Dynamic Study Module - Oxidative Phosphorylation
- Which ATP synthase subunit catalyzes the phosphorylation of ADP?
Beta
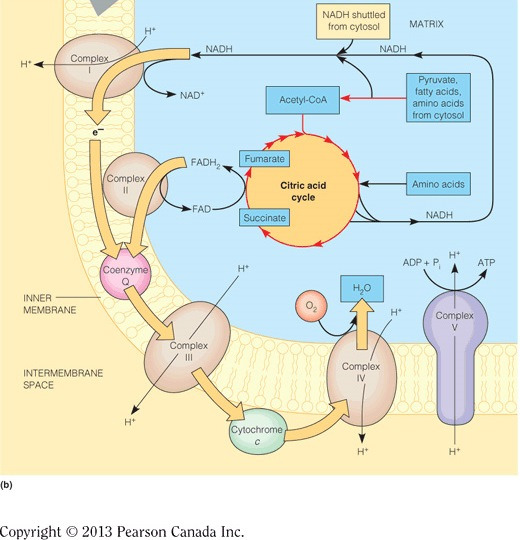
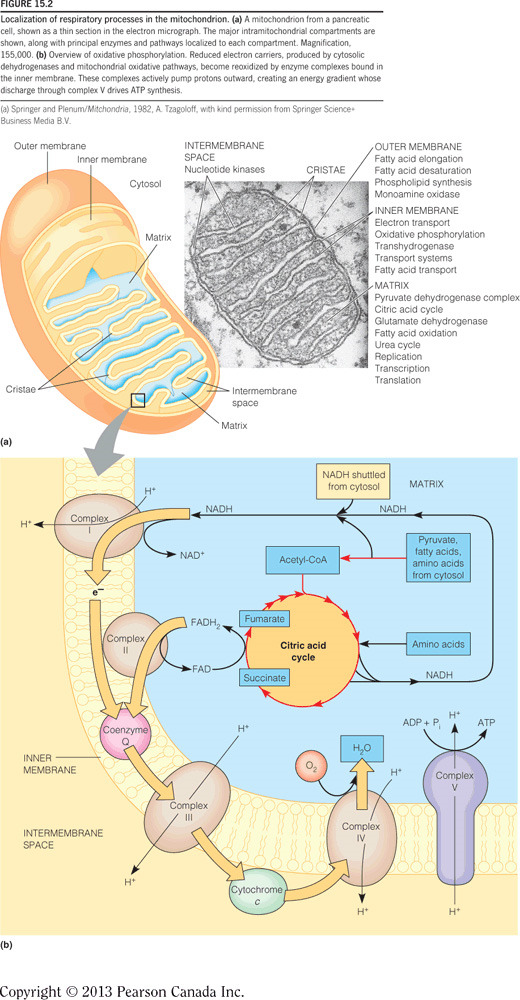
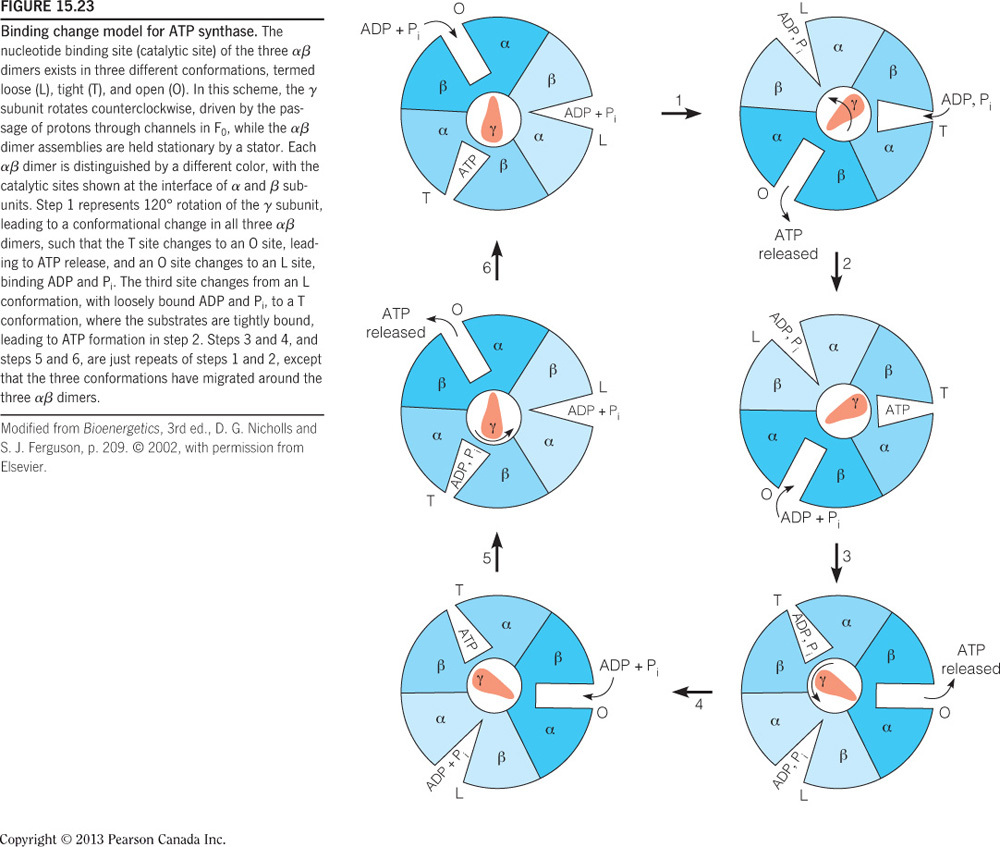
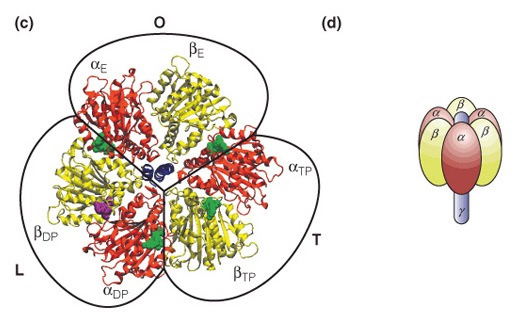
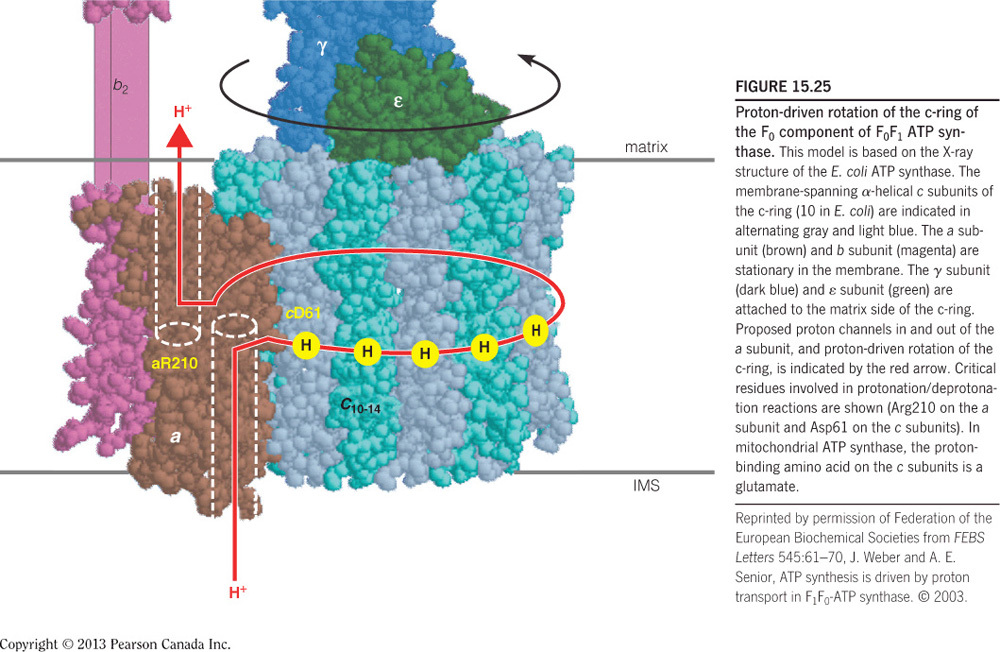
ADP and Pi bind at the interface of one alpha-beta dimer but catalysis occurs in the beta subunits. C10 binds protons in the intermembrane space and subsequently rotates, and since gamma is attached to c10, gamma also rotates. The rotation of gamma pushes the alpha-beta dimers into one of three different conformations. A full rotation will push each dimer once and therefore one ATP molecule is made per gamma 360-degree rotation.
- How many electrons enter Complex I from the complete oxidation of pyruvate?
8
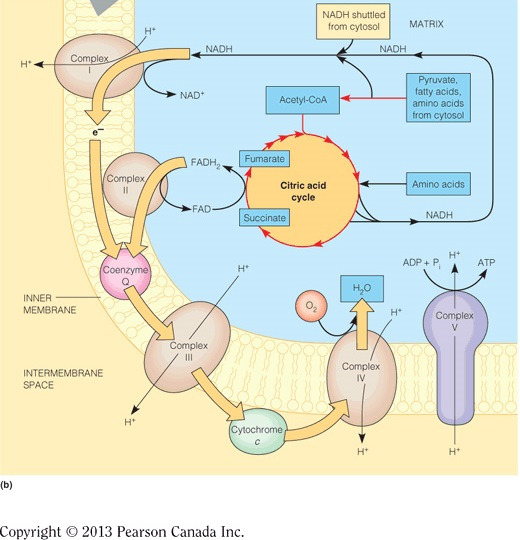
Complete oxidation of pyruvate yields a total of four NADH molecues, one through the pyruvate dehydrogenase complex and three through the citric acid cycle. Each NADH carries two electrons and therefore a total of eight electrons enter into Complex I.
- Which of the following represents the flow of electrons through Complex III?
QH2 → cyt b → Fe-S → cyt c1 → cyt c
The flow of electrons is as follows: QH2 → cyt b → Fe-S → cyt c1 → cyt c
Complex III accepts electrons one at a time from QH2, which are passed through the heme in cytochrome b, then through a series of Fe-S clusters, and then to cytochrome c1. Cyt c1 finally passes electrons, one at a time, to the intermembrane space mobile electron carrier, cyt c. The figure below demonstrates the flow of electrons through all of the complexes in the electron transport chain.
- How many total protons are pumped into the intermembrane space when electrons from NADH are used to reduce oxygen?
10
The electrons stripped from NADH at Complex I will pass to Q, which generates enough energy to pump four protons. QH2 will pass these electrons to Complex III, where they will be delivered to cytochrome c. This passing of electrons generates enough energy to pump another four protons through Complex III. Finally, when cytochrome c passes electrons to Complex IV, which are ultimately used to reduce oxygen to water, another two protons are pumped. In all, 10 protons (4 + 4 + 2 = 10) are pumped per NADH. The figure below illustrates the total number of protons pumped when all complexes are expressed and placed in the correct order.
- When the mitochondrion is actively synthesizing ATP, which region of the mitochondrion has the most basic pH?
Mitochondrial matrix
ATP synthase must utilize the electrochemical gradient, generated by the electron transport chain, to synthesize ATP. Therefore the intermembrane space will have a higher concentration of proton, and thus a lower and more acidic pH. The mitochondrial matrix will have a higher, more basic pH.
- What determines the sequence of electron carriers in the electron transport chain?
Electrons are passed through the complexes in order of increasing reduction potential.
All of the electron carriers in the electron transport chain are in order of increasing reduction potential (i.e., movement is towards the molecule/carrier that wants electrons the most). The highest reduction potential is a positive number, which directly correlates to a negative Gibbs free energy according to the following equation: ∆Go’ = -n F ∆Εo’, where F = 96,500 J/mol K, n = number of electrons, and ∆Εo’ is the change in reduction potential. If electrons are passed towards the more positive reduction potential, then ∆Εo’ will be (+) and Gibbs will be (-).
- ATP synthase derives energy for making ATP from the flow of protons from the __ to the __.
intermembrane space; matrix
ATP synthase derives energy for making ATP from the flow of protons from the intermembrane space to the matrix. In the figure below, ATP synthase is the purple and yellow protein complex. The ETC moves electrons favorably through the complexes and this energy is used to pump protons into the intermembrane space, generating an electrochemical gradient (i.e., separation of charge across a membrane and a separation of concentration). The energy in the electrochemical gradient is harnessed by ATP synthase, which enables protons to flow down their gradient (into the matrix) and toward the positively charged side of the inner membrane. All of this releases energy to drive the phosphorylation of ADP.
- If a person were deficient in iron, which protein complexes in the electron transport chain would be directly affected?
All complexes would be affected.
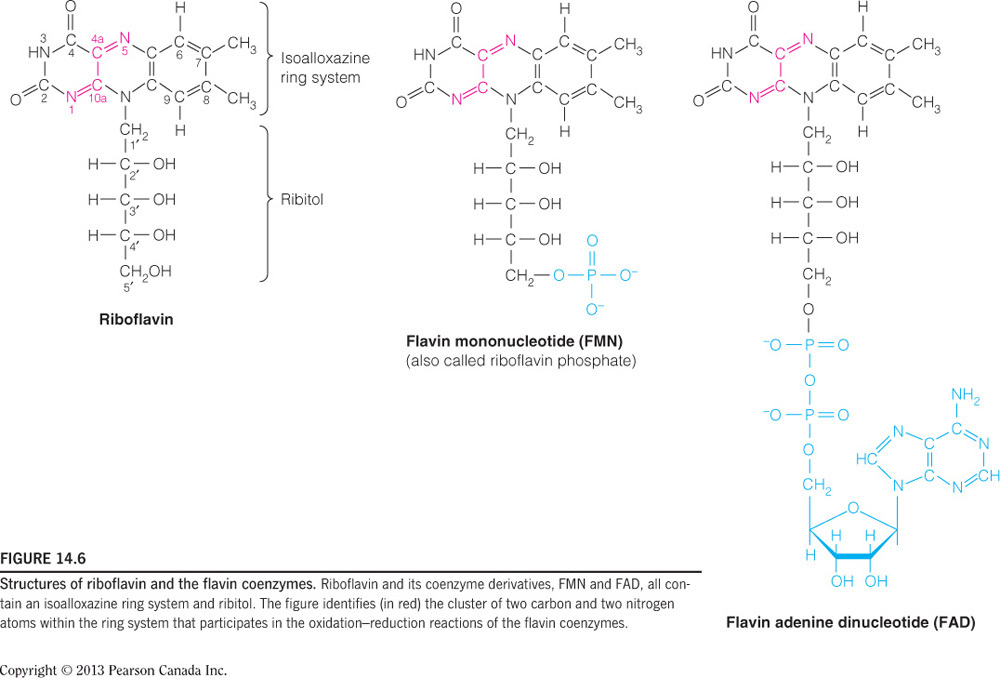
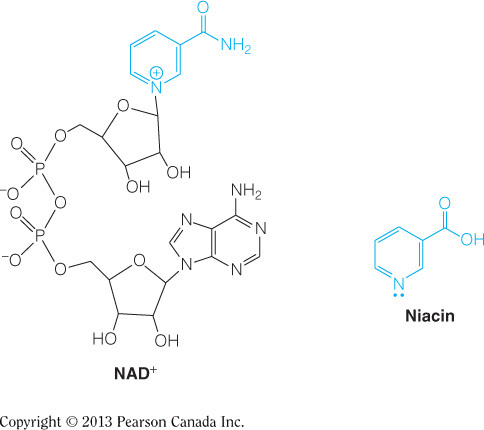
All the complexes rely on iron in different ways. Complexes III and IV utilize hemes (one-part iron and one-part porphyrin) and would be directly affected by an iron deficiency. Complexes I and II use iron-sulfur clusters to pass electrons. Additionally, Complex II oxidizes FADH2, which would be affected by a vitamin B2 (riboflavin) deficiency. Complex I initially passes electrons from NADH to the flavin mononucleotide (FMN), which is also a derivative of riboflavin. Complex I also uses NADH, which would be affected by vitamin B3 (niacin) deficiency.
- How many ATP molecules can be synthesized when the electrons from NADH reduce oxygen, assuming the process is 100 percent efficient? Assume ATP synthesis is ∆Go’= +30 kJ/mol.
7
- Which ATP synthase subunit binds protons from the intermembrane space?
binds protons in the intermembrane space and subsequently rotates and since gamma is attached to c10, gamma also rotates. The rotation of gamma pushes the alpha-beta dimers into one of three different conformations. A full rotation will push each dimer once and therefore one ATP molecule is made per gamma 360-degree rotation. The alpha-beta dimer binds and phosphorylates one ADP at a time and releases ATP.
- If a person were deficient in vitamin B2, which protein complexes in oxidative phosphorylation would be directly affected?
Complex I and Complex II
Complex II oxidizes FADH2, which would be affected by a vitamin B2 deficiency. FAD/FADH2 is a riboflavin molecule covalently bound to ADP, as seen in the figure below. Complex I initially passes electrons from NADH to the flavin mononucleotide (FMN), which is also a derivative of riboflavin, as seen in the figure below. Complex I uses NADH, which would be affected by vitamin B3 (niacin) deficiency. Complexes III and IV utilize hemes and would be directly affected by an iron deficiency.
- Which of the pathways contain steps that oxidize NADH to NAD+?
Oxidative phosphorylation
Oxidative phosphorylation oxidizes NADH at Complex I, and the electrons are passed through the electron transport chain to generate an electrochemical gradient in the mitochondria. Glycolysis, the citric acid cycle, and beta-oxidation do the reverse and generate NADH by reducing NAD+. The figure below demonstrates connectivity between these processes.
- Suppose you isolate a novel bacterial species and discover that this species only expresses Complex I, II, and IV. How would the number of ATP molecules synthesized be affected per NADH?
Approximately one less ATP would be synthesized per NADH.
The electrons will pass from Complex I to IV,because the reduction potentials are still in increasing order. However, the lack of Complex III means that four fewer protons will be pumped per NADH. This means that approximately one less ATP will be synthesized for each NADH (because three protons = one ATP) and only two molecules of ATP can be synthesized per NADH. Compare this to 10 protons that are normally synthesized per NADH (approximately three ATPs). The decrease is due to the inefficient transfer of electrons. The figure below illustrates the total number of protons pumped when all complexes are expressed and placed in the correct order.
- Which protein complex in oxidative phosphorylation containing hemes would be greatly impacted by site-directed mutagenesis of a conserved Cys?
Complex III
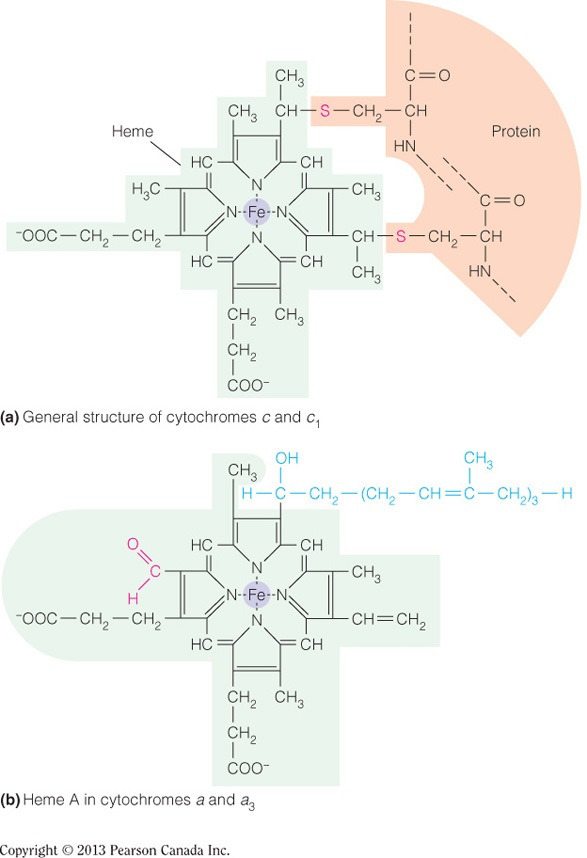
The type c hemes, found in Complex III, are covalently linked to the protein via thiol groups from conserved cysteine residues (as illustrated in the figure below). Complexes I and II do not contain hemes and neither does ATP synthase. Type a hemes, found in Complex IV, are associated via hydrophobic interactions between the isoprenoid functional group and hydrophobic residues.
- In mammalian cells, where does the electron transport chain take place?
Inner membrane of mitochondria
In mammalian or eukaryotic cells, the electron transport chain is embedded in the inner membrane of the mitochondria (see figure). In cells lacking mitochondria, like bacteria, the electron transport chain is embedded in the cytoplasmic membrane.
- Which of the following is the ETC mobile electron carrier that is soluble in the intermembrane space?
Cytochrome c
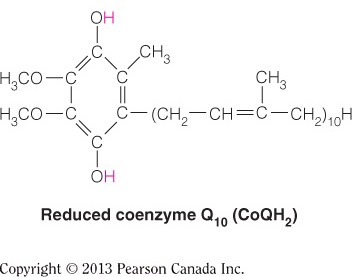
Cytochrome c is a mobile electron carrier that transports electrons from Complex III to Complex IV in the intermembrane space, which is an aqueous environment. Therefore, cytochrome c is the hydrophilic electron carrier. QH2 is the mobile electron carrier soluble in the inner membrane that passes electrons to Complex III. This molecule has a hydrophobic isoprenoid functional group that enables it to be soluble in the inner membrane. Cytochromes b and c1 are part of Complex III and are not mobile. Cytochrome a3 is one of the cytochromes in Complex IV, which is also not a mobile electron carrier.
- Which protein complex in oxidative phosphorylation directly uses ?
Complex IV
Complex IV (aka cytochrome c oxidase) transfers electrons from cytochrome c to cytochrome a3, where oxygen is reduced to water (see figure below). Complex I oxidizes NADH, Complex II oxidizes FADH2, and Complex III transfers electrons from QH2 to cytochrome C. All of these complexes, except II, pump protons to generate an electrochemical gradient and the energy produced is harnessed by ATP synthase to phosphorylate ADP.
- How many different conformations can the alpha-beta dimers of ATP synthase assume?
3
The alpha-beta dimers change conformation three times per turn as dictated by gamma. The three conformations are (1) open (O), which binds ADP and Pi; (2) loose (L), which locks in ADP and Pi; and (3) tight (T), wherein ATP is formed. The figure below illustrates the different conformations. Note that the alpha-beta subunits are not rotating but gamma is rotating in the middle of these dimers and pushes each into a new conformation.
- Most of the oxygen that we take in by breathing is converted to _____?
water
Oxygen is only directly used in the electron transport chains as the final electron acceptor. In complex IV, oxygen is reduced to water. Carbon dioxide is produced in the catabolic pathways that oxidize hydrocarbons (i.e., citric acid cycle, pyruvate dehydrogenase complex, etc.).
- If a person were deficient in vitamin B3, which protein complex in oxidative phosphorylation would be directly affected?
Complex I
Complex I oxidizes NADH, which would be affected by a vitamin B3 (niacin) deficiency. NAD+/NADH is a derivatized niacin molecule covalently bound to ADP, as seen in the figure below. Complex II uses FAD as a substrate, which would be affected by vitamin B2 (riboflavin) deficiency. Complexes III and IV utilize hemes and would be directly affected by an iron deficiency.
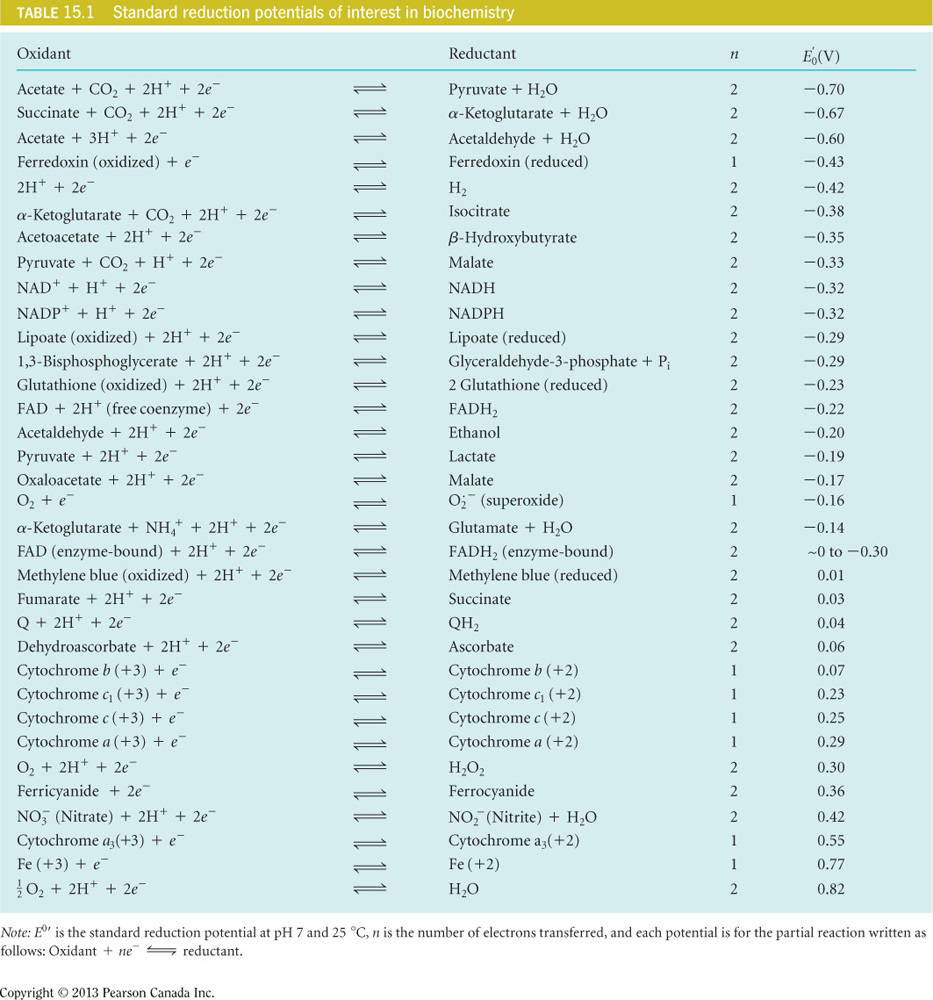
What is the free energy when the electrons from NADH reduce oxygen?
(F= 96,500 J/mol K)

-220 kJ/mol
Here , n = 2 because two electrons are passed as seen in the table. F = 96,500 J/mol V. is determined by adding the reduction potentials of the two half reactions appropriately. In the table, all reactions are set up to show acceptance of electrons. In oxidation-reduction reactions, only one molecule can accept electrons and the other can donate electrons. For this reason, one of the half reactions needs to be flipped. The problem states that the electrons from NADH reduce oxygen and therefore you will flip the half-reaction for NADH and also flip the sign on the reduction potential to become +.32 V. Adding the two half reactions will now cancel the electrons (which are balanced) and then you can add the reduction potentials: 0.82 V + 0.32 V = 1.14 V = . Plugging these values into the equation gives a free energy of -220 kJ/mol of energy that is released with electrons that are passed from NADH to oxygen.
- Which of the following is the ETC mobile electron carrier that is soluble in the inner membrane?
QH2
QH2 is the mobile electron carrier soluble in the inner membrane that passes electrons to complex III. This molecule has a hydrophobic isoprenoid functional group, as illustrated by the figure below, that enables it to be soluble in the inner membrane. Cytochrome c is a mobile electron carrier that transports electrons from Complex III to Complex IV in the intermembrane space, which is an aqueous environment and therefore cytochrome c is the hydrophilic electron carrier. Cytochromes b and c1 are part of Complex III and are not mobile. Cytochrome a3 is one of the cytochromes in Complex IV, which is also not a mobile electron carrier.
- How many electrons enter Complex II from complete oxidation of pyruvate?
2
Complete oxidation of pyruvate through the pyruvate dehydrogenase complex and citric acid cycle will yield one FADH2 molecule, which carries two electrons. Therefore, two electrons enter into Complex II.
- In mammalian cells, where is the ATP synthase protein complex located?
Inner membrane of mitochondria
In mammalian or eukaryotic cells, ATP synthase is embedded in the inner membrane of the mitochondria, with the phosphorylation subunits on the matrix side (see figure). In cells lacking mitochondria, like bacteria, ATP synthase is embedded in the cytoplasmic membrane.
- How many total ATP molecules can be generated for each NADH, assuming oxidative phosphorylation is 100 percent efficient?
3.3
Assuming oxidative phosphorylation is 100 percent efficient, then one NADH will equal 10 protons pumped through the electron transport chain. Three protons are need for one ATP and therefore 3.3 ATPs are yielded from one NADH. The process, however, is not 100 percent efficient and therefore, when talking about oxidative phosphorylation, this number is approximated to 2.5 ATP molecules per NADH.
- Which of the following represents the flow of electrons through Complex IV?
cyt c → cyt a → cyt a3 → O2
The flow od electrons is as follows: cyt c → cyt a → cyt a3 → O2
Complex IV transfers accepted electrons from cyt c and passes them to cyt a and then to cyt a3, where oxygen is reduced to water. The figure below demonstrates the flow of electrons through all of the complexes in the electron transport chain.
- Which of the pathways contain steps that oxidize NADH to NAD+?
Oxidative phosphorylation
Oxidative phosphorylation oxidizes NADH at Complex I, and the electrons are passed through the electron transport chain to generate an electrochemical gradient in the mitochondria. Glycolysis, the citric acid cycle, and beta-oxidation do the reverse and generate NADH by reducing NAD+. The figure below demonstrates connectivity between these processes.
- Suppose you isolate a novel bacterial species and discover that this species only expresses Complex I, II, and IV. How would the number of ATP molecules synthesized be affected per NADH?
Approximately one less ATP would be synthesized per NADH.
The electrons will pass from Complex I to IV, because the reduction potentials are still in increasing order. However, the lack of Complex III means that four fewer protons will be pumped per NADH. This means that approximately one less ATP will be synthesized for each NADH (because three protons = one ATP) and only two molecules of ATP can be synthesized per NADH. Compare this to 10 protons that are normally synthesized per NADH (approximately three ATPs). The decrease is due to the inefficient transfer of electrons. The figure below illustrates the total number of protons pumped when all complexes are expressed and placed in the correct order
- Which of the following is NOT a two-electron carrier?
Cytochrome c
Cytochrome c is a one-electron carrier (not a two-electron carrier) that transports electrons one at a time from Complex III to IV. QH2 is a two-electron carrier that can accept electrons from either Complex I or Complex II. These electrons are dropped off at Complex III one at a time. The two electrons from NADH are passed to QH2 in Complex I and the two electrons from FADH2 are also passed to QH2 in Complex II.
- Oxidative phosphorylation refers to __.
the electron transport chain coupled to ATP synthesis
Oxidative phosphorylation refers to the coupling of the electron transport chain, where oxidation of NADH and FADH2 occurs, with ATP synthesis, where phosphorylation of ADP occurs. Glycolysis, the citric acid cycle, and beta-oxidation supply the reduced coenzymes, NADH and FADH2, to the electron transport chain.
- Which protein complex in oxidative phosphorylation oxidizes NADH?
Complex I
Complex I (aka NADH dehydrogenase) oxidizes NADH on the matrix side of the inner mitochondrial membrane (see the figure below). Here, the two electrons stripped from NADH are initially passed to flavin mononucleotide (FMN), then are passed through a series of Fe-S clusters, and are finally transferred to the mobile electron carrier: coenzyme Q (Q). Complex II oxidizes FADH2. Complex III transfers electrons from QH2 to cytochrome C. Complex IV transfers electrons from cytochrome C to heme a3, where oxygen is reduced to water. All of these complexes, except II, pump protons to generate an electrochemical gradient; the energy from which is harnessed by ATP synthase to phosphorylate ADP.
- Which of the following enzymes would NOT be expected to contribute electron carriers to oxidative phosphorylation?
Lactate dehydrogenase
Lactate dehydrogenase oxidizes NADH in the cytosol, and it therefore would not contribute reduced coenzymes to the electron transport chain, which occurs in the inner mitochondrial membrane. Malate dehydrogenase (TCA cycle), succinate dehydrogenase (TCA cycle), and GAP dehydrogenase (glycolysis) all yield the reduced coenzyme NADH, which enters the electron transport chain at Complex I. Succinate dehydrogenase (TCA cycle and part of complex II) passes electrons from succinate to coenzyme Q.
- Which protein complex in oxidative phosphorylation generates ?
None of the listed protein complexes in oxidative phosphorylation generates CO2.
None of the protein complexes in oxidative phosphorylation generates carbon dioxide. The catabolic pathways, like glycolysis and the TCA cycle, are where carbons become oxidized. The electrons gained from oxidizing these carbons are transferred to NADH and FADH2. These electrons are finally used to reduce oxygen in the electron transport chain. Complex I oxidizes NADH, Complex II oxidizes FADH2, Complex III transfers electrons from QH2 to cytochrome c, and Complex IV transfers electrons from cyt c to heme a3 where oxygen is reduced to water. All of these complexes, except II, pump protons to generate an electrochemical gradient, the energy from which is harnessed by ATP synthase to phosphorylate ADP.
- Which protein complex in oxidative phosphorylation generates an NTP ( where N = any nitrogenous base ) ?
ATP synthase
ATP synthase (in purple in the figure) harnesses the electrochemical gradient energy generated by the electron transport chain to phosphorylate ADP and thereby generate ATP. Complex I oxidizes NADH, Complex II oxidizes FADH2, Complex III transfers electrons from QH2 to cytochrome c, and Complex IV transfers electrons from cyt c to cytochrome a3, where oxygen is reduced to water. All of these complexes, except II, pump protons to generate an electrochemical gradient and the energy produced is harnessed by ATP synthase to phosphorylate ADP.
- When the mitochondrion is actively synthesizing ATP, which region of the mitochondrion has the most basic pH?
Mitochondrial matrix
ATP synthase must utilize the electrochemical gradient, generated by the electron transport chain, to synthesize ATP. Therefore the intermembrane space will have a higher concentration of proton, and thus a lower and more acidic pH. The mitochondrial matrix will have a higher, more basic pH.
- The ADP/Pi binding and catalytic subunits of ATP synthase can best be described as __.
trimer of dimers
The subunits that bind ADP and Pi and then catalyze the reaction are a dimer pair called alpha-beta (as seen in the figure below). These are heterodimers, as beta and alpha have different primary sequences. In one ATP synthase, there are three alpha-beta dimers and therefore the best description of these are trimer of dimers. A homohexamer indicates that all of the six subunits have the same primary sequence. The alpha-beta trimer of dimers could also be described as a heterohexamer, but this, however, is less descriptive.
Which of the following is the overall reaction for the reduction of oxygen by NADH?

NADH + O2 + H+ → NAD+ + H2O
In the table, all reactions are set up to show acceptance of electrons. In oxidation-reduction reactions, only one molecule can accept electrons and the other can donate electrons. For this reason, one of the half reactions needs to be flipped. The problem states that the electrons from NADH reduce oxygen and therefore you will flip the half-reaction for NADH and also flip the sign on the reduction potential to become +.32 V. The flipped half reaction for NADH will be : NADH + O2 + H+ → NAD+ + H2O. When the two half reactions are added, the electrons and protons on both sides of the equation cancel each other out and can be removed in the final equation.
- The function of thermogenin, a protein expressed in brown adipose tissue, is to __.
uncouple the ETC from ATP synthase, so the chemiosmotic potential generates heat instead of ATP
Thermogenin is a protein expressed in the mitochondria of brown adipose cells. This protein is embedded in the inner membrane and functions to uncouple ETC from ATP synthesis, by allowing the protons in the intermembrane space to freely diffuse down their concentration gradient into the mitochondrial matrix. This generates energy that is not captured to phosphorylate ADP; instead, it is dissipated as heat.
- How many total protons are pumped into the intermembrane space when electrons from FADH2 are used to reduce oxygen?
6
The electrons stripped from FADH2 at Complex II will pass to Q. QH2 will pass these electrons to Complex III, where they will be delivered to cytochrome c. This passing of electrons generates enough energy to pump another four protons through complex III. Finally, when cytochrome c passes electrons to Complex IV, which are ultimately used to reduce oxygen to water, another two protons are pumped. In all, six protons (4 + 2 = 6 ) are pumped per FADH2. The figure below illustrates the total number of protons pumped when all complexes are expressed and placed in the correct order. Although Complex II is not shown, it resides in between complex I and III.
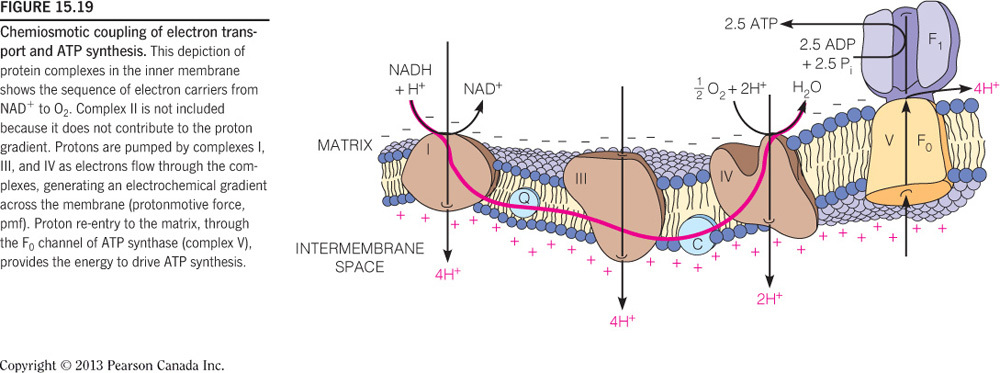
- How many total ATP molecules can be generated for each FADH2, assuming oxidative phosphorylation is 100 percent efficient?
2
Assuming oxidative phosphorylation is 100 percent efficient, then one FADH2 will equal six protons pumped through the electron transport chain. Three protons are need for one ATP and therefore two ATPs are yielded from one FADH2. The process, however, is not 100 percent efficient and therefore, when talking about oxidative phosphorylation, this number is approximated to 1.5 ATP molecules/FADH2.
- Which protein complex in oxidative phosphorylation oxidizes FADH2?
Complex II
Complex II (aka succinate-coenzyme Q reductase) oxidizes FADH2 in the inner mitochondrial membrane (see figure below). This protein is part of the citric acid cycle, where succinate is oxidized to fumarate and the electrons are passed to FAD. The two FADH2 electrons are then stripped and initially passed through a series of Fe-S clusters before finally being transferred to the mobile electron carrier: coenzyme Q (QH2). Complex I oxidizes NADH, Complex III transfers electrons from QH2 to cytochrome c, and Complex IV transfers electrons from cytochrome c to cytochrome a3, where oxygen is reduced to water. All of these complexes, except II, pump protons to generate an electrochemical gradient and the energy produced is harnessed by ATP synthase to phosphorylate ADP.
- Suppose you isolate a novel bacterial species and discover that this species only expresses Complex I, II, and IV. How would the number of ATP molecules synthesized be affected per NADH?
Approximately one less ATP would be synthesized per NADH.
The electrons will pass from Complex I to IV,because the reduction potentials are still in increasing order. However, the lack of Complex III means that four fewer protons will be pumped per NADH. This means that approximately one less ATP will be synthesized for each NADH (because three protons = one ATP) and only two molecules of ATP can be synthesized per NADH. Compare this to 10 protons that are normally synthesized per NADH (approximately three ATPs). The decrease is due to the inefficient transfer of electrons. The figure below illustrates the total number of protons pumped when all complexes are expressed and placed in the correct order.
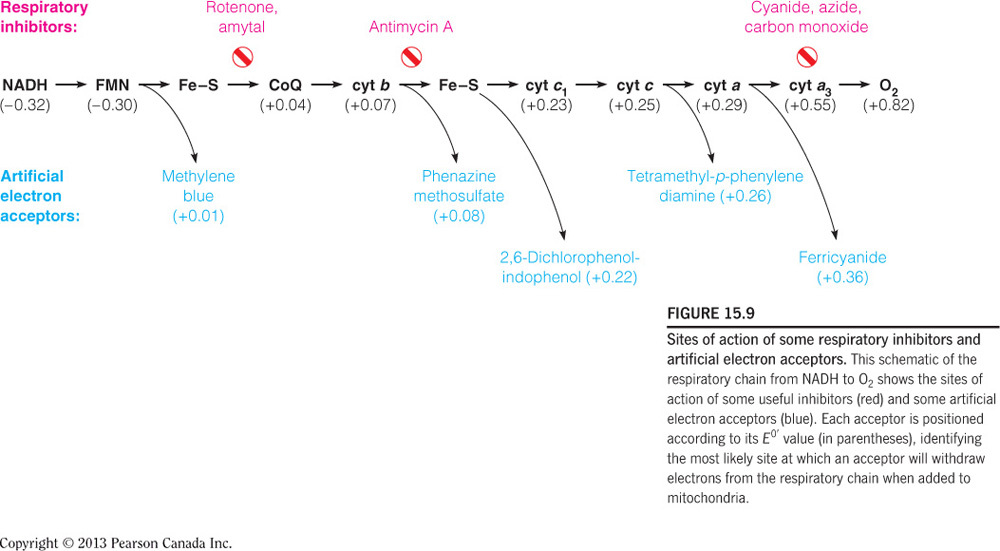
- Chemical asphyxiation occurs on which protein complex in oxidative phosphorylation?
Complex IV
Chemical asphyxiation occurs when a competitive inhibitor molecule (i.e., a chemical) binds to cytochrome a, which is a part of Complex IV. This causes asphyxiation (inability of molecular oxygen to be used by the body) because cytochrome a is the only cytochrome in the electron transport chain that binds oxygen. Inhibitors of this cytochrome, like CO, can also bind to other cytochromes; however, because oxygen doesn’t bind there, it is not considered chemical asphyxiation. The figure below illustrates that there are several modes of inhibition of the electron transport chain, but again only those inhibitor binding at cyt a3 will result in chemical asphyxiation.
- Which of the following ATP synthase subunits rotate?
Gamma and
The rotary subunit on ATP synthase is , which rotates when protons binds. Gamma** is bound to c10 and therefore it also rotates (as seen in the figure below). The rotational movement of gamma pushes the alpha/beta dimers into new conformations but these dimers do not rotate. The alpha-beta dimer binds and phosphorylates one ADP at a time and releases ATP.