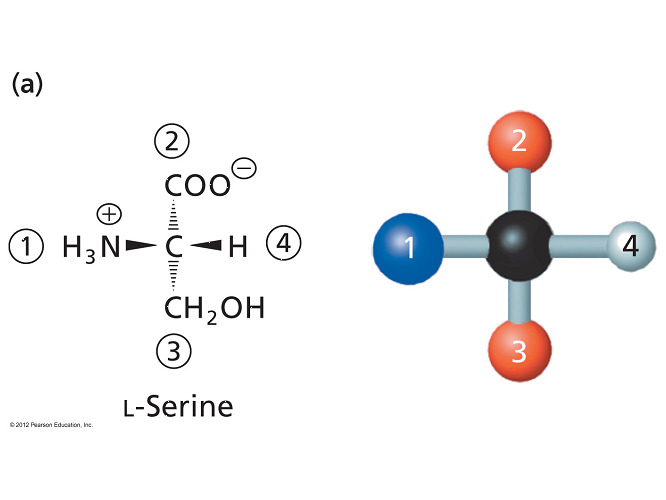
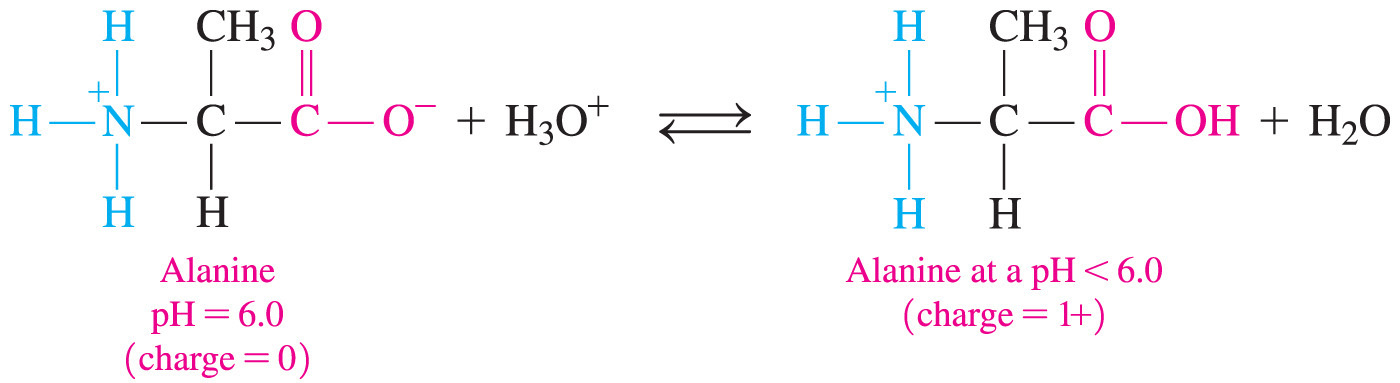- Amino acids can act as either acids or bases. Which term correctly describes this behavior?
amphoteric
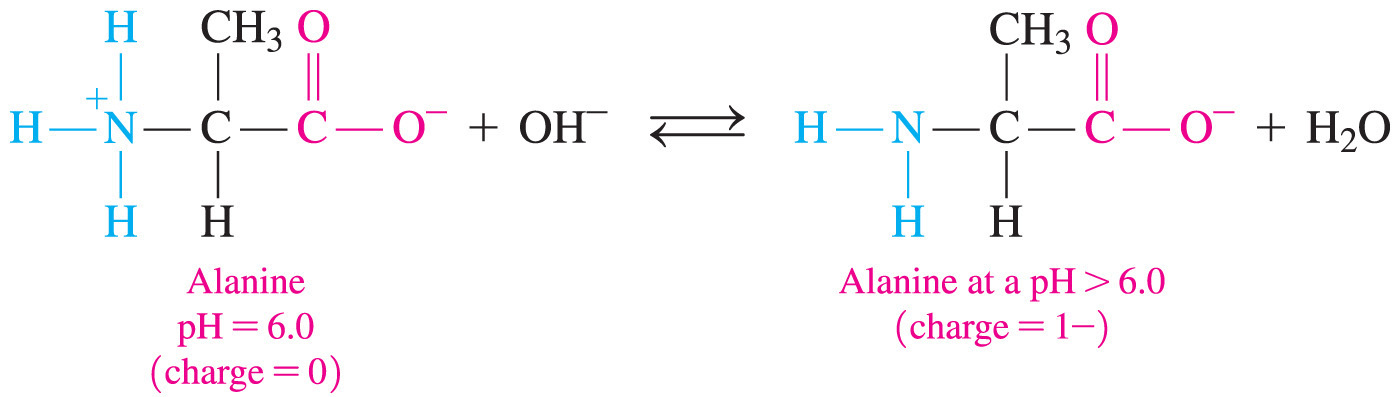
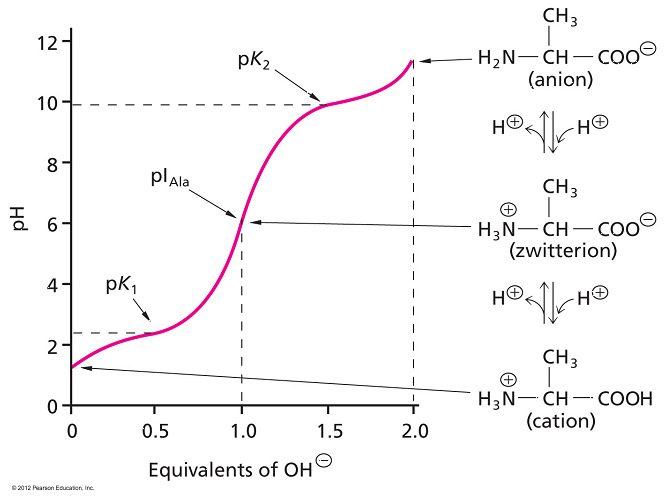
- An amphoteric substance is one that can act as either an acid or a base.
- Amino acids are amphoteric because they can either lose a proton (acid) or accept a proton (base).
- The term isoelectric describes a compound with no net electric charge.
- The term amphiphilic describes a compound with both hydrophilic and hydrophobic properties.
- The term aliphatic describes a compound that is non-aromatic and has an open-chain structure.
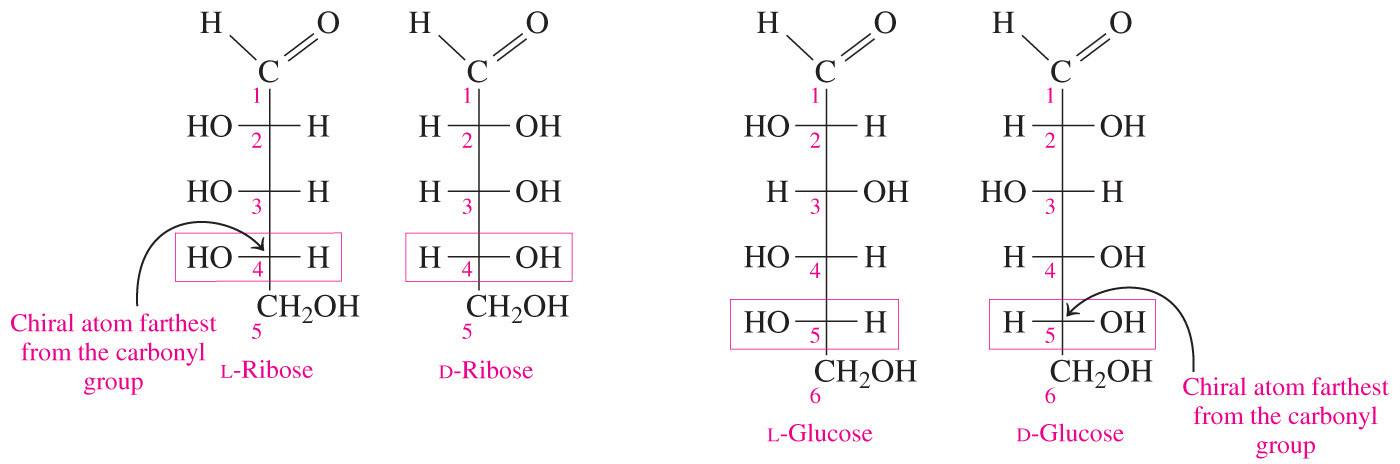
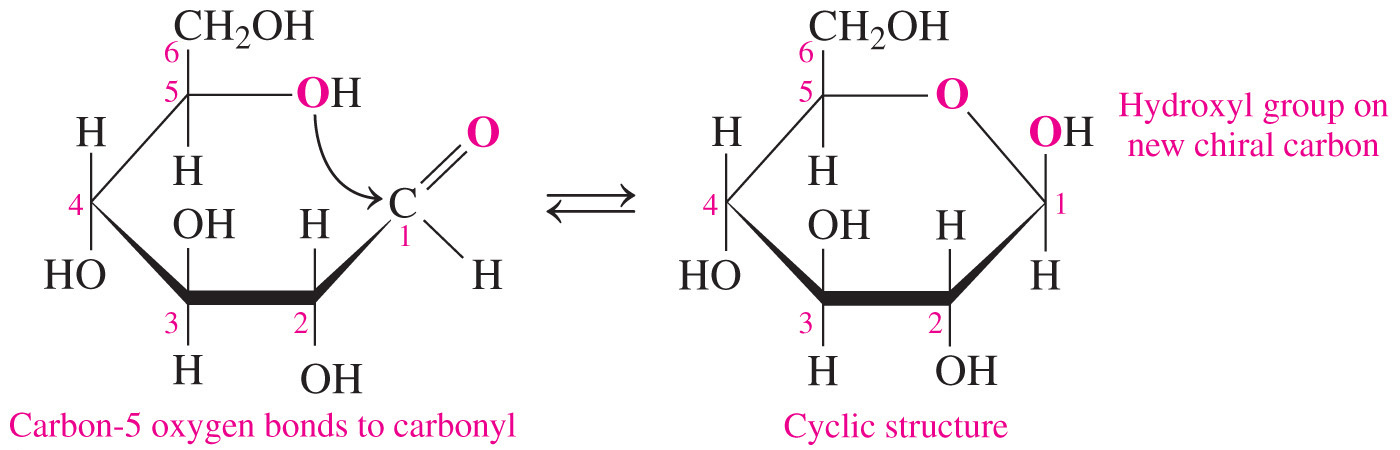
- Which statement accurately describes the relationship between the cyclic and open-chain structures of a monosaccharide?
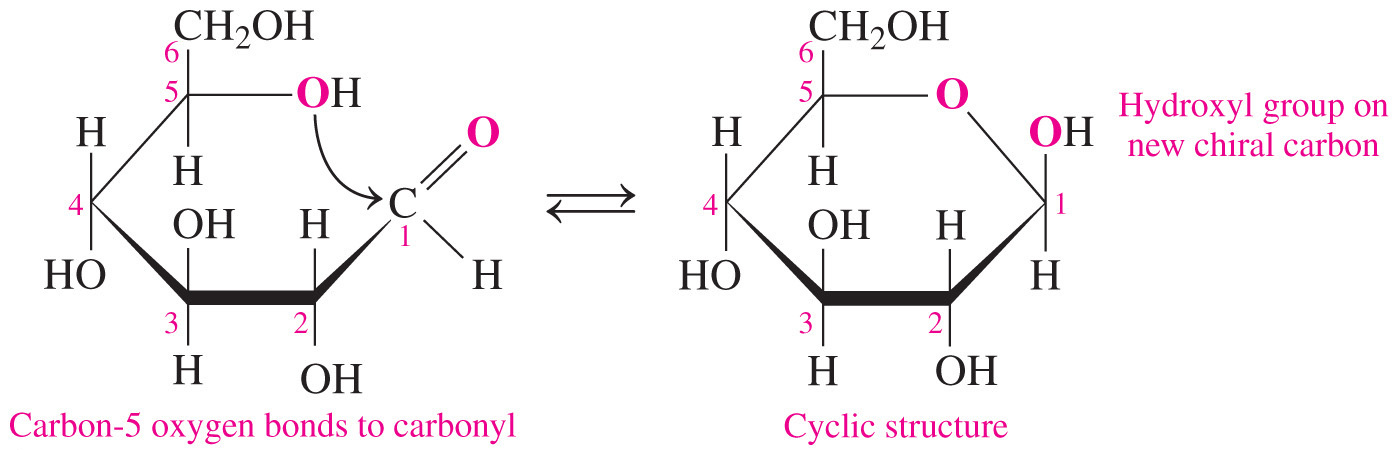
The cyclic form has one more chiral center than the open-chain form.
- Chirality is indicated by a tetrahedral carbon atom with four different substituents.
- Carbonyl carbons are not chiral because they are not tetrahedral.
- The cyclic form of a monosaccharide has one more chiral center than the open-chain form.
- The new bond created in the conversion from the open-chain form to the cyclic form is chiral.
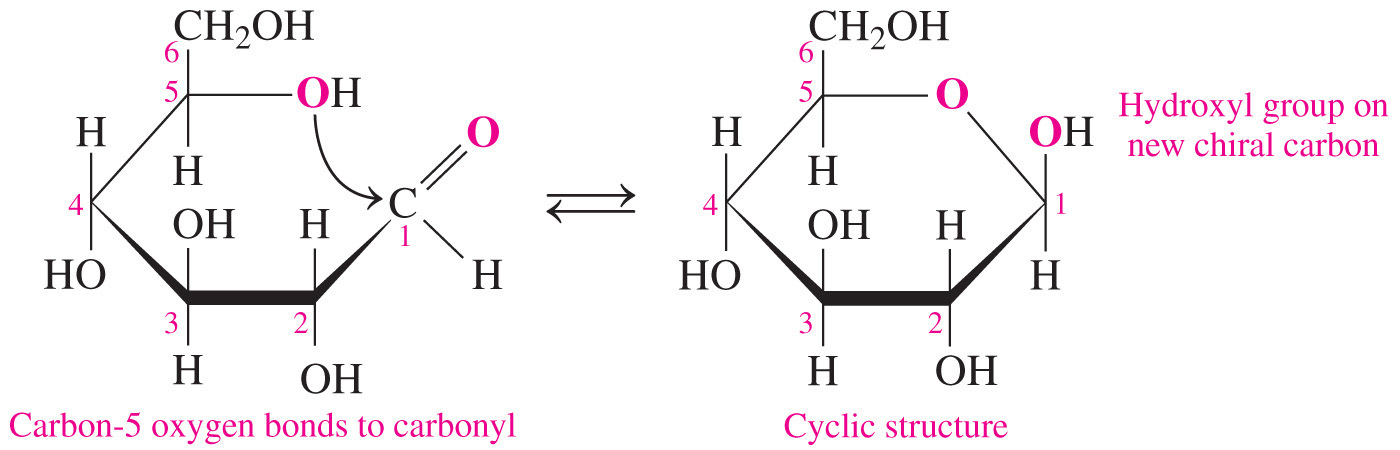
- The process of converting the open-chain form to the cyclic form involves the formation of a hemiacetal bond in aldoses (or hemiketal bond in ketoses) between the carbonyl group and the hydroxyl group on the highest-number chiral carbon (also called the penultimate carbon).
- The statements indicating that cyclic form has one less chiral center than the open-chain form, or even the same number of chiral centers, are inaccurate.
- It is also not true that the open-chain form is less predominant in nature.
- The majority of all monosaccharides exist in the cyclic form due to the high reactivity between carbonyl and hydroxyl groups.
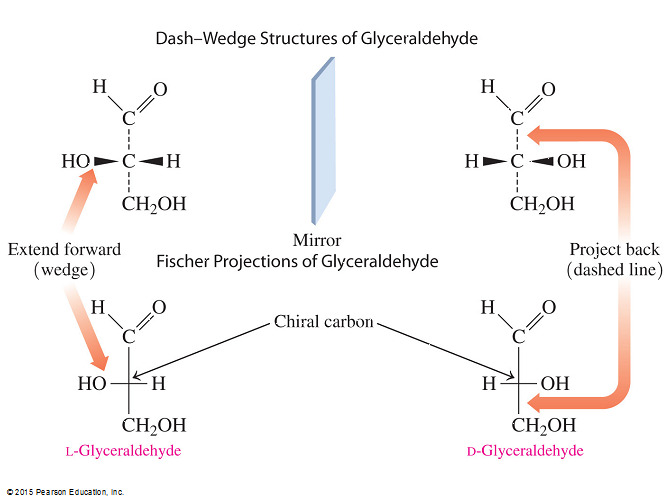
- The open-chain structure of a monosaccharide is represented as a Fischer projection, a two-dimensional representation of a three-dimensional organic molecule.
- A Haworth projection is a representation of the cyclic structure of a monosaccharide.
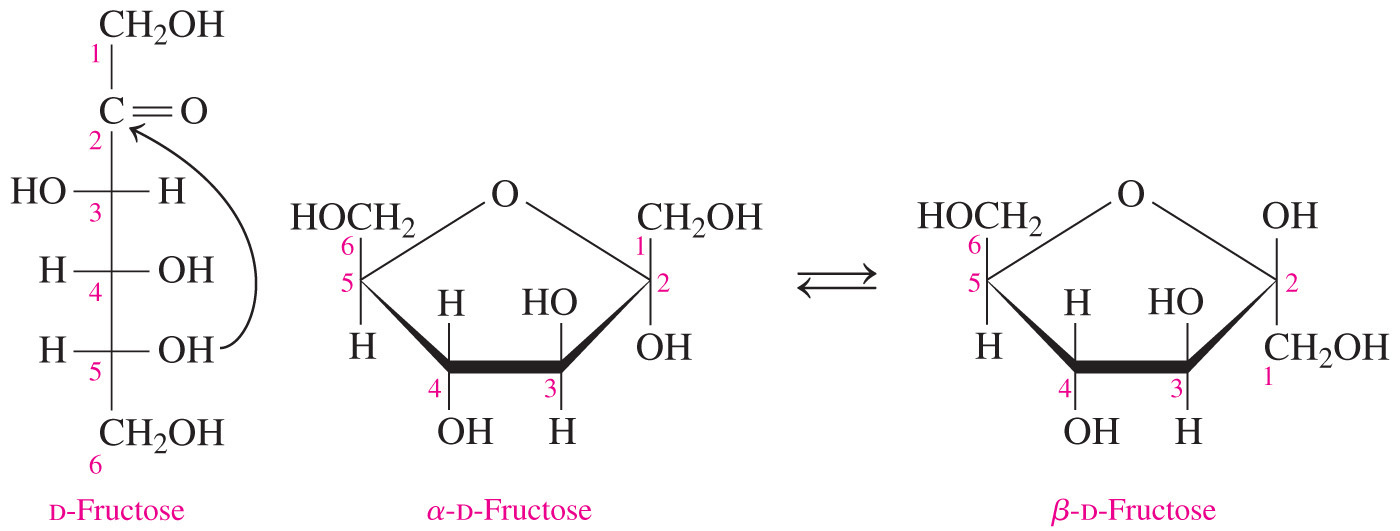
- Which of these statements about glucose or fructose is true?
Carbon-1 of glucose is part of the ring.
- Carbon-1 of glucose is part of the ring.
- Glucose forms a six-membered pyranose ring and it has only one achiral carbon (carbon-6) that is not part of the ring.
- Fructose forms a five-membered furanose ring and it has two achiral carbons (carbon-1 and carbon-6) that are not included in the ring.
- The other three answer choices are all incorrect.
- Carbon-1 of fructose, and carbon-6 of fructose and glucose all appear outside of the ring.
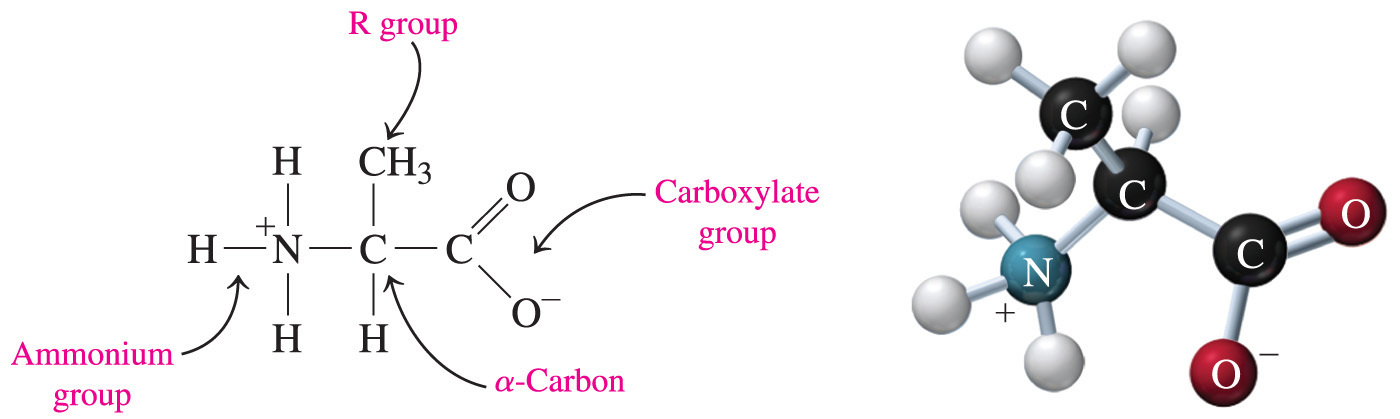
- Which of the following statements is true for the standard amino acids found in proteins?
They are all α amino acids.
- All of the standard amino acids found in proteins are α amino acids because they all have an alpha carbon that is adjacent to a carboxyl group.
- Proteins in animal and plant tissue are made from L-forms of amino acids and not the D-form.
- However, all of the 20 amino acids are not L because they are not all chiral.
- The simplest amino acid, glycine, has a hydrogen atom as a side chain and thus it does not have four different attachments to its tetrahedral carbon.
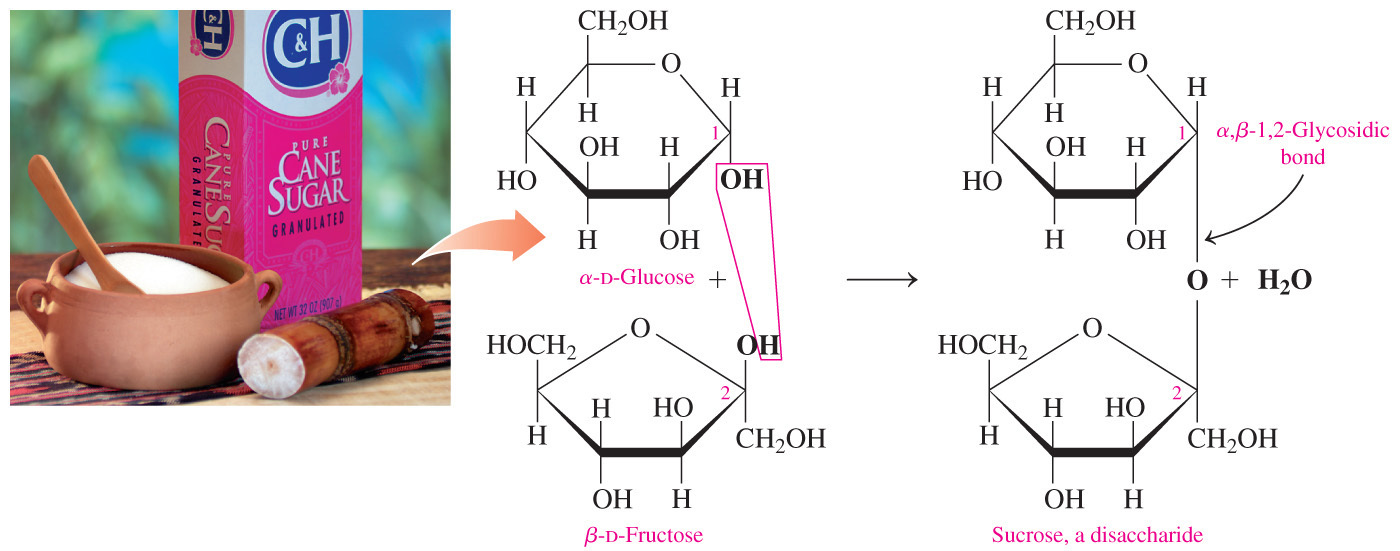
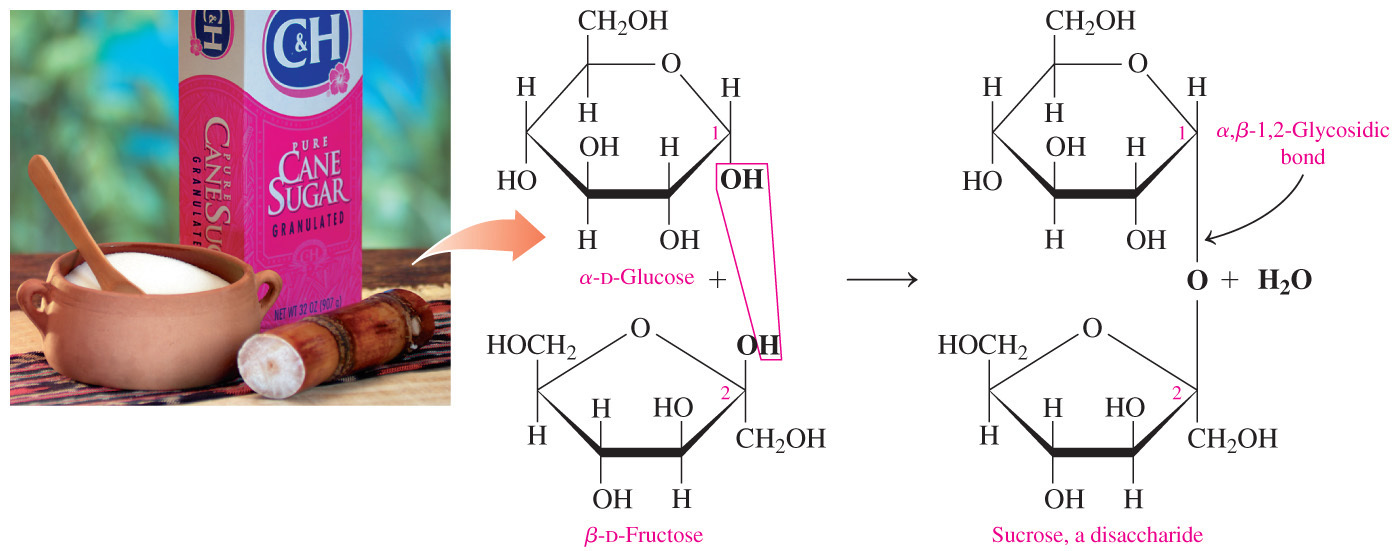
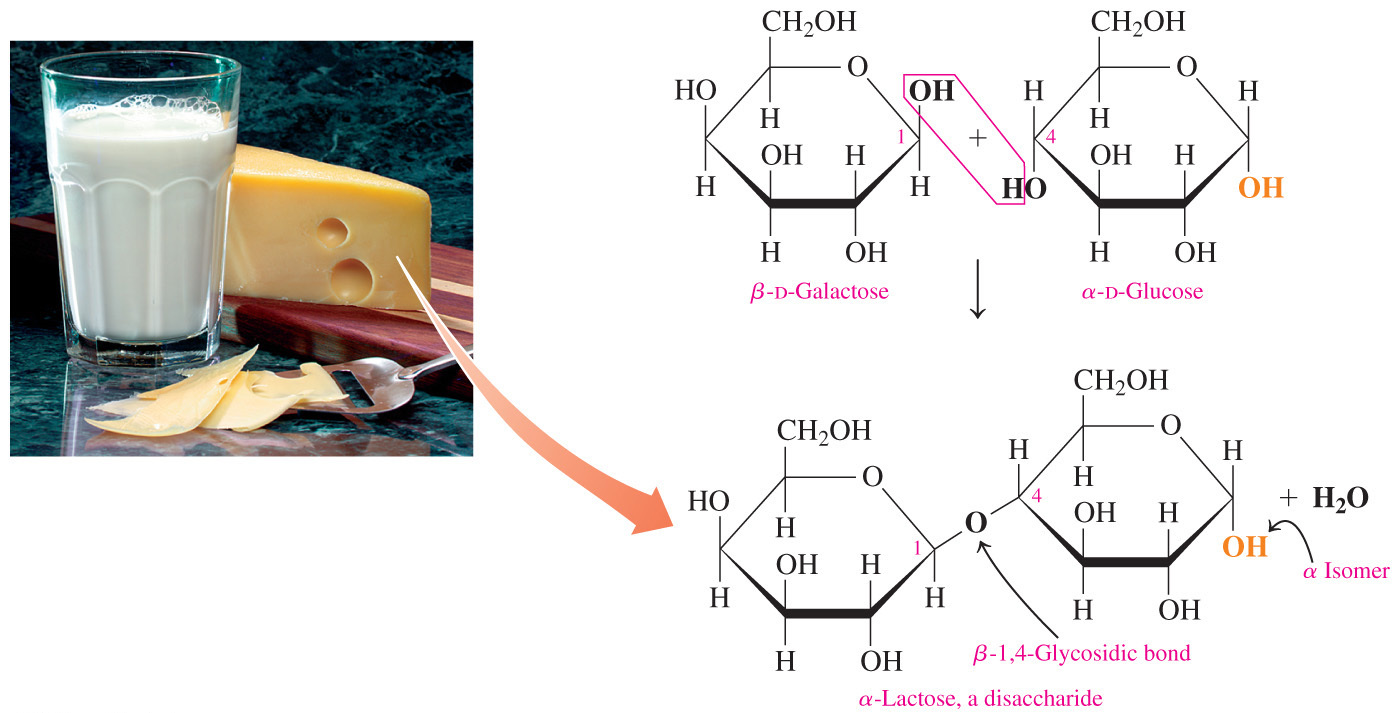
- Which statement correctly describes the distinction between the glycosidic bond of a reducing disaccharide versus the glycosidic bond of a non-reducing disaccharide?
In a reducing disaccharide, only one of the carbons in the glycosidic bond can be anomeric but in a non-reducing disaccharide, both carbons in the glycosidic bond must be anomeric.
- If a disaccharide is a reducing sugar, only one of the carbons in the glycosidic bond can be an anomeric carbon, or the carbon that was the carbonyl carbon in the acyclic form.
- A free anomeric carbon must be available for oxidation in order for a disaccharide to be a reducing sugar.
- This is why it is called a reducing sugar: because it can serve as reducing agent.
- If a disaccharide is a non-reducing sugar, both carbons in the glycosidic bond must be anomeric carbons.
- A non-reducing sugar cannot be oxidized due to the absence of a free anomeric carbon.
- The process of converting the open-chain form to the cyclic form results in the formation of an additional chiral carbon that is referred to as the anomeric carbon.
- It is the carbon that was previously a carbonyl in the open-chain form.
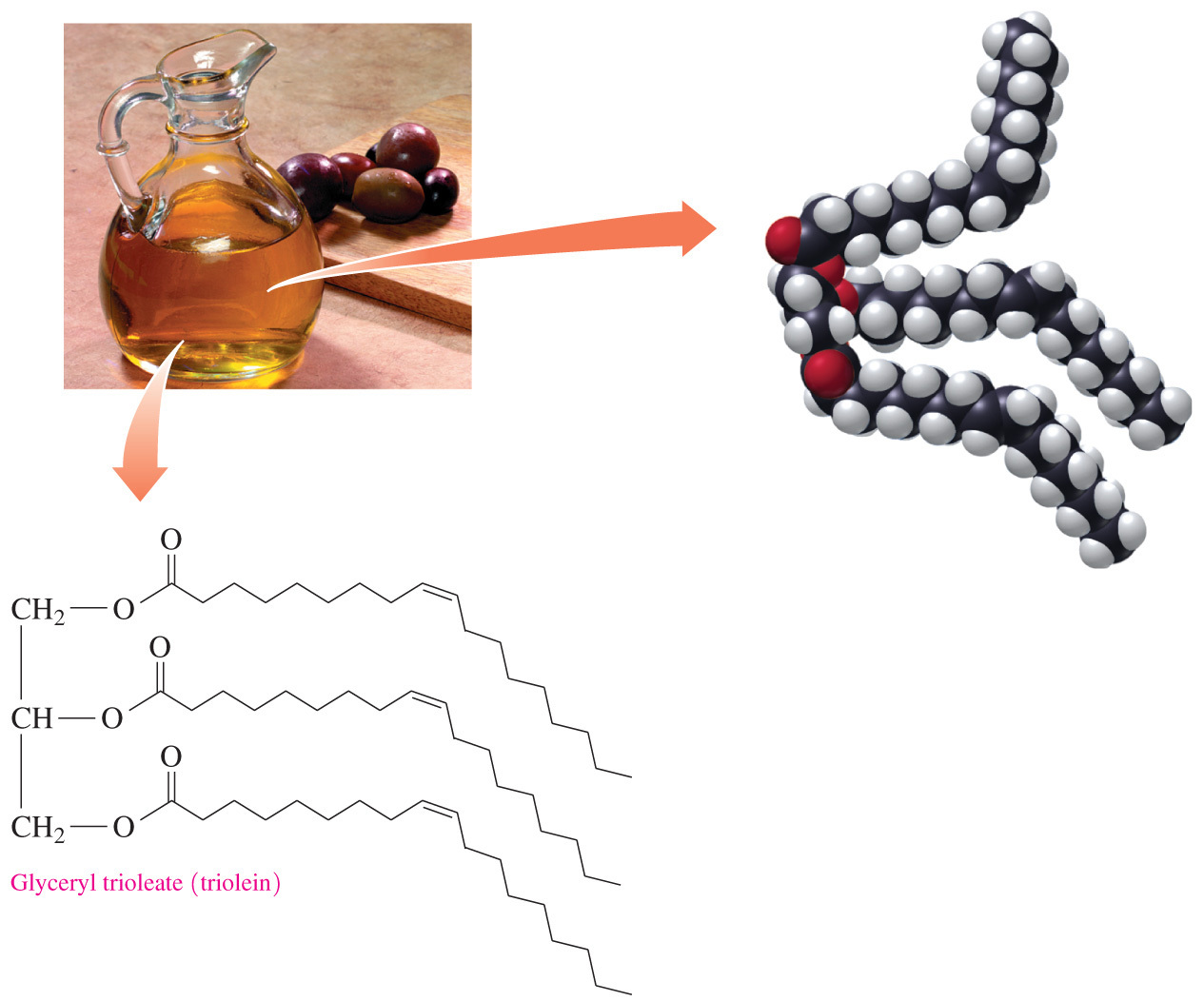
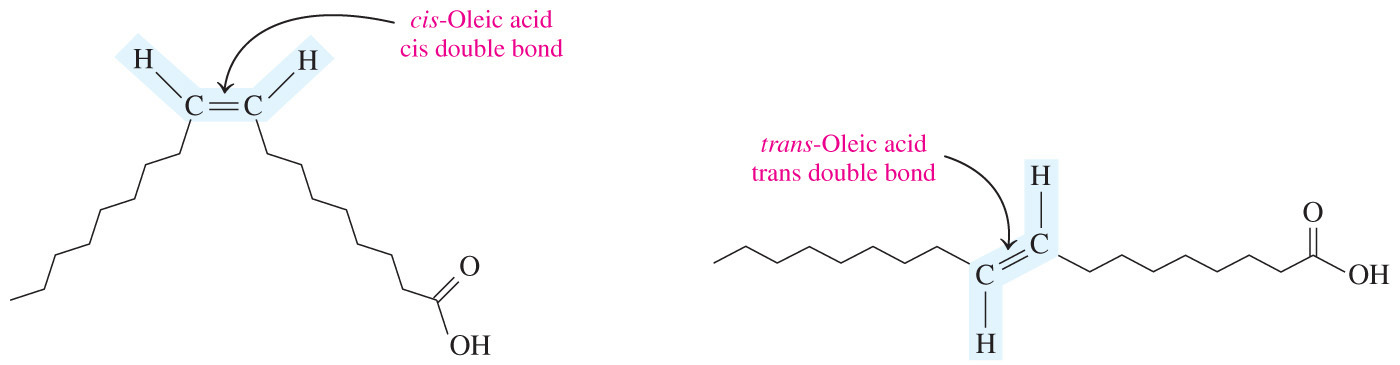
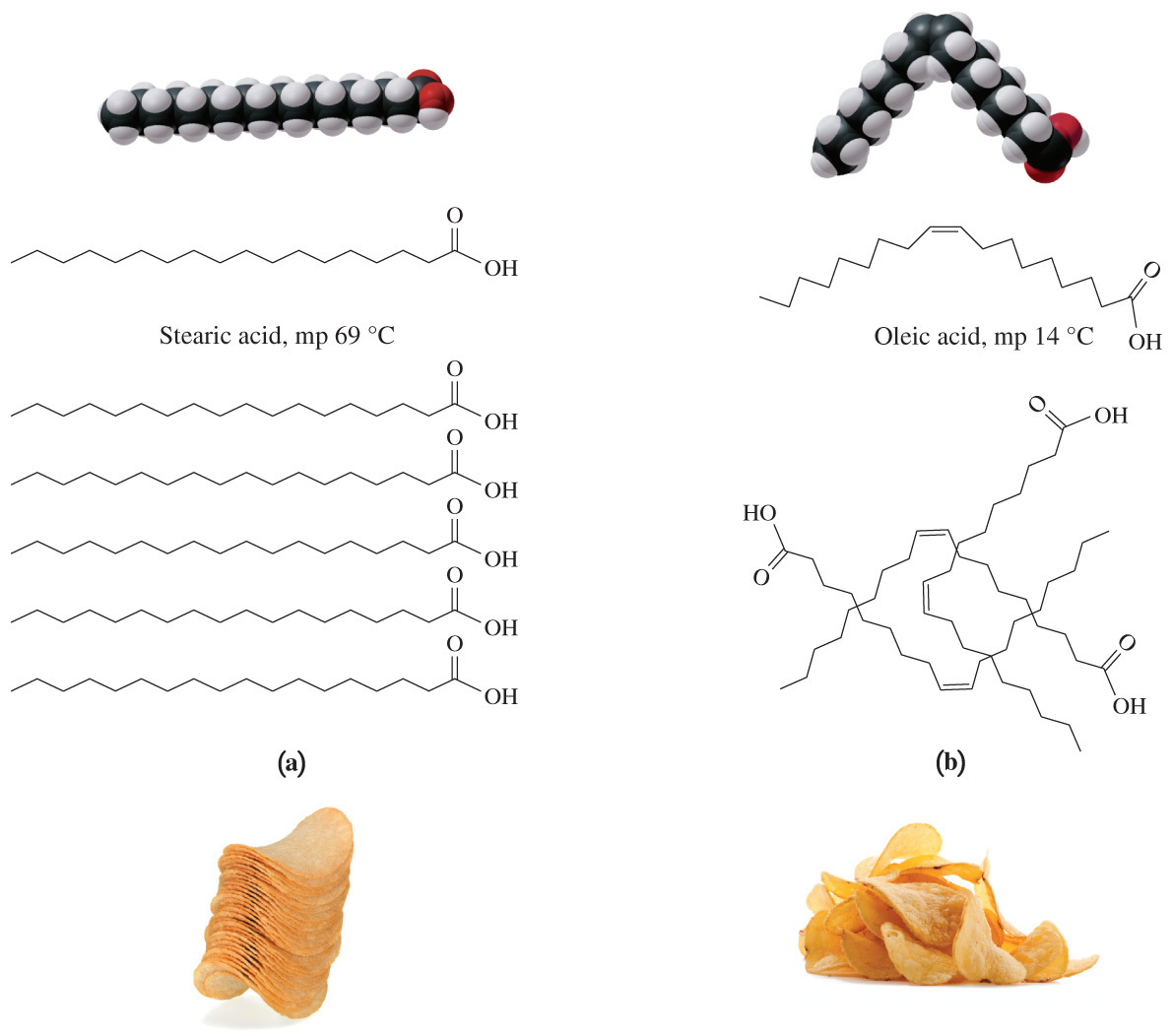
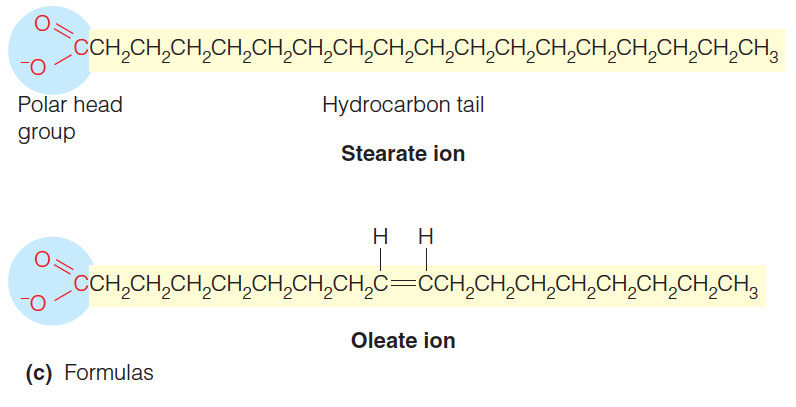
- Triglycerides that contain unsaturated fatty acids are usually __ at room temperature because the acyl chains do not fit closely together due to the kinks from the __.
liquids; cis double bonds
- Triglycerides that contain unsaturated fatty acid are usually liquids at room temperature because the acyl chains do not fit closely together due to the kinks from the cis double bonds.
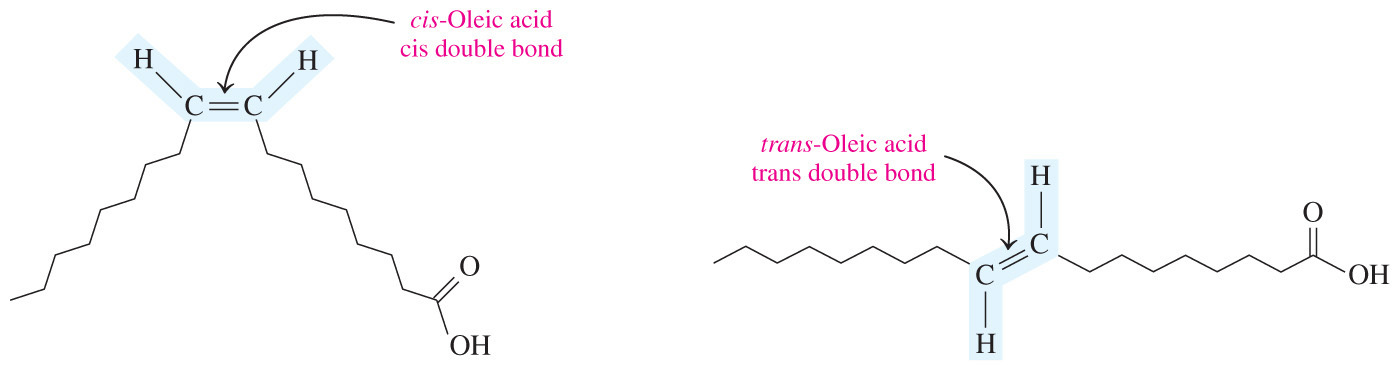
- In a cis double bond, adjacent hydrogen atoms are on the same side of the double bond.
- This creates a bend or kink in the chain and limits the ability of fatty acids to pack closely.
- As a result, the melting points of unsaturated fatty acids are lower than the melting points of saturated fats because it does not take as much heat energy to fully separate (or melt) the unsaturated fatty acid molecules.
- Therefore, triglycerides that contain unsaturated fatty acids are normally liquids at room temperature.
- Triglycerides that contain unsaturated fatty acid are not usually in the trans form.
- In addition, trans fatty acids are not liquids at room temperature because of the nature of their double bond.
- (Adjacent hydrogens are bound to opposite sides of the double bond, giving them a shape that is similar to straight saturated fatty acids.)
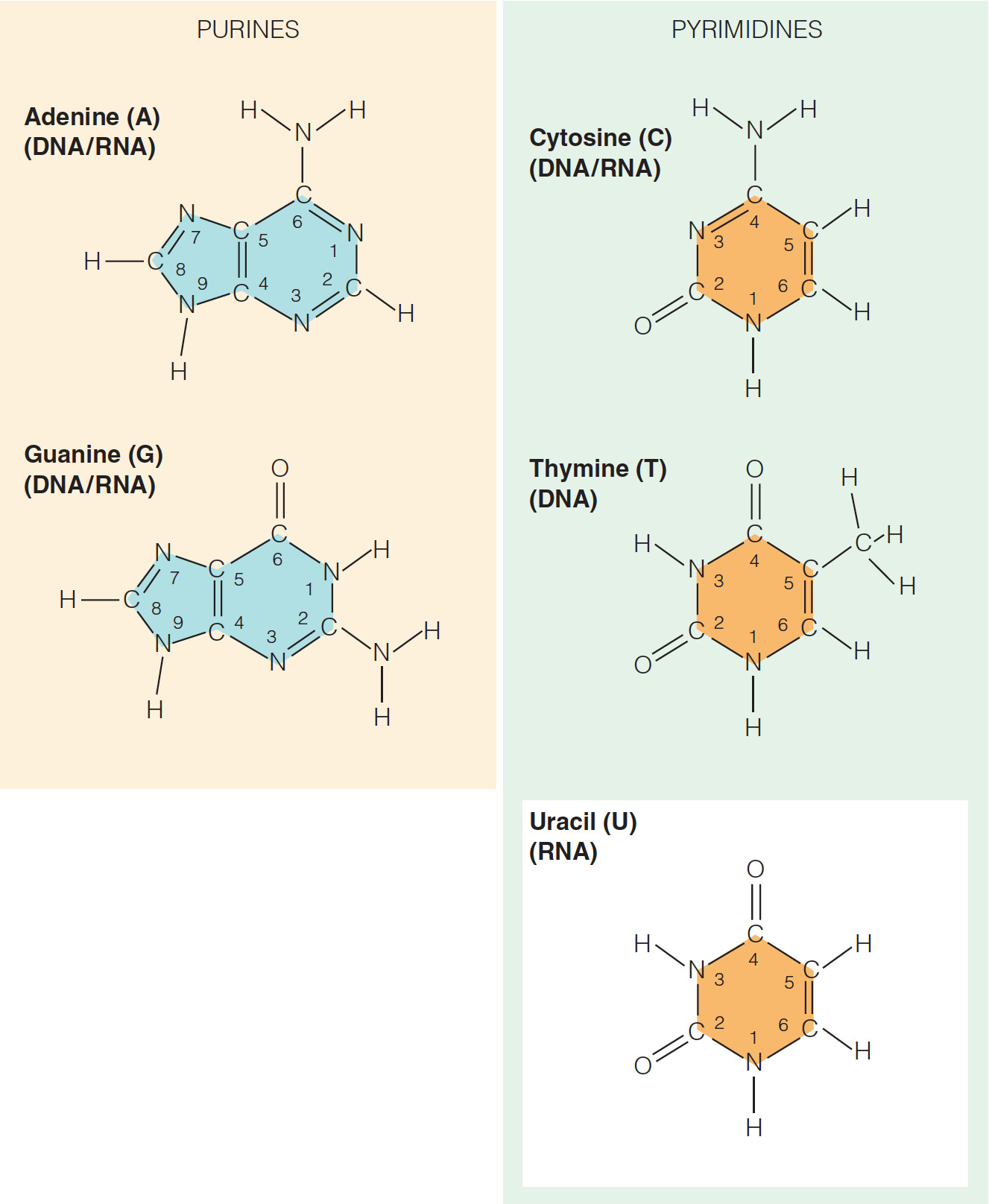
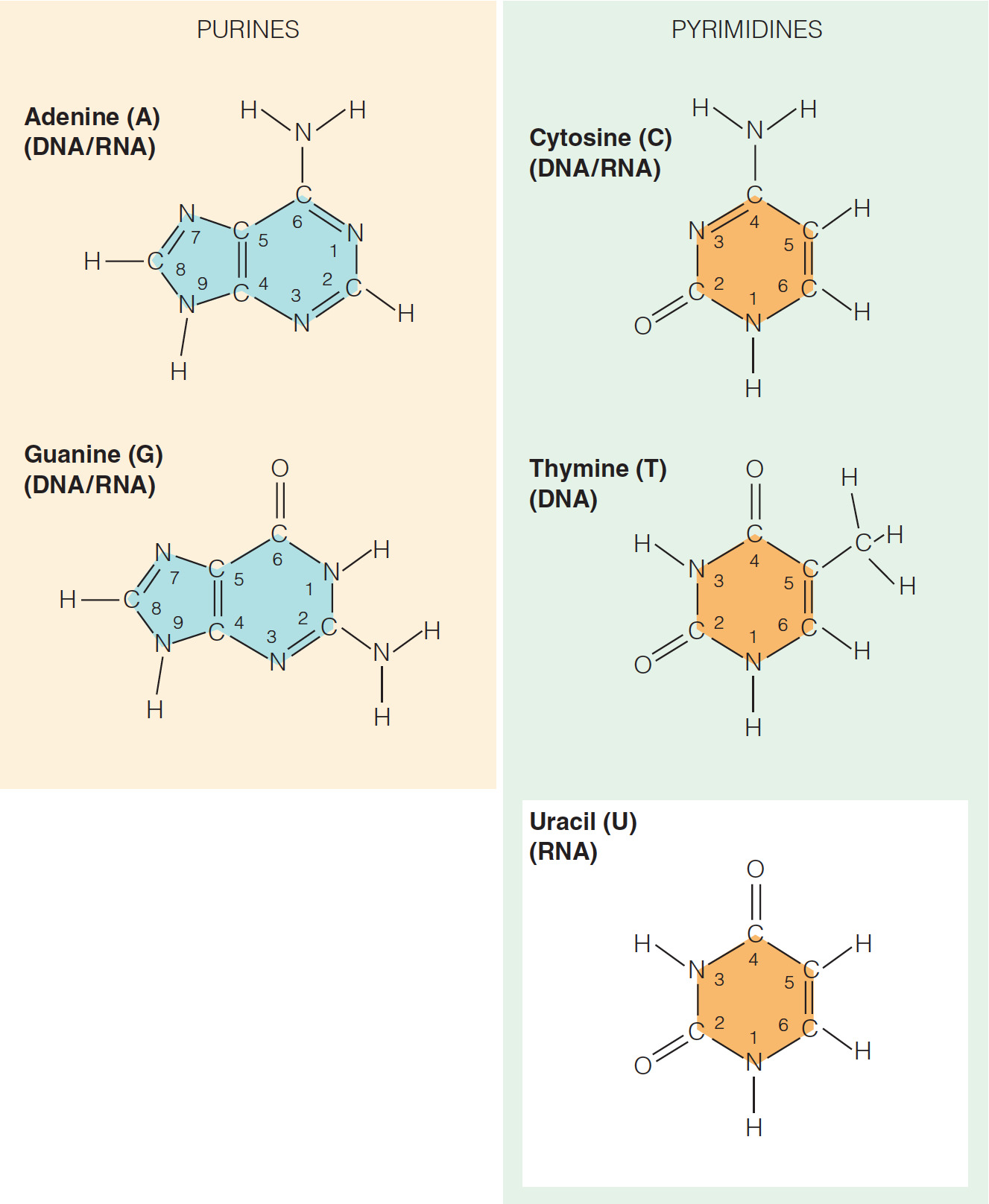
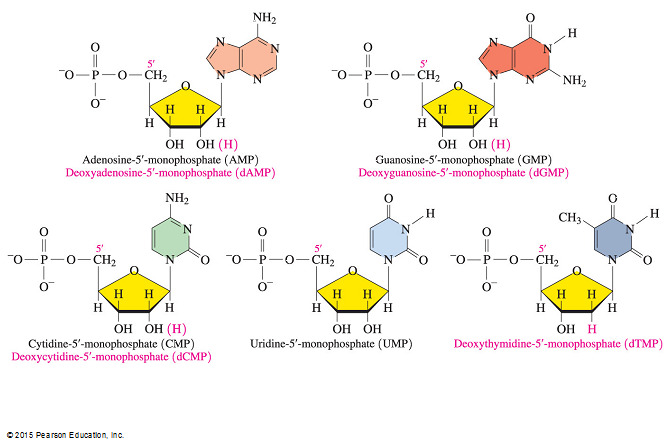
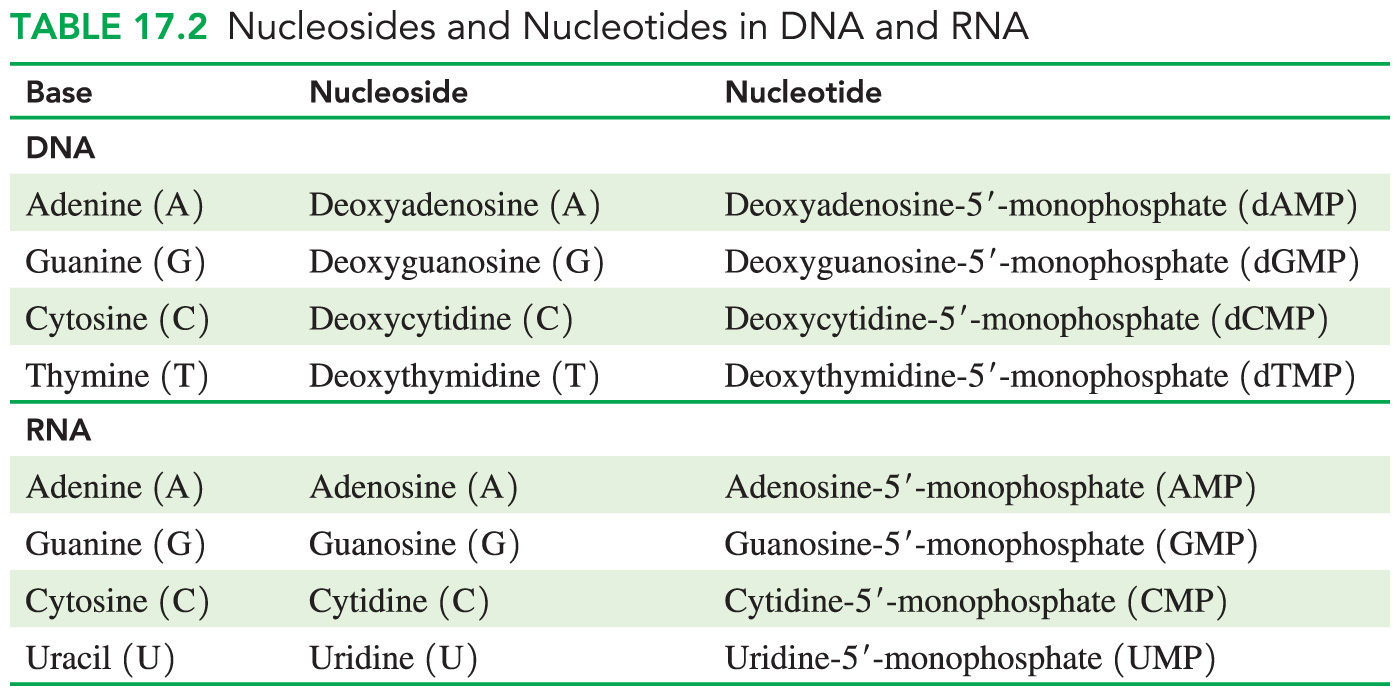
- Which statement does NOT describe a structural similarity between the nucleotides of DNA and RNA?
Both DNA and RNA contain the bases adenine, cytosine, guanine, and uracil.
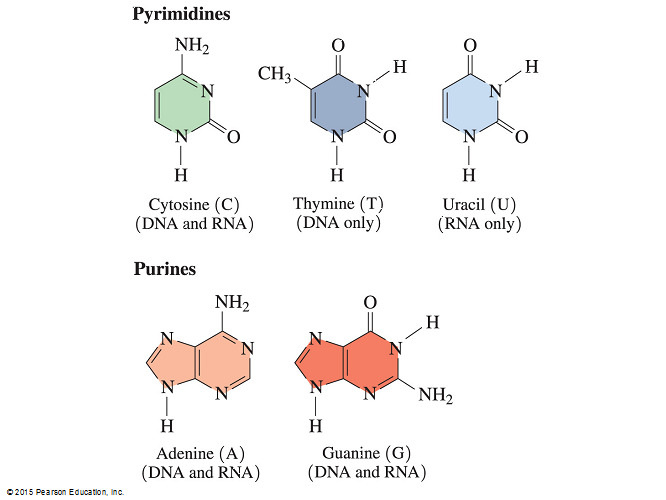
- It is not true that both DNA and RNA contain the bases adenine, cytosine, guanine, and uracil because while both DNA and RNA contain the bases cytosine and guanine, only RNA contains the base uracil. DNA contains a thymine base instead of uracil.
- The difference between uracil and thymine is that thymine has a methyl group in the C5 position while uracil does not.
- As a result, thymine is also called 5-methyl uracil.
Structural similarities between DNA and RNA include the following:
- Both contain nitrogenous bases that are cyclic compounds with at least one nitrogen atom in the ring structure (heterocyclic amines).
- Both contain a pentose sugar that is attached to a phosphate group via a phosphodiester linkage.
- Both are polymers of nucleotides that are also referred to as nucleoside 5’-monophosphates because nucleosides contain only the sugar-base adduct and no phosphate group.
Consider this information concerning the fatty acid composition of four different triglycerides:
In triglyceride A, three saturated fatty acids are attached to glycerol.
In triglyceride B, one saturated and two unsaturated fatty acids are attached to glycerol.
In triglyceride C, two saturated and one unsaturated fatty acids are attached to glycerol.
In triglyceride D, three unsaturated fatty acids are attached to glycerol.
Which of the following statements is correct?
Triglyceride A has a higher melting point than triglyceride C.
- The melting point of a triglyceride increases as the number of saturated fatty acids increase.
- A triglyceride (triglyceride A) with three saturated fatty acids attached to glycerol has a higher melting point than the triglyceride (triglyceride C) with two saturated fatty acids and one unsaturated fatty acid attached to glycerol.
- The other answers are all incorrect statements because the first triglyceride mentioned in each statement has fewer saturated fatty acids than the second triglyceride mentioned in the statement.
- Saturated fatty acids contain no double bond and thus their hydrocarbon tails are straight and can stack tightly upon one another.
- This allows stronger interaction between the carbon atoms.
- As a result, the melting point of saturated fatty acids are higher than unsaturated because it takes more heat energy to fully separate (or melt) the molecules.
- This is why triglycerides that contain saturated fatty acids are normally solids at room temperature.
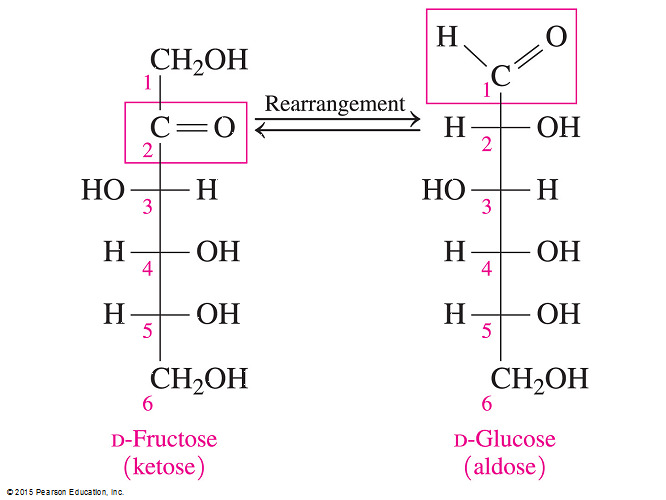
- Which of the following statements is true about glucose but NOT about fructose?
It is an aldose.
- Monosaccharides can be either polyhydroxyaldehydes (aldoses) or polyhydroxyketones (ketoses).
- Fructose is not an aldose but it is a ketose. In the two-dimensional Fischer projection, the carbonyl group is on C2 for fructose but C1 for glucose.
- Glucose is an aldose.
- The other statements are all correct.
- Both glucose and fructose are monosaccharides; they are both six-carbon sugars (hexoses); and they are the building blocks for the disaccharide sucrose.
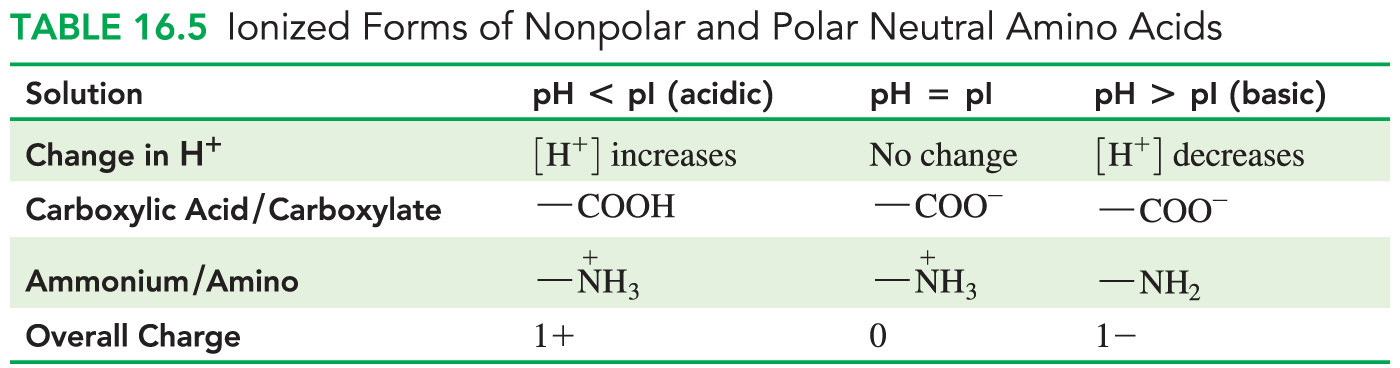
- Which statement is true concerning amino acids that contain an uncharged side chain or R group?
This type of amino acid never exists in a unionized form.
- The R groups of nonpolar or polar neutral amino acids are never charged.
- Amino acids with acidic R groups can become negative and those with basic R groups can become positive, depending on the pH of the solution.
- All amino acids have both an acidic carboxyl group (that loses its proton and becomes negatively charged at very low pH) and a basic amino group (that does not lose its proton and remains positively charged until very high pH).
- An amino acid that contains an uncharged R group never exists in a unionized form because the carboxyl group would have to be protonated while the amino group is deprotonated.
- This conflicts with the Bronsted-Lowry definition of acid (proton donor) and base (proton acceptor).
- Amino acids commonly exist as a zwitterion—a compound with no overall electrical charge.
- This is because there is an internal transfer of a hydrogen ion from the –COOH group to the –NH2 group, resulting in an ion with both a negative charge and a positive charge.
The remaining answer choices are all incorrect because amino acids with nonpolar or polar neutral R groups never exist in a unionized form, regardless of whether the pH is low, neutral, or high.
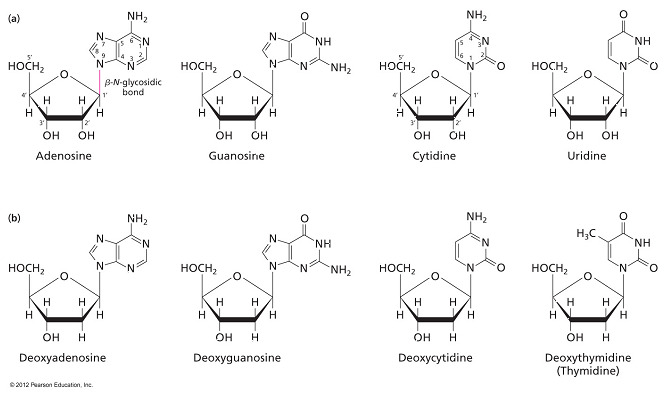
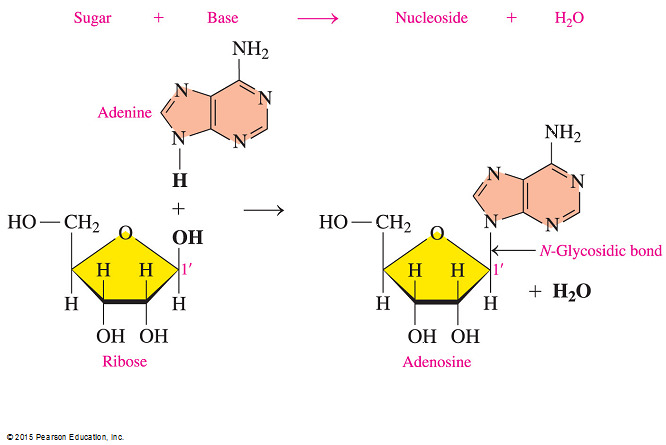
- Which statement is true concerning the linkage between the sugar and the base in a nucleoside?
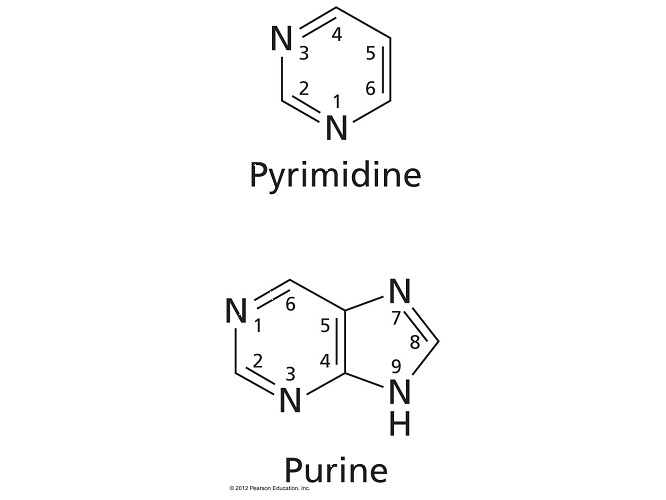
In a purine, an N-glycosidic linkage forms between C1’ of the sugar and N-9 of the base. In a pyrimidine, an N-glycosidic linkage forms between C1’ of the sugar and N-1 of the base.
- In a nucleoside that contains a pyrimidine base, the nitrogen in the first position of the base forms a N-glycosidic bond to C1’ of the sugar.
- The two answer choices indicating that a pyrimidine base has C1’ of the sugar linked to its N-9 are incorrect because single-ringed pyrimidine bases only have six members (nitrogens at positions 1 and 3).
- The answer choice indicating that a purine base has C1’ of the sugar linked to N-1 is also incorrect.
- Double-ringed purines have nitrogens at positions 1, 3, 7, and 9 but only N-9 has a hydrogen that can bond via dehydration synthesis.
- The monosaccharide glucose has a molecular formula What is the molecular formula for a polymer made by linking 10 glucose molecules together by dehydration synthesis?
- In dehydration synthesis, a molecule of water is removed from monomers of organic compounds in order to join them together by a chemical reaction to make polymers.
- A polymer of 10 glucoses formed by dehydration synthesis has the molecular formula
- Polysaccharides are made of many sugar molecules joined together by a glycosidic linkage or bond.
- The number of bonds needed is always one less than the number of monomers held together.
- For example, one bond is needed to create a disaccharide and nine bonds are needed to create a polymer made by linking 10 glucose molecules together.
- Because one water molecule is lost for each bond, the molecular formula for a disaccharide composed of glucose () is .
- The loss of nine molecules, or 18 hydrogens and nine oxygens, results in a molecular formula of
- The answer is incorrect because it implies that 10 water molecules are lost.
- The answer is incorrect because it implies that no water molecules are lost.
- The answer is incorrect because it implies that one water molecule is lost.
- Which answer lists a base, a nucleotide, and a nucleoside (in that order)?
Guanine, GMP, and guanosine
- “Guanine, GMP, and guanosine” is the correct answer, listing a base (guanine), a nucleotide (GMP), and a nucleoside (guanosine).
- The base component of a nucleotide can be guanine, cytosine, adenine, or thymine in DNA (or uracil in RNA).
- A nucleotide consists of a nitrogenous base, a sugar (ribose or deoxyribose), and one to three phosphate groups.
- A nucleotide containing the base guanine is referred to as GMP, GDP, or GTP, depending on the number of phosphate groups attached.
- A nucleoside is a nucleotide without the phosphate group.
- Examples include guanosine, cytidine, adenosine, thymidine, and uridine.
- The answer “Guanine, guanosine, and GMP” is incorrect because this answer lists a base, a nucleoside, and a nucleotide.
- The answer listing “Guanosine, GMP, and guanine” is incorrect because this answer lists a nucleoside, a nucleotide, and a base.
- The answer listing “Guanosine, guanine, and GMP” is incorrect because this answer lists a nucleoside, a base, and a nucleotide.
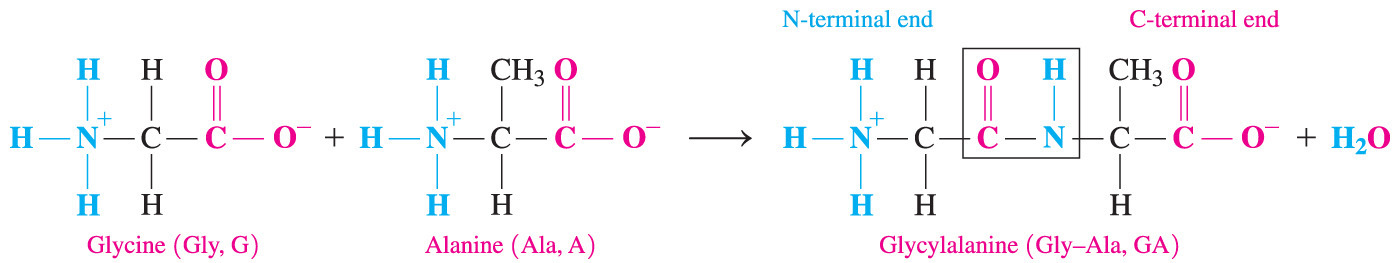
- Which statement is true concerning the peptide bond that forms between two amino acids?
The backbone carboxyl group from one amino acid and the backbone amino group from the other amino acid form a peptide bond.
- An amide bond forms between a carboxyl group and an amino group.
- A peptide bond is an amide bond that forms when the backbone carboxyl group from one amino acid attaches to the backbone amino group of the adjacent amino acid, due to loss of water (dehydration synthesis).
- The other answers are incorrect because the side chains or R groups of amino acids are never involved in peptide bond formation.
- In addition, amide bonds never form between two amino groups or two carboxyl groups.
- The presence of which of the following classifies a biomolecule as a fatty acid?
A long hydrocarbon chain attached to a carboxyl group
- Fatty acids contain a long hydrocarbon chain that is attached to a carboxyl group.
- The presence of the long aliphatic and nonpolar hydrocarbon tail causes fatty acids to be insoluble in water.
- The presence of a sugar, a base, and a phosphate classifies a biomolecule as a nucleotide.
- The presence of at least one amino and one carboxyl group classifies a biomolecule as an amino acid
- The presence of many hydroxyl groups and at least one carbonyl classifies a biomolecule as a monosaccharide.
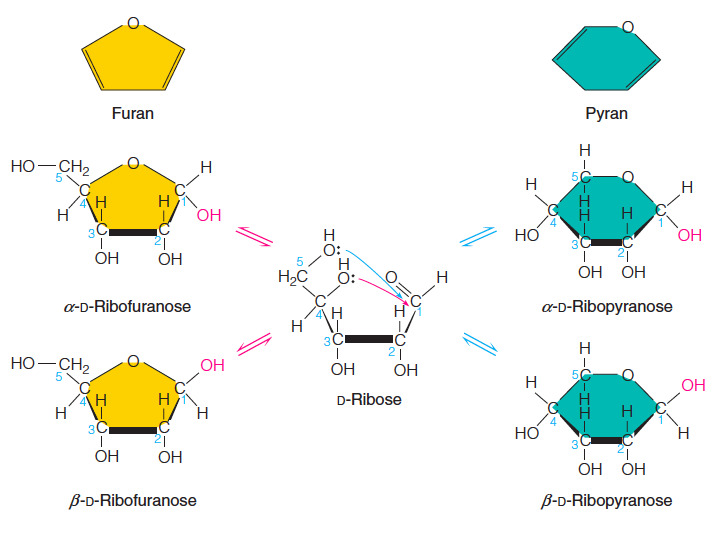
- Which statement correctly describes the difference between a pyranose and a furanose sugar?
All of these statements are true.
Aldohexoses form pyranose sugars and ketohexoses form furanose sugars
A pyranose contains a six-membered ring and a furanose contains a five-membered ring.
A pyranose has five chiral carbons and a furanose has four chiral carbons.
All of the statements correctly describe the difference between pyranose and furanose sugars.
- Pyranose sugars are derivatives of the organic compound pyran (a six-membered ring consisting of five carbon atoms and one oxygen atom) and furanose sugars are derivatives of the organic compound furan (a five-membered ring consisting of four carbon atoms and one oxygen atom).
- In both pyranoses and furanoses, all of the carbons in the ring are chiral or they are tetrahedral with four different substituents.
- Thus, a pyranose has five chiral carbons and a furanose has four chiral carbons.
- The process of converting the open-chain form to the cyclic form results in the formation of an additional chiral carbon that is referred to as the anomeric carbon.
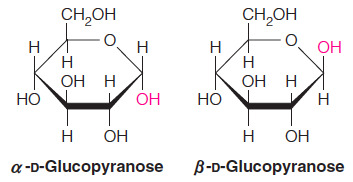
- In the simple three-dimensional representation of the cyclic monosaccharide, called a Haworth projection, the anomer has the hydroxyl group pointed downward and the anomer has it pointed upward.
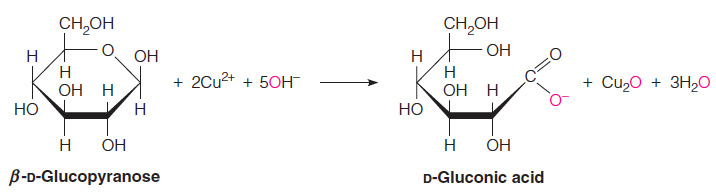
- Which of the following statements about monosaccharides is NOT true?
Monosaccharides are non-reducing sugars.
- The statement indicating that monosaccharides are non-reducing sugars is NOT true.
- All monosaccharides are reducing sugars because they have a free anomeric carbon that can be oxidized, and thus they can serve as reducing agents.
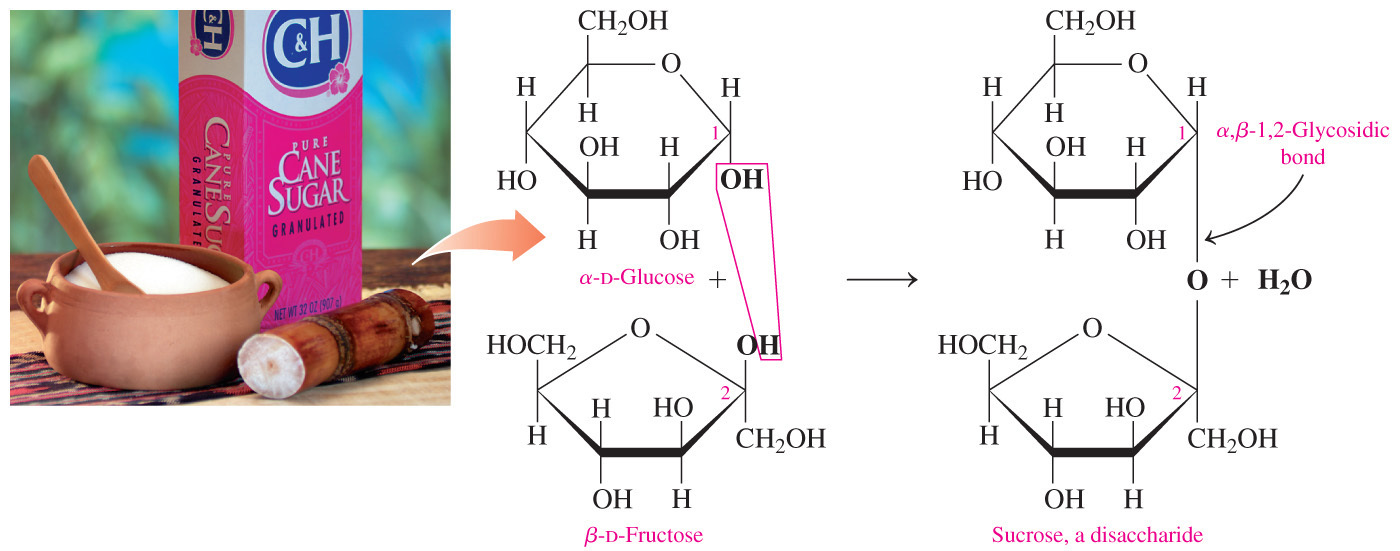
- When both (for a disaccharide) or almost all (for a polysaccharide) of the anomeric carbons are involved in the formation of the glycosidic bond, a sugar is classified as non-reducing.
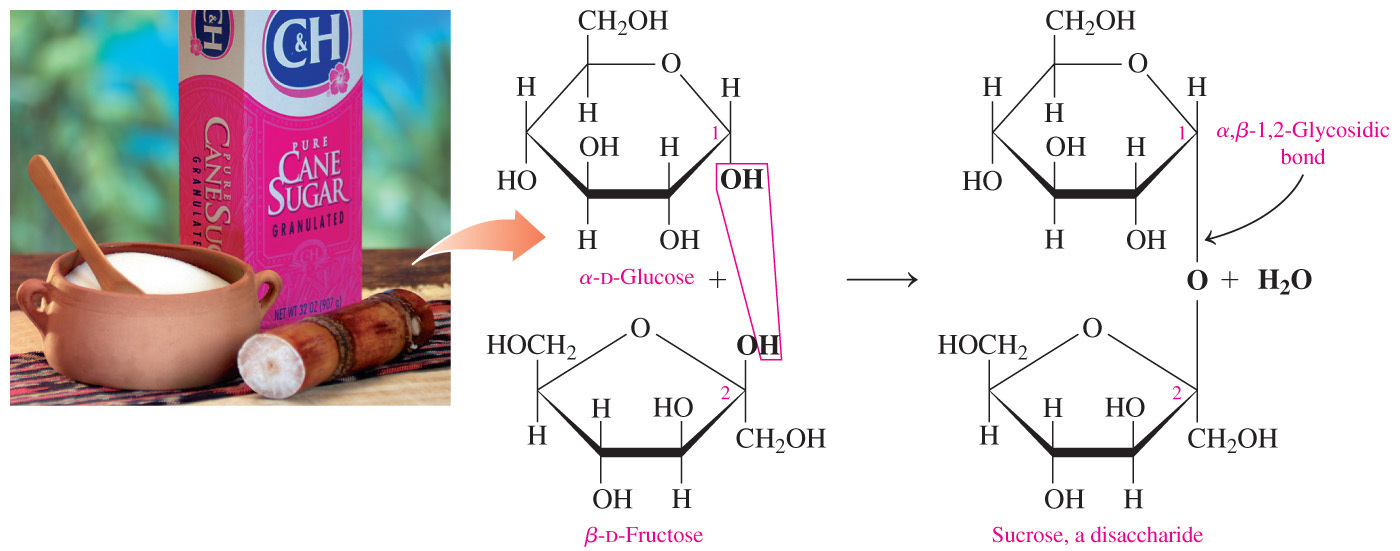
- Sucrose is a non-reducing disaccharide because carbon-1 of glucose is joined to carbon-2 of fructose and they both are anomeric carbons.
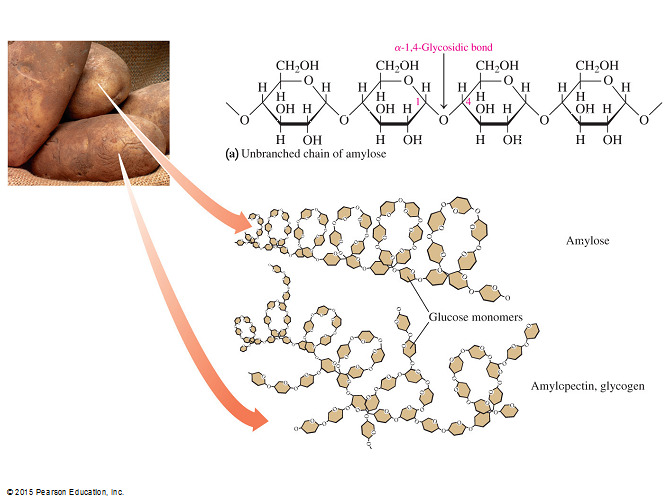
- Polysaccharides such as starch, glycogen, and cellulose are all non-reducing because only the terminal glucose in each molecule has its anomeric carbon free for oxidation.
- All the other statements are true for monosaccharides.
- They are not decomposable into simpler sugars by hydrolysis; they have the general formula , and they contain one carbonyl group and two or more hydroxyl groups.
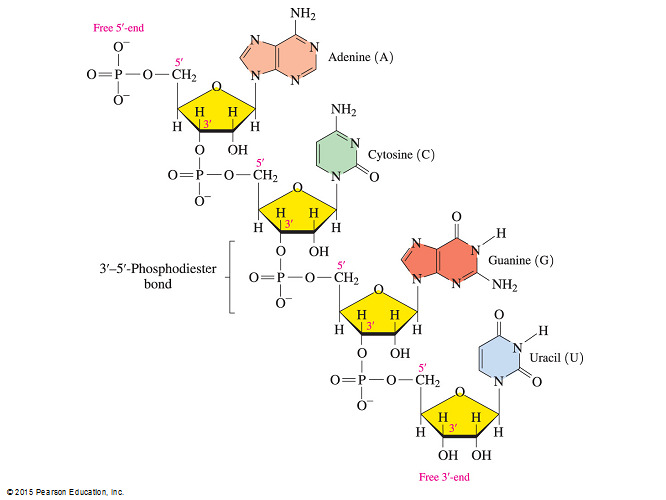
- Which statement is true concerning complementary base pairing between DNA nucleotides?
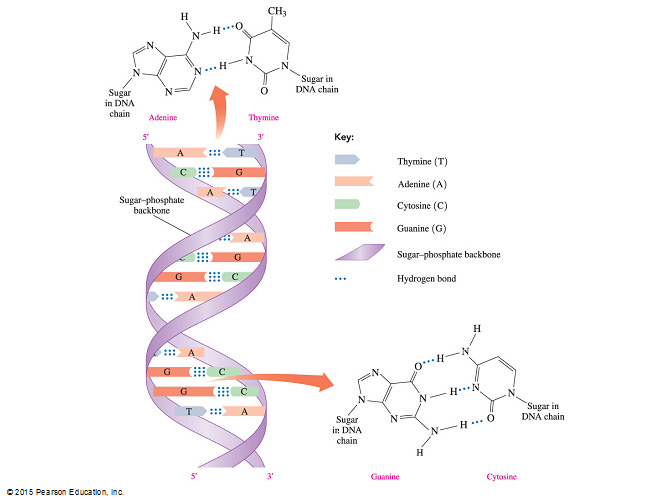
In the DNA double helix, adenine pairs with thymine and cytosine pairs with guanine.
- Two strands of DNA are held together in the shape of a double helix by the bonds between base pairs.
- Hydrogen bonding between the nitrogenous bases is one noncovalent attraction that maintains the double helical structure.
- Each base always pairs with a specific complementary base.
- For example, adenine pairs with thymine and cytosine pairs with guanine.
- Cytosine and thymine (as well as uracil in RNA) are the smaller bases that are classified as pyrimidines (a pyrimidine is a single six-member ring).
- Adenine and guanine are the larger bases that are classified as purines (a purine is a six-member ring fused to a five-member ring).
It is not true that any base can pair with any other base in the DNA double helix.
The nucleotides in a base pair must be complementary, which means their shape will allow them to bond together with hydrogen bonds.
Purine bases are complementary only with pyrimidine bases and the pairings found in nature, adenine and thymine (A-T) or guanine and cytosine (G-C), are the only combinations that result in the proper duplex structure.
The A-T pair forms two hydrogen bonds and the C-G pair forms three.
- The answer that states that adenine pairs with cytosine and guanine pairs with thymine is incorrect; A-C and G-T pairings are energetically unfavorable because they give a mismatched pattern of hydrogen donors and acceptors.
- The answer that states that adenine pairs with guanine and cytosine pairs with thymine is incorrect.
- Purine-purine base pairings (for example, A-G) are energetically unfavorable because the large molecules are too close, which leads to overlap, repulsion, and the inability for these pairs to fit within the helix.
- In addition, pyrimidine-pyrimidine base pairings (for example, C-T) are energetically unfavorable because the small molecules are too far apart and there is too much space for hydrogen bonds to form between them.
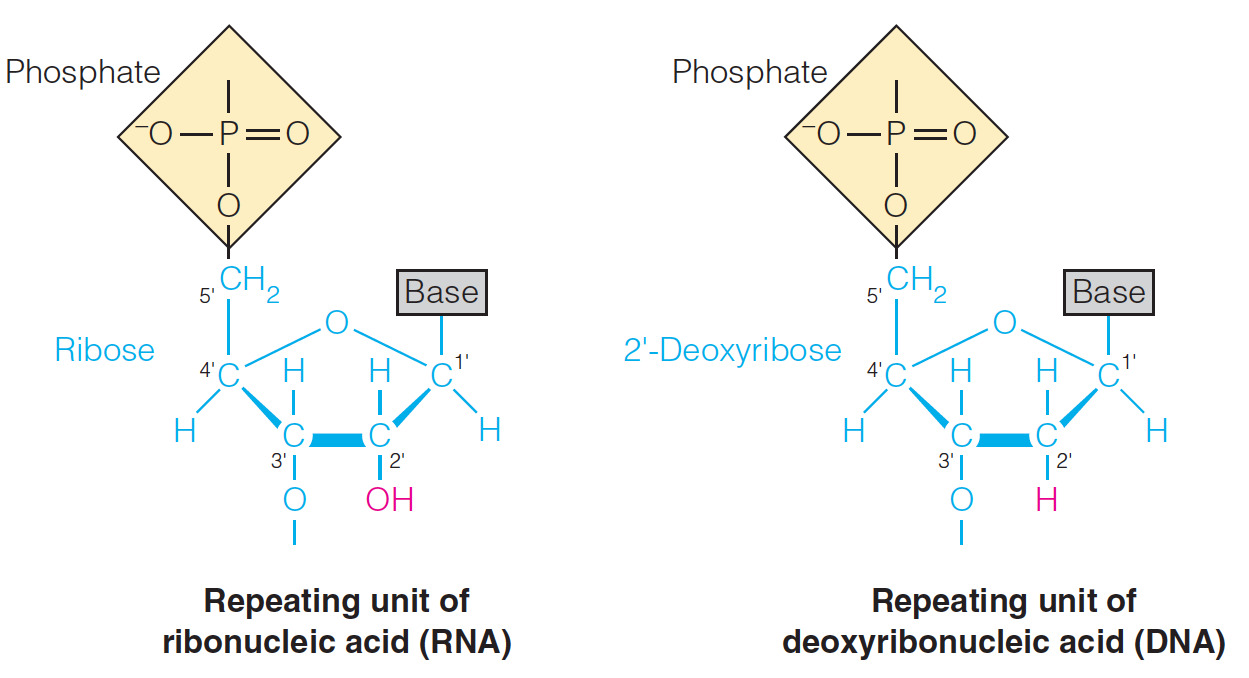
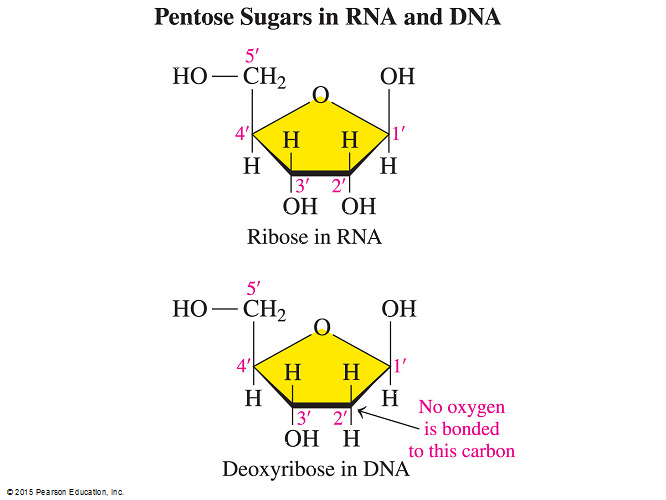
- Which statement describes a structural difference between the nucleotides of DNA and RNA?
The C2’ of the ribose sugar bonds to a hydroxyl group in RNA but to a hydrogen atom in DNA.
- Both DNA and RNA are polymers made up of repeating monomer units that are known as nucleotides.
- However, DNA and RNA differ structurally because in RNA carbon number 2 (C2’) of the ribose sugar is bonded to a hydroxyl group whereas in DNA C2’ bonds to a hydrogen atom instead.
- Carbons in the sugar are designated by primes to distinguish them from the carbons in the base.
- The nucleotides of both DNA and RNA consist of three components: a nitrogenous base, a pentose sugar, and a phosphate group.
- DNA and RNA differ from each other in the nature of the sugar component.
- RNA contains the sugar ribose and DNA has deoxyribose due to the replacement of 2’-OH with hydrogen.
- The answer indicating that C2’ of the ribose sugar in RNA bonds to the hydrogen and C2’ of the ribose sugar in DNA bonds to the hydroxyl is thus incorrect.
- The other two answer choices are also incorrect because, in both DNA and RNA, the 5’-OH of the ribose sugar bonds to a phosphate group that is involved in forming a phoshodiester linkage with the 3’-OH of the next repeating unit of the nucleic acid.
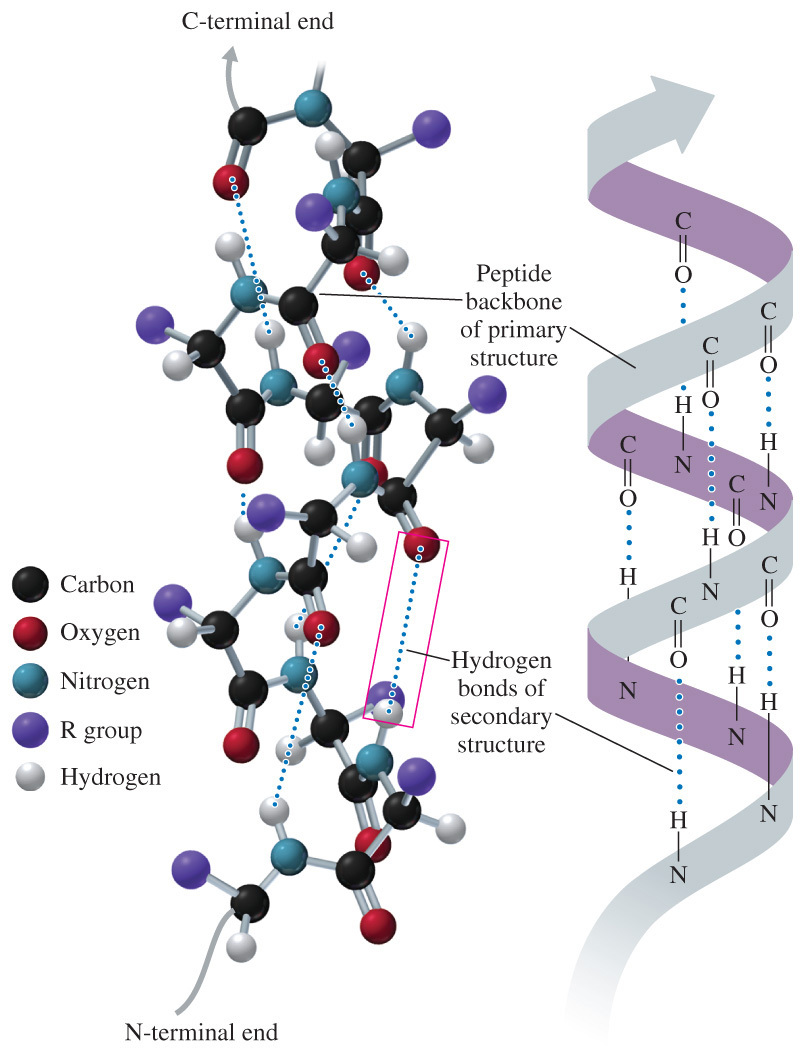
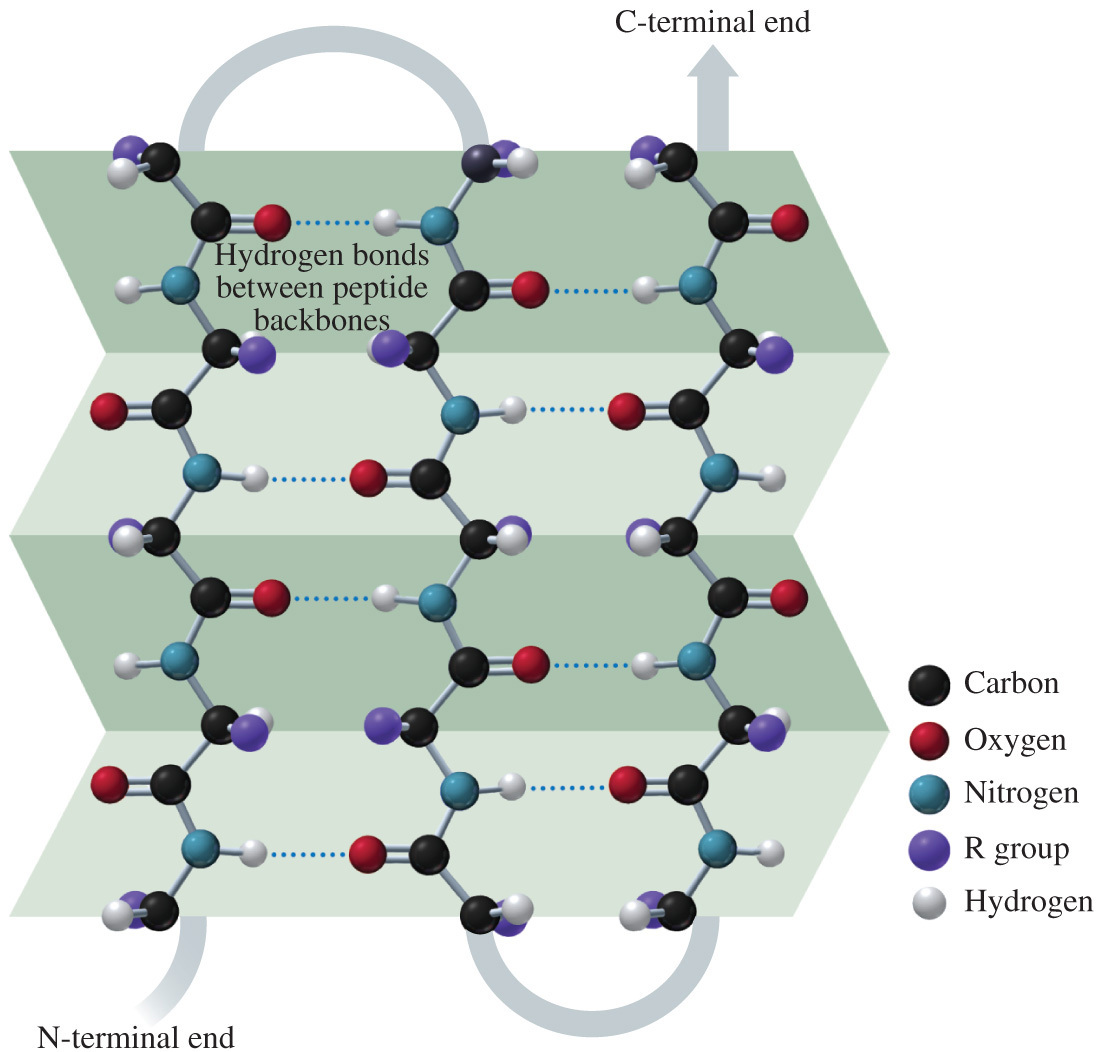
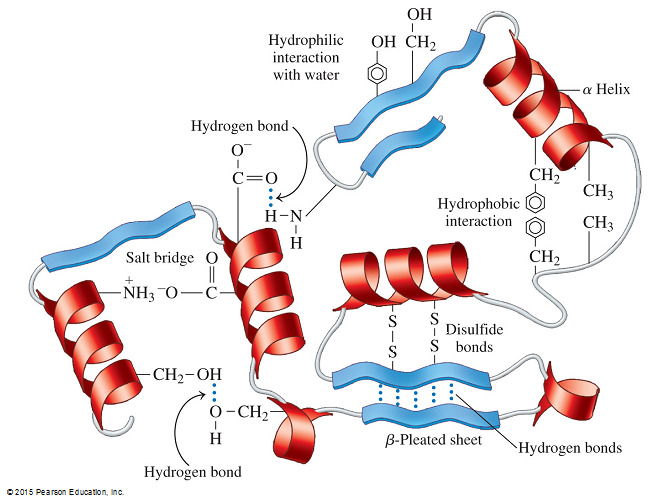
- Which of the following statements correctly describes the use of hydrogen bonding in proteins?
Secondary structures of proteins are held together by hydrogen bonding between the C=O and N-H of the backbone. Tertiary structure is held together by hydrogen bonding between the side chains of amino acids.
- Secondary structures of proteins are held together by hydrogen bonding between the C=O and N-H of the backbone.
- Tertiary structure is held together by hydrogen bonding between the side chains of amino acids.
- The α-helix and ß-pleated sheet are both examples of secondary protein structures that are stabilized by hydrogen bonding between the backbone.
- This structure results in local folding.
- Backbone hydrogen bonding does not hold together the tertiary structure of a protein.
- In addition to hydrogen bonding between amino acid side chains, a protein’s tertiary structure is stabilized by other forces, including hydrophobic and hydrophilic interactions, salt bridges, and covalent cross linking.
- Which of the following statements about monosaccharides is NOT true?
Monosaccharides are non-reducing sugars
- The statement indicating that monosaccharides are non-reducing sugars is NOT true.
- All monosaccharides are reducing sugars because they have a free anomeric carbon that can be oxidized, and thus they can serve as reducing agents.
- When both (for a disaccharide) or almost all (for a polysaccharide) of the anomeric carbons are involved in the formation of the glycosidic bond, a sugar is classified as non-reducing.
- Sucrose is a non-reducing disaccharide because carbon-1 of glucose is joined to carbon-2 of fructose and they both are anomeric carbons.
- Polysaccharides such as starch, glycogen, and cellulose are all non-reducing because only the terminal glucose in each molecule has its anomeric carbon free for oxidation.
- All the other statements are true for monosaccharides.
- They are not decomposable into simpler sugars by hydrolysis; they have the general formula and they contain one carbonyl group and two or more hydroxyl groups.
- What type of stereochemistry do most naturally occurring monounsaturated fatty acids exhibit?
Cis
Most naturally occurring monounsaturated fatty acids exhibit cis stereochemistry.
Trans fats are commonly a result of hydrogenation, a laboratory process commonly used in the food industry.
- Therefore, trans fats are not often found in nature.
- In a cis double bond, adjacent hydrogen atoms are on the same side of the double bond.
- This creates a bend or kink in the chain and limits the ability of fatty acids to be closely packed.
- As a result, the melting point of unsaturated fatty acids is lower than the melting point of saturated fats because it does not take as much heat energy to fully separate (or melt) the kinked and less closely packed molecules.
- Trans-fatty acids have higher melting points because adjacent hydrogens bond to opposite sides of the double bond.
- They have a shape that is similar to straight saturated fatty acids.
- Fatty acids are not represented by D and L stereochemistry because none of the carbons are chiral.
- In which pair does the first fatty acid have a lower melting point than the second?
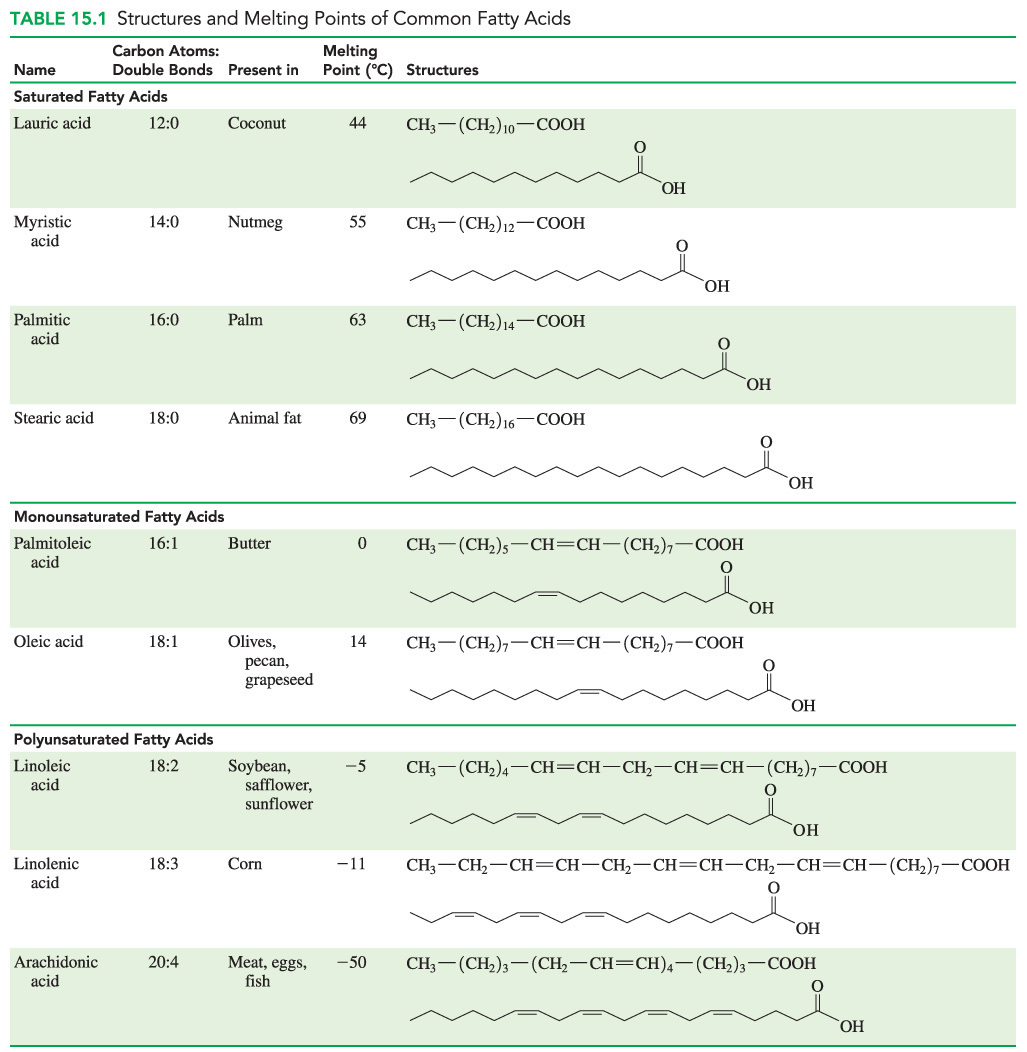
Linolenic acid (18 carbons, three double bonds) and arachidic acid (20 carbons, zero double bonds)
- Fatty acids with fewer carbons have lower melting points.
- Also, fatty acids with more double bonds have lower melting points.
- Linolenic acid (18 carbons, three double bonds) has a lower melting point than arachidic acid (20 carbons, zero double bonds).
- Linolenic acid has fewer carbons and more double bonds than arichidic acid.
- Oleic acid (18 carbons, one double bond) does not have a lower melting point than linoleic acid (18 carbons, two double bonds) because oleic acid has fewer double bonds.
- Stearic acid (18 carbons, zero double bonds) does not have a lower melting point than palmitoleic acid (16 carbons, one double bond) because stearic acid has more carbons and fewer double bonds.
- Myristic acid (14 carbons, zero double bonds) does not have a lower melting point than lauric acid (12 carbons, zero double bonds) because myristic acid has more carbons.
- Which list includes only pyrimidine bases?
Cytosine, thymine, and uracil
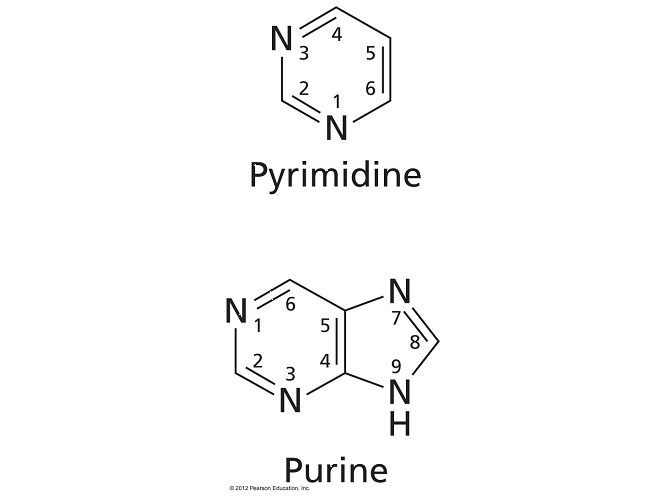
- All of the bases in nucleic acids are derivatives of the organic compounds pyrimidine and purine.
- Cytosine and thymine (as well as uracil in RNA) are classified as pyrimidine bases.
- Pyrimidine bases are the smaller bases that each have a single six-member ring. Adenine and guanine are purines, a bicyclic base where a six-member ring is fused to a five-member ring.
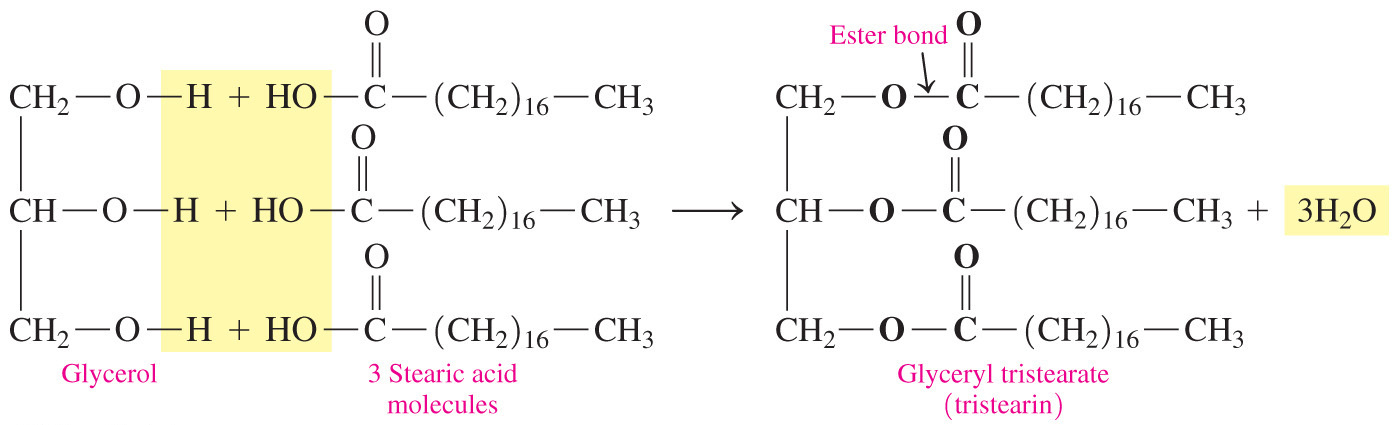
- Which type of functional group is present in triglycerides?
Ester
- Triglycerides contain an ester linkage that forms as a result of a dehydration synthesis reaction between glycerol and three fatty acids.
- A hydroxyl and a carboxyl react to form an ester.
- Two of the other answer choices are the functional groups found in glycerol (alcohol) and the fatty acid (carboxylic acid) before they combine to make the triglyceride. Ketones contain a carbonyl group and not a carboxyl; therefore, ketones is also an incorrect answer choice.