Dynamic Study Module - Citric Acid Cycle
- Citrate synthase is inhibited by high concentrations of ___.
NADH
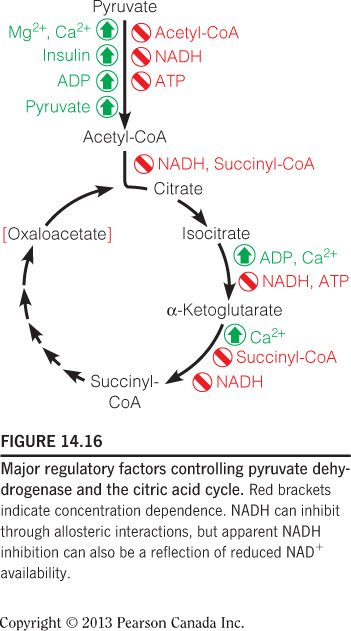
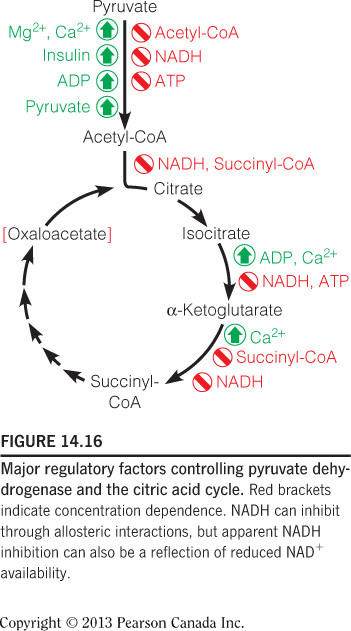
High [NADH] inhibits citrate synthase, as this is a signal that oxygen concentration is low and/or ATP no longer needs to be made. High [NADH] also inhibits the other two irreversible reactions in the citric acid cycle. High [ADP] and [Ca2+] are allosteric activators of the citric acid cycle at other steps. High [ATP] inhibits isocitrate dehydrogenase. This figure summarizes all of the regulatory affector molecules and their respective enzyme targets.
- Which enzyme-catalyzed reaction in the citric acid cycle generates CO2?
The reaction catalyzed by isocitrate dehydrogenase
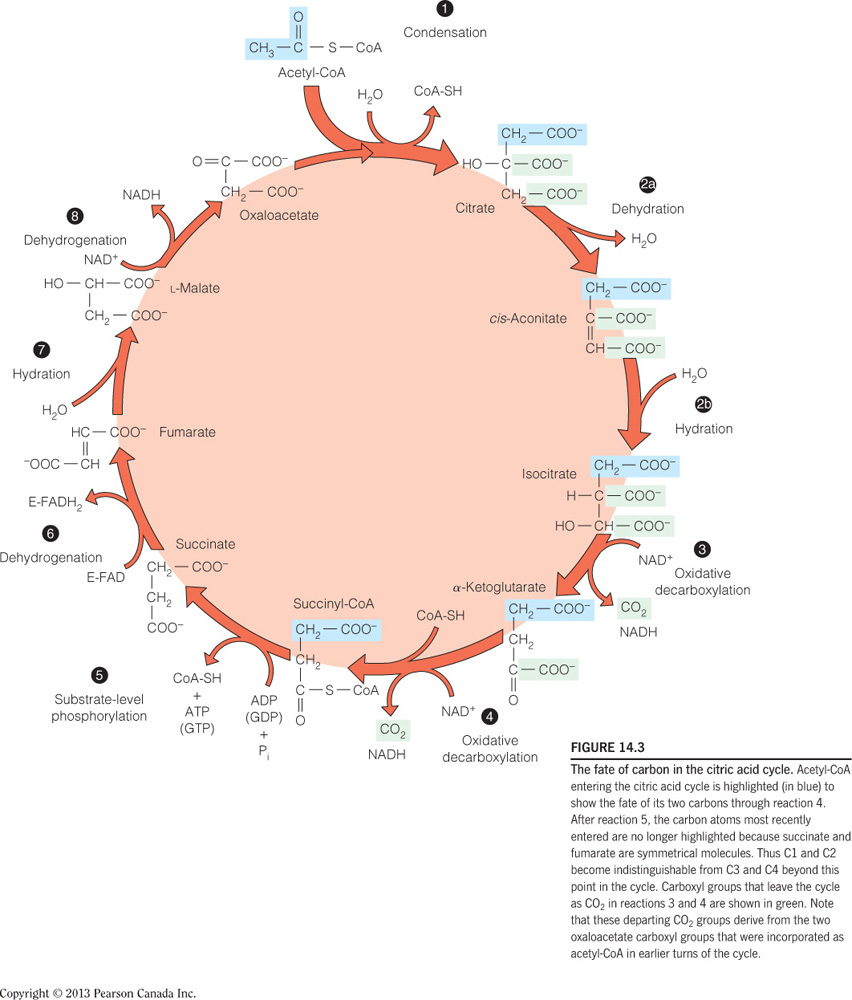
Carbon dioxide is generated in two reactions of the citric acid cycle. One is catalyzed by isocitrate dehydrogenase and the other by alpha-ketoglutarate dehydrogenase. NADH is a product of the reaction catalyzed by malate dehydrogenase, ATP (GTP) and CoA are products of the succinyl-CoA synthetase reaction, and CoA is a product of the reaction catalyzed by citrate synthase.
- The first reaction of the citric acid cycle, where acetyl-CoA and __ combine to form citrate, is catalyzed by __.
OAA; citrate synthase
The first enzyme-catalyzed reaction of the citric acid cycle is catalyzed by citrate synthase. This reaction is irreversible and it combines OAA and acetyl-CoA to produce a six-carbon intermediate called citrate. Isocitrate dehydrogenase catalyzes the second irreversible reaction in TCA, where isocitrate and NAD+ are substrates. Alpha-ketoglutarate dehydrogenase catalyzes the third irreversible reaction, where alpha-ketoglutarate and NAD+ are substrates.
How many carbon atoms do each of the following molecules contain?
(1) citrate (2) alpha-ketoglutarate (3) OAA (4) Acetyl group on CoA
6, 5, 4, 2
During the first reaction of the citric acid cycle, a four-carbon molecule (OAA) combines with the two-carbon carrier (acetyl-CoA) to form the six-carbon intermediate citrate. During the oxidation of intermediates in the citric acid cycle, oxidized carbons leave as carbon dioxide, and after the first carbon dioxide leaves, a five-carbon intermediate (alpha-ketoglutarate) remains. A second carbon dioxide molecule leaves in the next reaction to form succinyl-CoA (which has four carbons). All of the remaining intermediates then have four carbons until OAA is regenerated and another acetyl-CoA combines with it to restart the process.
- Which of the following coenzymes is NOT tightly/covalently bound to the pyruvate dehydrogenase complex (PDC)?
NAD+
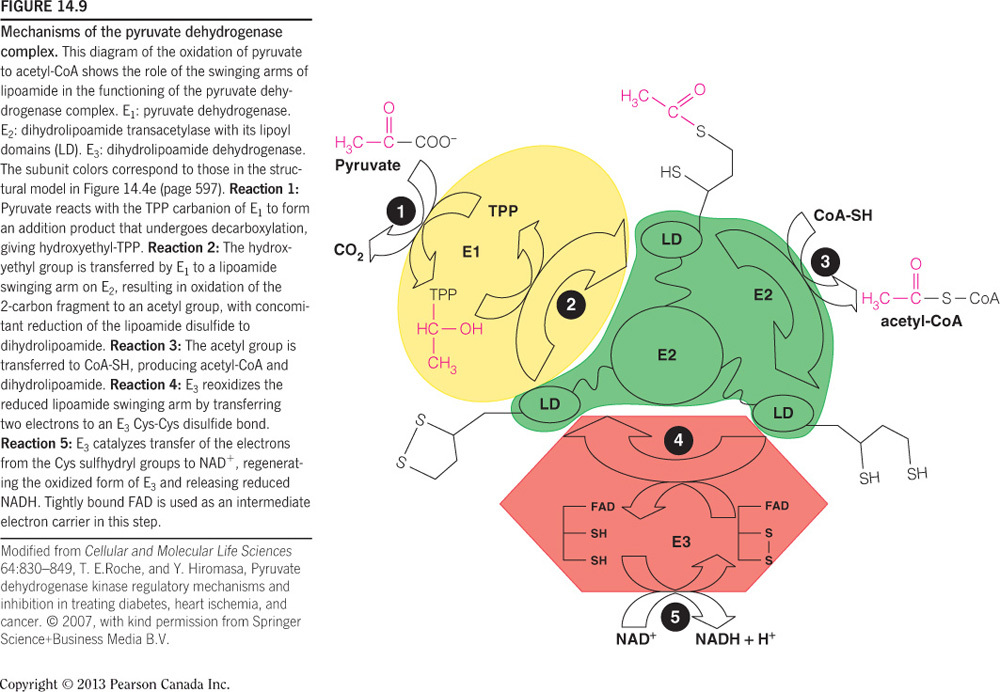
NAD+ and CoA are the two coenzymes that are not covalently or tightly associated with the PDC. Both of these molecules enter and leave their respective subunits. CoA is associated with E2 and NAD+ is associated with E3. TPP is tightly bound to E1, lipoic acid (i.e., lipoamide) is covalently bound to E2, and FAD is tightly bound to E3.
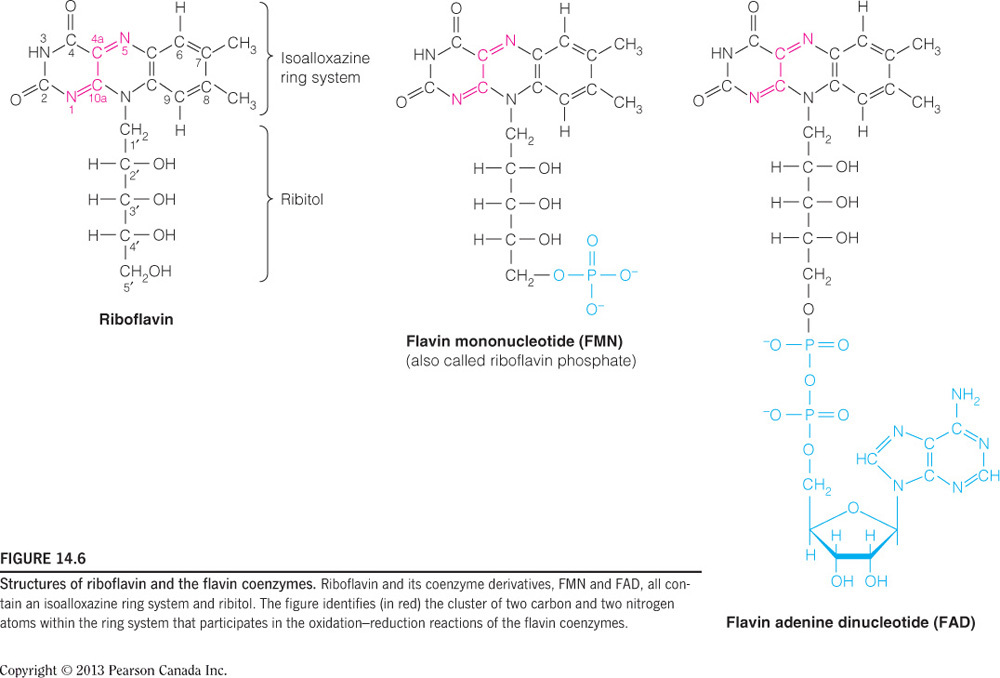
- If a person were deficient in vitamin B2, what enzyme-catalyzed reaction in the citric acid cycle would be directly affected?
The reaction catalyzed by succinate dehydrogenase
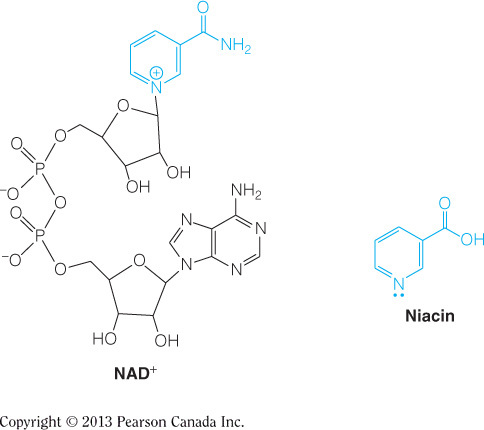
Succinate dehydrogenase uses FAD as a substrate, which is a nucleotide covalently bound to vitamin B2 (riboflavin). Malate dehydrogenase uses NAD+, which would be affected by a vitamin B3 (niacin) deficiency. Isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase would also be directly affected by a deficiency in B3. CoA is a product of reactions catalyzed by citrate synthase and succinyl-CoA dehydrogenase, which would be affected by a vitamin B5 deficiency.
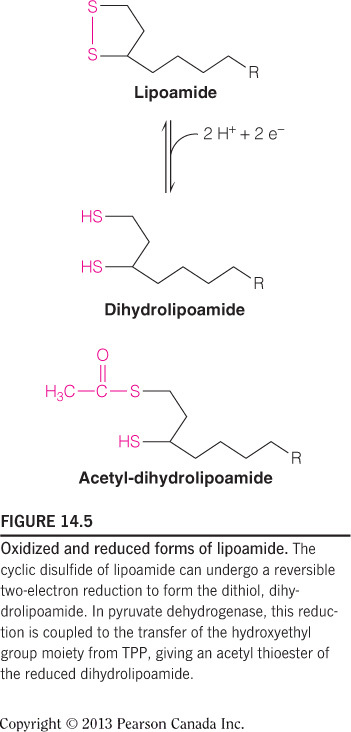
- During the reaction catalyzed by the pyruvate dehydrogenase complex (PDC), the acetyl group taken from pyruvate is covalently linked to what type of atom in lipoamide?
Sulfur
Lipoamide accepts the acetyl group from thiamin pyrophosphate (TPP) by forming a thioester bond; therefore the acetyl group is linked to a sulfur atom. This figure shows the carbonyl of the acetyl group covalently bound to the thiol sulfur of lipoamide.
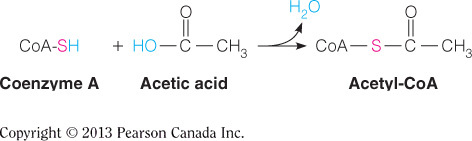
- The acetyl group in acetyl coenzyme A is covalently linked to what type of atom?
Sulfur
CoA accepts the acetyl group from lipoamide by forming a thioester bound; therefore, the acetyl group is covalently bound to a sulfur atom. This figure shows the carbonyl of the acetyl group covalently bound to the thiol sulfur of CoA.
- Which enzyme-catalyzed reaction in the citric acid cycle generates NAD+?
None of the citric acid cycle reactions generate NAD+.
None of the citric acid cycle reactions generate NAD+. Malate dehydrogenase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase use NAD+ as a substrate for their respective oxidation-reduction reactions. Succinate dehydrogenase uses FAD as a substrate for its oxidation-reduction reaction. It is notable that NAD+ is indirectly regenerated for use as a substrate in the citric acid cycle when NADH is oxidized at Complex I of the electron transport chain. As this reaction occurs on the mitochondrial matrix side of the inner membrane, the ETC provides an indirect source of NAD+ as long as molecular oxygen is available.
- Which of the following enzyme-catalyzed reactions in the citric acid cycle generates NADH?
The reaction catalyzed by malate dehydrogenase
Malate dehydrogenase catalyzes the oxidation of malate to OAA, where two electrons are removed to reduce NAD+ to NADH. Isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase also catalyze oxidation-reduction reactions that generate NADH. The reaction using succinyl-CoA synthase generates ATP (GTP) and CoA. Succinate dehydrogenase also catalyzes an oxidation-reduction reaction but FAD is reduced to FADH2.
- If a person were deficient in vitamin B5, which enzyme-catalyzed reaction in the citric acid cycle would be directly affected?
The reaction catalyzed by alpha-ketoglutarate dehydrogenase
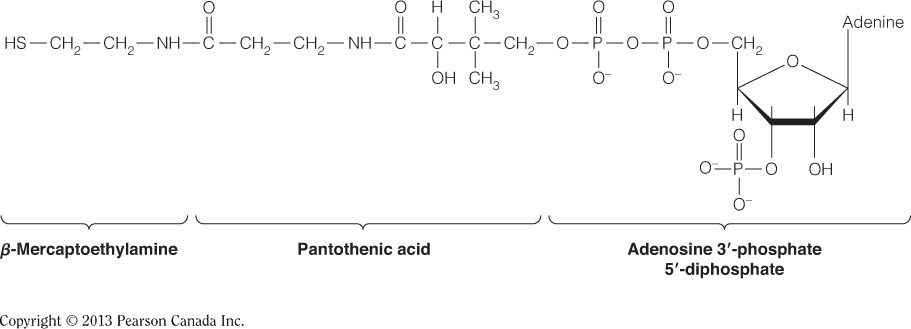
Vitamin B5 is pantothenic acid, which is added to a nucleotide to become coenzyme A (CoA). The only enzyme in the citric acid cycle that directly uses CoA as a substrate is alpha-ketoglutarate dehydrogenase. CoA is a product of reactions catalyzed by citrate synthase and succinyl-CoA dehydrogenase. Malate dehydrogenase uses NAD+, which would be affected by a vitamin B3 (niacin) deficiency, and succinate dehydrogenase uses FAD as a substrate, which would be affected by a vitamin B2 (riboflavin) deficiency.
- Which of the following enzymes does NOT catalyze an irreversible reaction in the citric acid cycle?
Succinate dehydrogenase
Succinate dehydrogenase catalyzes the sixth step in the citric acid cycle where succinate is oxidized by the fumarate. The Gibbs free energy of this reaction is nearly zero and therefore it is not irreversible. This reaction is pulled forward by the preceding reaction catalyzed by citrate synthase, which is irreversible, due to the reaction having a large negative Gibbs free energy. Isocitrate dehydrogenase (step 3) and alpha-ketoglutarate dehydrogenase (step 4) also catalyze irreversible reactions and large, negative Gibbs free energy changes are associated with each.
- Of the choices below, which is the LEAST oxidized molecule?
Citrate
The citric acid cycle is a pathway that oxidizes the carbon intermediates and moves those electrons to reduce NAD+ and FAD to NADH and FADH2, respectively. The final carbon intermediate in the pathway, OAA, therefore is the most oxidized and the first carbon intermediate, citrate, is the least oxidized. This is a list of the intermediates (in order from least to most oxidized) and it follows the order of the pathway: citrate alpha-ketoglutarate succinate malate oxaloacetate. This figure shows all of the carbon intermediates and demonstrates that they are being oxidized throughout the cycle.
- If a person were deficient in vitamin B3, what enzyme-catalyzed reaction in the citric acid cycle would be directly affected?
The reaction catalyzed by malate dehydrogenase
Malate dehydrogenase uses NAD+, which would be affected by a vitamin B3 (niacin) deficiency. NAD+ is niacin covalently bound to ADP. Isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase would also be directly affected by a deficiency in B3. CoA is a product of reactions catalyzed by citrate synthase and succinyl-CoA dehydrogenase, which would be affected by a vitamin B5 deficiency. Succinate dehydrogenase uses FAD as a substrate, which would be affected by a vitamin B2 (riboflavin) deficiency.
- A-ketoglutarate dehydrogenase is regulated by which of the following?
All of the listed choices are correct.
High [succinyl-CoA] allosterically inhibits alpha-ketoglutarate, which is an example of product inhibition since succinyl-CoA is a product of the reaction catalyzed by alpha-ketoglutarate. Product inhibition is a form of allosteric inhibition. Also, high [Ca2+] is an allosteric activator for this enzyme. This figure summarizes all of the regulatory affector molecules and their respective enzyme targets.
- Where in a eukaryotic cell does the citric acid cycle occur?
Mitochondrial matrix
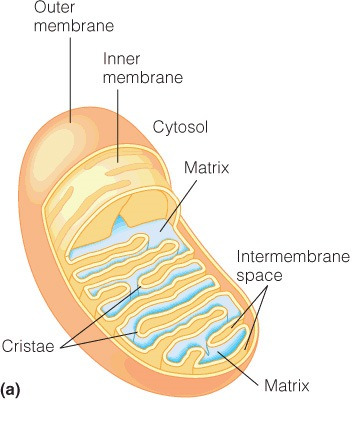
The citric acid cycle occurs in the mitochondrial matrix of eukaryotic cells and in the cytosol of prokaryotic cells. The only exception is that one enzyme, succinate dehydrogenase, is embedded in the inner membrane of the mitochondria. Various other metabolic pathways occur in other cellular compartments, like the cytosol, peroxisome, and others. The figure shows a mitochondrion; the matrix is the water-filled, innermost region.
- Which enzyme-catalyzed reaction in the citric acid cycle generates an NTP (where N = any nitrogenous base)?
The reaction catalyzed by succinyl-CoA synthetase
Succinyl-CoA synthetase uses the energy from thioester bond hydrolysis (ΔG°’ = -36 kJ/mol) to drive the generation of ATP (GTP) (ΔG°’ = +30.5 kJ/mol) via substrate-level phosphorylation. The NTP generated is dependent on tissue type. The heart, the brain, and skeletal muscle generate ATP whereas the kidneys and the liver generate GTP. This reaction also yields CoA. Alpha-ketoglutarate dehydrogenase, citrate synthase, and isocitrate dehydrogenase catalyze the irreversible reactions, however none of these generate an NTP.
- Does the citric acid cycle directly require oxygen (O2)?
No, oxygen (O2) is not a substrate for any of the enzyme-catalyzed reactions.
No, oxygen (O2) is not a substrate for any of the enzyme-catalyzed reactions within the pathway. Molecular oxygen is indirectly necessary for the citric acid cycle via the electron transport chain. The electron transport chain oxidizes NADH to NAD+, which is necessary for the citric acid cycle to continue running. Oxygen is the final electron acceptor in the electron transport chain, and is therefore needed to oxidize NADH. In this way, molecular oxygen is indirectly required and the citric acid cycle is considered an aerobic pathway. Carbon dioxide is a product of the citric acid cycle and NADH and GTP (ATP) are produced in reactions that do not directly require oxygen.
- Which enzyme-catalyzed reaction in the citric acid cycle generates FADH2?
The reaction catalyzed by succinate dehydrogenase
The reaction catalyzed by succinate dehydrogenase generates FADH2. All of the enzymes listed catalyze oxidation-reduction reactions but only the succinate fumarate reaction moves electrons from succinate to reduce FAD to FADH2. Malate dehydrogenase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase reduce NAD+ to NADH.
- Which of the following is NOT a product of the citric acid cycle?
Acetyl-CoA
Acetyl-CoA is a substrate of the first reaction catalyzed by the enzyme citrate synthase. Carbon dioxide and NADH are products of reactions catalyzed by isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase. NADH is also a product of the malate dehydrogenase-catalyzed reaction, but carbon dioxide is not a product for this reaction. ATP (GTP) is a product of the reaction catalyzed by succinyl-CoA synthetase.
- Which enzyme-catalyzed reaction in the citric acid cycle generates H2O?
None of the listed choices are correct.
None of the enzyme-catalyzed reactions in citric acid cycle generates water. However, three reactions use water and these are catalyzed by citrate synthase, fumarase, and aconitase.
- How many turns of the citric acid cycle are required to oxidize the carbons from acetyl-CoA?
2
Two turns of the citric acid cycle are required to oxidize the carbons from acetyl-CoA. Although the full oxidation of acetyl-CoA yields two carbon dioxide molecules, which are two of the products of one turn of the cycle, these carbon dioxide molecules are the carbons from the previous turn. The incoming carbons from acetyl-CoA will continue throughout an entire round of the citric acid cycle, and only leave as carbon dioxide during the subsequent turn. This figure highlights the acetyl-CoA carbons as they move through the citric acid cycle.
- Carbon atoms enter the citric acid cycle in the reaction involving which enzyme?
Citrate synthase
New carbons enter the citric acid cycle as acetyl-CoA in the first step, catalyzed by citrate synthase. Malate dehydrogenase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase all catalyze oxidation-reduction reactions within the citric acid cycle.
- Consider the reaction that generates an NTP in the citric acid cycle. Which of the following is a driving force for this reaction?
Hydrolysis of the thioester bond releases energy that is coupled to the formation of the phosphoanhydride bond in ATP (GTP).
Succinly-CoA synthetase uses the energy from thioester bond hydrolysis (ΔG°’= -36 kJ/mol) to drive the generation of ATP (GTP) (ΔG°’= +30.5 kJ/mol) via substrate-level phosphorylation. The NTP generated is dependent on tissue type. The heart, the brain, and skeletal muscle generate ATP whereas the kidneys and the liver generate GTP. There is the same number of products as reactants; entropy is neither increased nor decreased based on this factor alone. Substrates would have more resonance; however, this factor alone would favor the substrates.
- Isocitrate dehydrogenase is down-regulated by which method?
Allosteric inhibition
High [NADH] inhibits isocitrate dehydrogenase, which is an example of product inhibition since this is the product of the isocitrate dehydrogenase-catalyzed reaction. Isocitrate dehydrogenase is not a zymogen nor is it under phosphorylation control. This enzyme is also feedback inhibited (not activated) by ATP, the product of oxidative phosphorylation. Also [ADP] allosterically up-regulates this enzyme.
- Which enzyme in the citric acid cycle is responsible for catalyzing the rate-limiting step?
Alpha-ketoglutarate dehydrogenase
Alpha-ketoglutarate dehydrogenase catalyzes the rate-limiting step in citric acid cycle, where alpha-ketoglutarate is oxidized to succinyl-CoA. This is the fourth reaction of the cycle and it has the largest negative change in Gibbs free energy. Three of the eight steps are irreversible. Citrate synthase and isocitrate dehydrogenase also catalyze irreversible reactions (steps 1 and 3).
- Which of the following statements is FALSE concerning the pyruvate dehydrogenase complexes (PDC or PDH)?
FADH2 is a product.
FADH2 is not a product; all of the other listed answers are true concerning the pyruvate dehydrogenase complexes. FAD is reduced to regenerate lipoamide. In return, FADH2 is re-oxidized (regenerated) by reducing NAD+ to NADH. Since this coenzyme is regenerated and does not dissociate from the PDC, it is not a product. The three products of the PDC-catalyzed reaction are carbon dioxide, acetyl-CoA, and NADH. TPP is a coenzyme that is associated with E1 and lipoic acid is a prosthetic group covalently bound to E2. This figure shows the movement of the acetyl-group through the PDC and the regeneration of lipoamide and FAD.
- Which of the following allosteric inhibitors for the pyruvate dehydrogenase complex (PDC) is a type of feedback inhibition?
ATP
ATP is a feedback inhibitor of the PDC, as ATP is a final product of oxidative phosphorylation. Feedback inhibition occurs when the final product, or intermediate near the end of a pathway, inhibits the beginning of the pathway. Electrons from oxidizing pyruvate to acetyl-CoA are stored in NADH, which is then oxidized to NAD+ in the electron transport chain. The chemiosmotic potential in the ETC powers ATP synthase, resulting in the production of ATP. Therefore ATP is a final product of this pathway. High [ATP] allosterically inhibits the PDC, an enzyme at the beginning, via feedback inhibition. NADH and acetyl-CoA are allosteric product inhibitors of the PDC. ADP and pyruvate are allosteric activators of the PDC.
- Fully oxidized carbon leaves the citric acid cycle as this chemical _____.
carbon dioxide
Fully oxidized carbon leaves the citric acid cycle as carbon dioxide. This occurs during two different enzyme-catalyzed reactions. CoA leaves the citric acid cycle in the first step, but this molecule only drops off the two-carbon molecule as an acetyl group. ATP is not oxidized in the citric acid cycle. OAA is the most oxidized intermediate in the citric acid cycle, but it carries neither of the carbons that leave as carbon dioxide. NAD+ is reduced to NADH using the electrons from oxidizing carbon intermediates.
- Why are citrate synthase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase good candidates for regulation of the citric acid cycle?
These enzymes catalyze the irreversible reactions in the citric acid cycle.
Citrate synthase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase are good candidates for regulation of the citric acid cycle because they catalyze the irreversible reactions. These enzymes can control the outputs of the pathway as they represent bottlenecks of the cycle. Only isocitrate dehydrogenase is feedback inhibited by [ATP]; however they are all inhibited by [NADH]. [ADP] and [Ca2+] are allosteric activators of the pathway. This figure summarizes all of the regulatory affector molecules and their respective enzyme targets.
- One-turn of the citric acid cycle yields
1 ATP (GTP), 3 NADH, 1 FADH2, and 2 CO2
One turn of the citric acid cycle includes four oxidation-reduction reactions—three generate NADH and one generates FADH2. The two carbons entering the citric acid cycle (from acetyl-CoA) are completely oxidized into two CO2 molecules. Only one ATP (GTP)** is generated during the cycle using substrate-level phosphorylation.
- How many carbon dioxide molecules will be released for the complete oxidation of the following molecules?
6, 3, 2
The complete oxidation of these molecules yields carbon dioxide, which has one carbon. Therefore, since glucose is six carbons, the complete oxidation will yield six carbon dioxides. Pyruvate has three carbons and acetyl-CoA has two carbons; therefore, they yield three and two carbon dioxides, respectively.