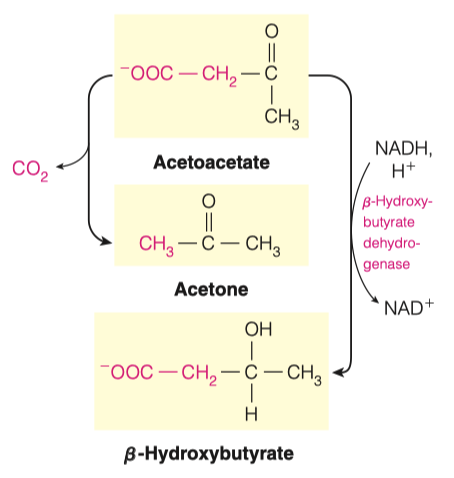
3.D. What reaction must take place to convert Acetoacetate to -Hydroxybutarate?
- Reduction
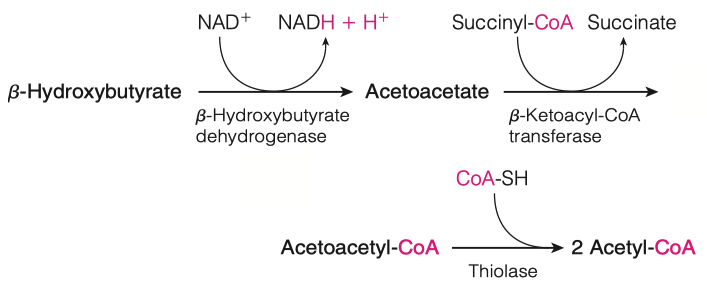
- What happens to the ketone bodies once they are generated in the liver?
- Ketone bodies are transported from liver to other tissues, where acetoacetate and -hydroxybutyrate can be reconverted to acetyl-CoA for energy generation.
- The reconversion involves enzymatic transfer of a CoA moiety from succinyl-CoA to acetoacetate, yielding acetoacetyl-CoA and succinate
Cholesterol Synthesis
- Cholesterol is a member of a group of lipids known as isoprenoids (generated from isoprene or its derivatives) and is subcategorized in the steroid family (based on a saturated tetracyclic hydrocarbon structure)
Ketogenesis happens in the liver, inside the mitochondria
-Oxidation happens in the liver
- so we might as well make ketones there because that is where we are getting our massive amounts of acetyl-CoA
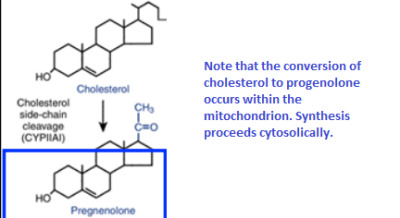
Cholesterol is also made in the Liver , inside the endoplasmic reticulum (ER)
This is how we regulate which process should take place
If we have excess acetyl-CoA , it can be moved into the cytosol or stay in the mitochondria
If its in the cytosol, it can be made into cholesterol
if stays in the mitochondria, it will be turned into ketone bodies
- Ketone bodies go out to blood stream and to tissues
5.A. Why is cholesterol considered a steroid alcohol?
5.B. What other features make cholesterol different from a standard saturated tetracyclic hydrocarbon?
- Can esterify with long chain fatty acids to form cholesterol esters such as cholesteryl
- Metabolic precursor of steroid hormones
- Aliphatic chain on C-17 , axial methyl groups at C-10 and C-13, a double bind in ring B , and a hydroxyl group ion ring A.
- Read over stage 1 of cholesterol formation (and use figure 16.34) to identify reactions 1 and 2.
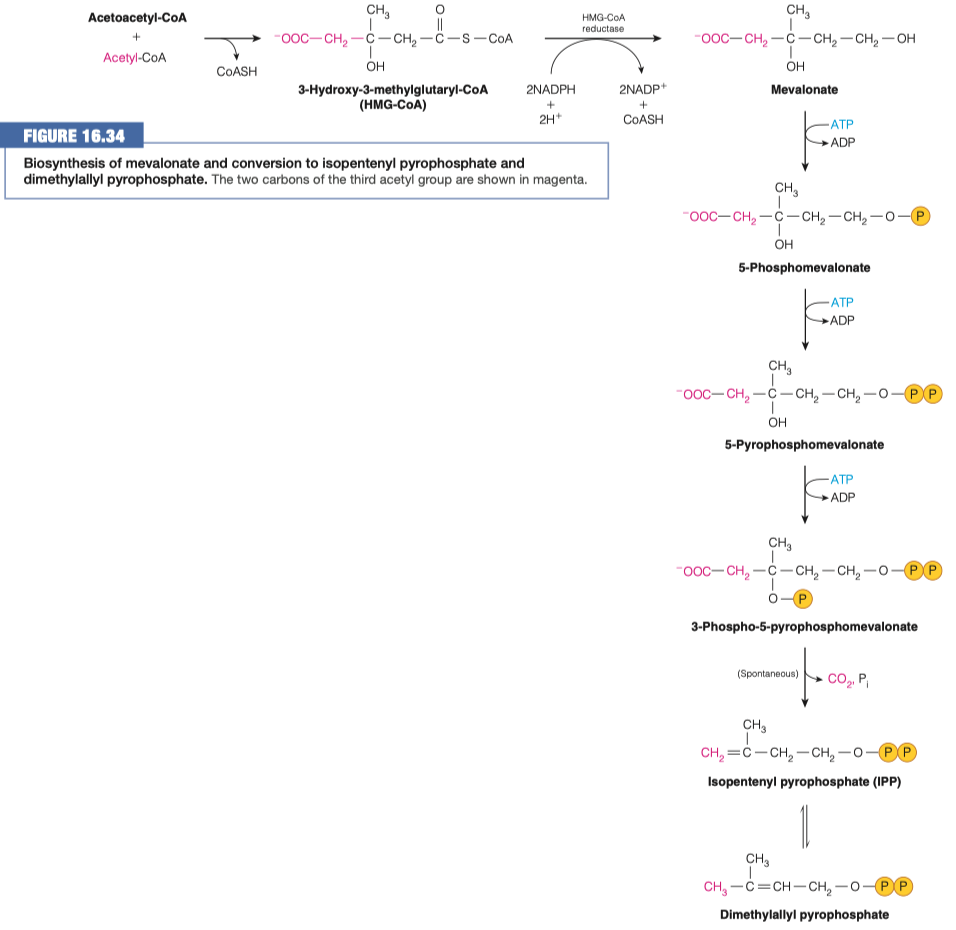
6.A. What other process is this similar to? What is different about it?
identical to reactions used in ketogenesis
It occurs in a different cell compartment.
- Ketogenesis occurs in mitochondria, whereas cholesterol biosynthesis occurs in the cytosol and the endoplasmic reticulum (ER).
step 1 is similar to ketogenesis
step 2 is different because it uses NADPH instead of NADH
- anytime we are doing a synthesis we tend to use NADPH
step 2 is also different because uses a different enzyme
- in cholesterol synthesis we use HMG-CoA reductase
- here, we are using HMG-lyase
6.B. What is the role of HMG-CoA reductase? How is it different from HMG-CoA lyase?
- HMG-CoA lyase that cleaves HMG-CoA is missing from the ER, where cholesterol biosynthesis begins
- HMG-CoA reductase an integral membrane protein in the ER catalyzes the reduction of HMG-CoA to mevalonate
- This multistep reaction requires two equivalents of NADPH (four electrons) to reduce the thioester to an alcohol.
- This is the major step that regulates the overall pathway of cholesterol biosynthesis.
- HMG-CoA lyase broke off an acetyl-CoA
- HMG-CoA reductase only broke off a CoA
- Read over stage 2 of cholesterol formation (and use figure 16.34) to identify reactions 3, 4, 5 and 6.
7.A. What is happening in these reactions?
- Reaction 3 = = Phosphorylation
- Reaction 4 = = Phosphorylation
- Reaction 5 = = Phosphorylation
- Reaction 6 = = Decarboxylation
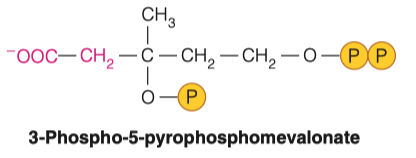
7.B. Where is the third phosphate group added?
- Other Oxygen , where the hydroxyl group was
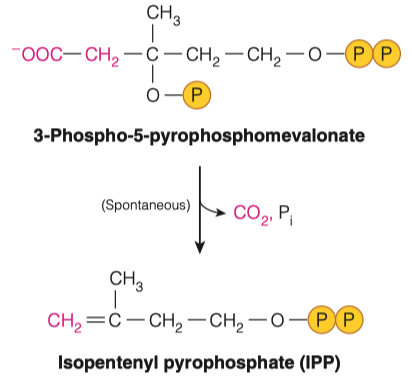
7.C. To make IPP from the molecule in the previous step, what must occur? How many carbons are left in the molecule?
- Decarboxylation
- This is important because it acts as a leaving factor with CO2 in order to generate IPP
- Isopentenyl pyrophosphate (IPP) will go through an isomerization reaction to form dimethylallyl pyrophosphate.
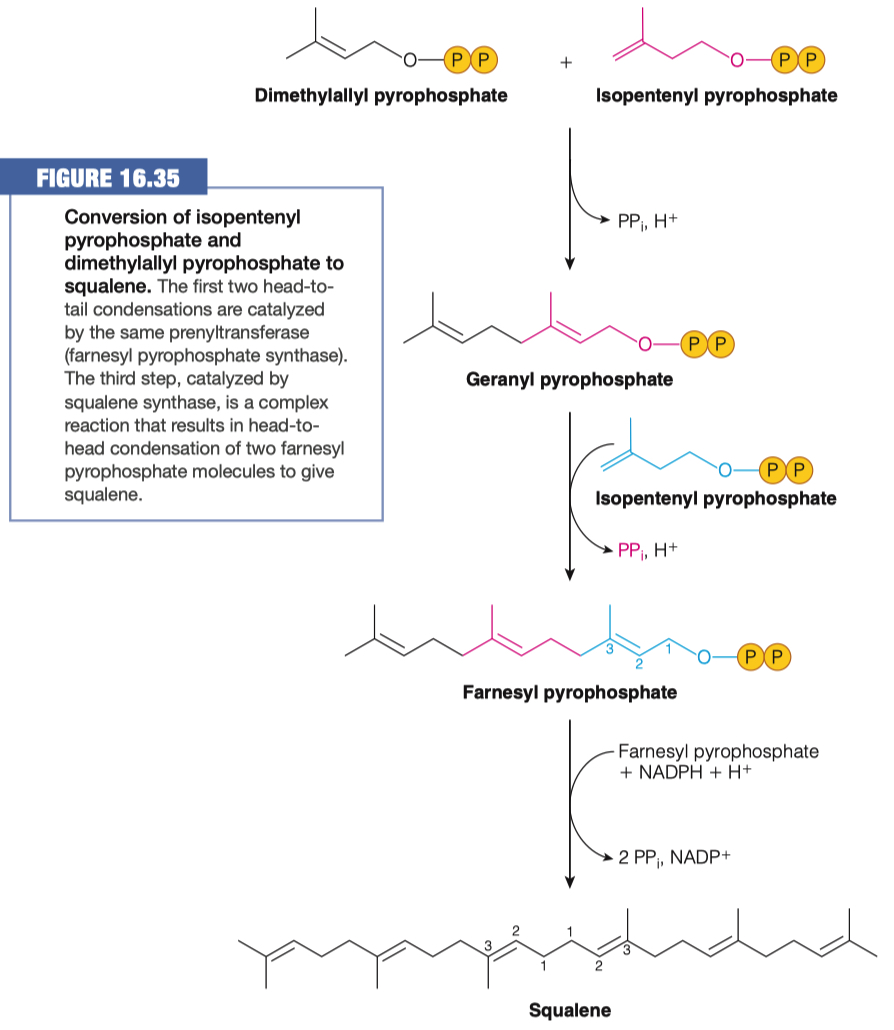
8.A. If one molecule of IPP and one molecule of dimethylallyl pyrophosphate are used in the next step to form geranyl pyrophopsphate (Figure 16.35) how many molecules of acetyl-CoA have been used? How many carbons are in this molecule?
Total Acetyl-CoA Molecules Used in Forming Geraynl pyrophosphate = 6
2 = to make Acetoacetyl-CoA
1 = to build HMG-CoA
HMG-CoA can build both Isopentenyl pyrophosphate and Dimethylallyl pyrophsosphate
- So multiply total by 2 = 6
Total Carbons in Geranyl pyrophosphate = 10
8.B. After the formation of Farnesyl pyrophosphate how many molecules of acetyl-CoA have been used? How many carbons are in this molecule?
- 6 , + 3 more to build another Isopentenyl pyrophosphate = 9 molecules of acetyl-CoA
- Total Carbons in Farnesyl pyrophosphate = 15
8.C. After the formation of squalene how many molecules of acetyl-CoA have been used? How many carbons are in this molecule? Does this make sense?
acetyl-CoA molecules
Total Carbons in Squalene = 30
Total Carbons in 18 acetyl-CoA = 36
- 6 carbons are spontaneously lost as carbon dioxide
- Squalene is cyclized to give rise to cholesterol
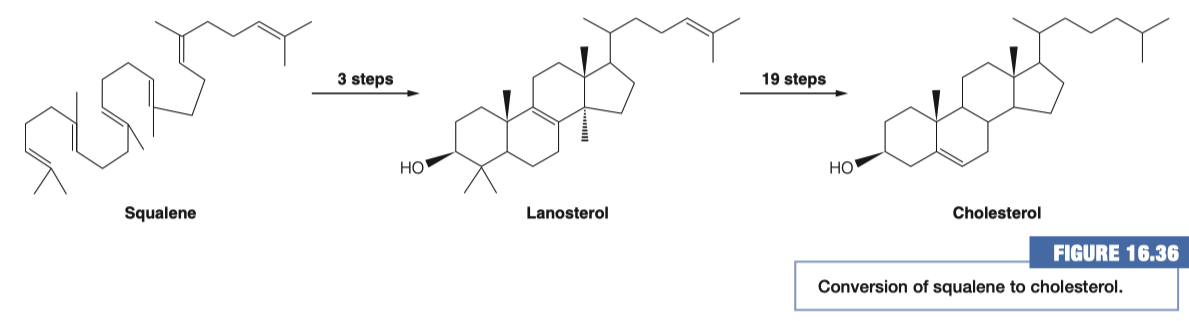
9.A. Where do these reactions take place?
- Endoplasmic Reticulum ( ER )
- everything previous to this step was done in the cytosol
9.B. How many demethylation must take place? Does this make sense based on the # of carbons in squalene and cholesterol?
3 Demethylations
Cholesterol has 27 carbons
Squalene has 30 carbons
- Therefore 3 demethylations must take place
Cholesterol Synthesis Regulation
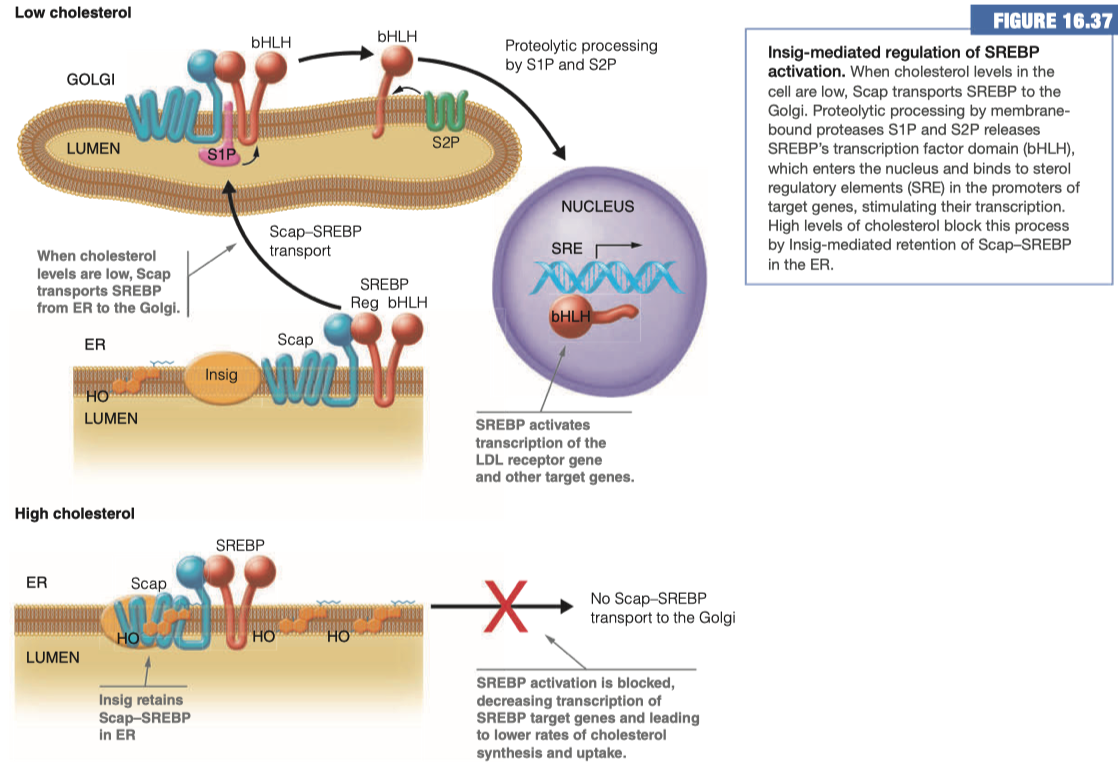
- Use figure 16.37 to how cholesterol formation is regulated in the cell.
We have "Insig" , "Scap" , and "SREBP" all as "players" here
In a HIGH cholesterol situation, Insig attracts Scap and secludes it at the membrane and prevents its interaction with SREBP
This means no Scap-SREBP transport to the Golgi Apparatus
- Which means no connection of the beta helix loop domain into the nucleus to promote the activity of any of our various cholesterol enzymes
In a LOW cholesterol situation the Insig does not interact with Scap.
When Scap and SREBP interact with each other, they are transported over to the Golgi
- Once in the Golgi, Scap-SREBP is chopped in half, and then it goes to the nucleus where it comes in contact with its receptor elements and promotes the activity or the expression of the multiple enzymes involved in cholesterol synthesis and/or uptake
In our body, we are determining how much cholesterol is in each cell.
Then we are regulating how much cholesterol is brought into the cell via these mechanisms, SCAP, SREBP, ect
- And we can decide if we need to make more, use more, or bring more into the cell
- What genes are being regulated by the process above?
SREBP target genes
- LDL receptor gene
- Other target genes
Any of the enzymes associated with cholesterol synthesis
HMG-CoA
Any of the enzymes that make:
- IPP
- Dimethylallyl pyrophosphate
- Geranyl pyrophosphate
- Ect
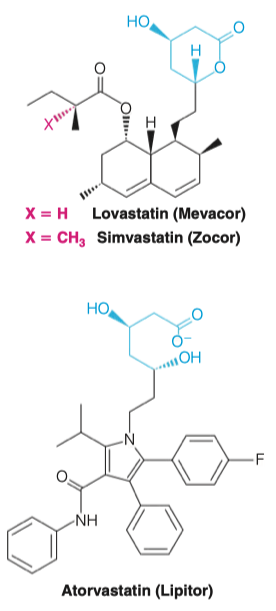
- What are statins? Which particular enzyme do they target and why?
Competitive HMG-CoA reductase inhibitors
- Each statin carries a mevalonate-like moiety (blue), explaining the competitive nature of its activity
Inhibition of HMG-CoA reductase depresses de novo cholesterol biosynthesis and, hence, intracellular cholesterol levels.
This in turn leads to increased production of LDL receptors, allowing more rapid clearance of extracellular cholesterol from the blood
Thus lowering blood cholesterol levels.
They prevent the transition from HMG-CoA to mevalonate
Very similar structure to HMG-CoA
The enzyme that normally uses HMG-CoA will use statins instead
This means it gets "confused" and doesn't end up converting HMG-CoA into mevalonate
If you are not making mavalonate:
- then you are not making IPP or dimethylallyl pyrophosphate
- you also cannot go through any of the remaining steps to make squaline or cyclize it into cholesterol
Statins are competitive inhibitors that we take to lower cholesterol levels inside our bodies through competitive inhibition
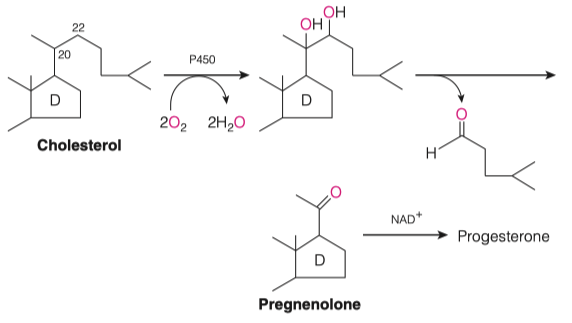
Cholesterol's Role in Steroid Hormone Production
- How are cholesterol and pregnenolone similar to one another?
Similar ring structures
Both cyclic hydrocarbons
Majority of the reaction takes place on ring structure "D"
Some hydroxylation event which facilitates the cleavage of the majority of the "tail" off of cholesterol
- This produces a carbonyl oxygen functional group, aka pregnenolone
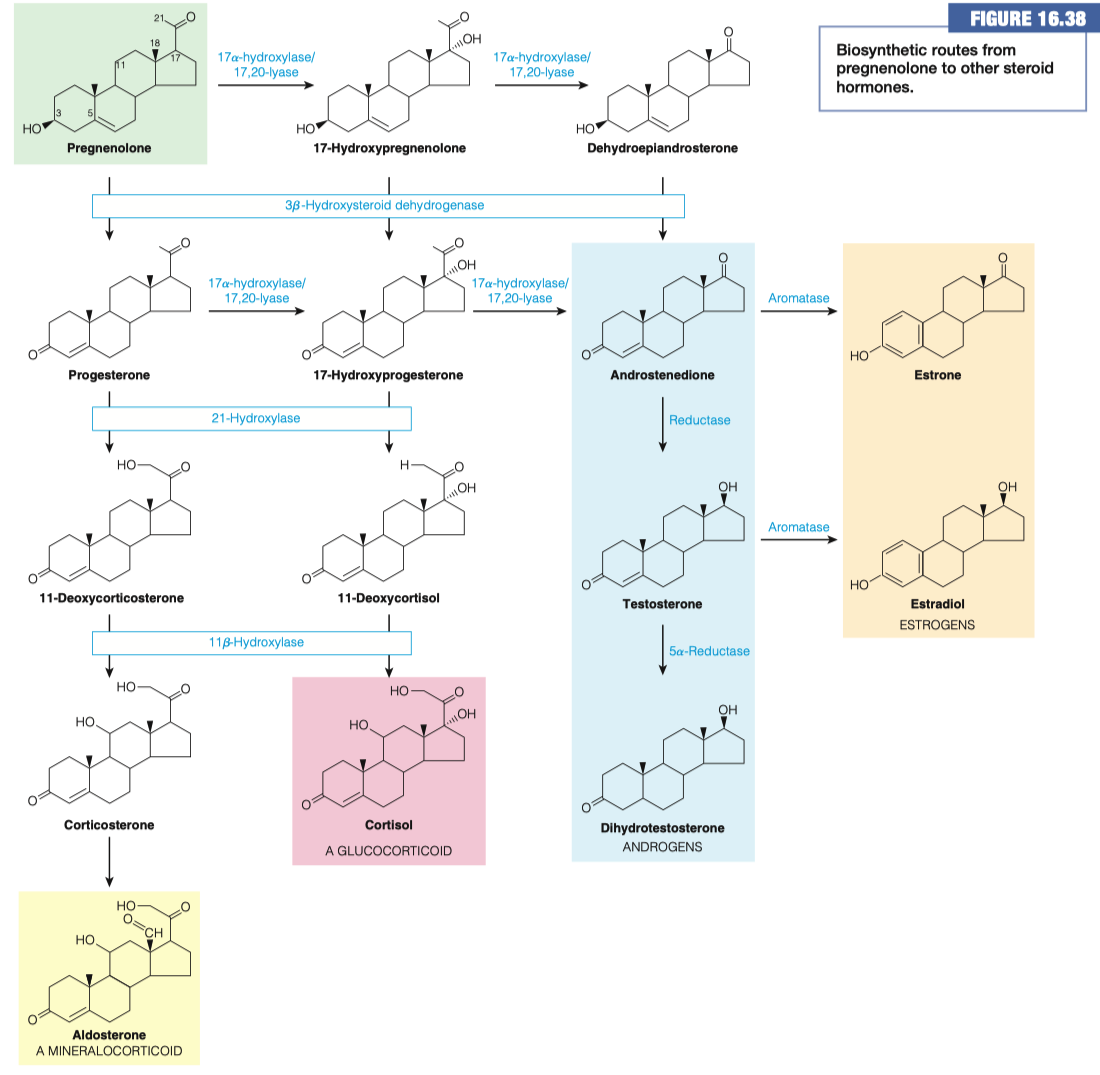
- Without spending too much time on it, review Figure 16.38 and explain how cholesterol is used in the synthesis of various steroid hormones.
Cholesterol is made into pregnenolone
- Pregnenolone is a precursor for all of the different steroids in our body
"Cholesterol is how we get our hormones"
The risk of using statins is they inhibit the production of cholesterol, which would lead to lower levels of steroids and hormones in the body
- Could cause major growth problems in younger populations