Gibbs Free Energy
$$\Delta G = \Delta H - \left(\ T * \Delta S\ \right)$$
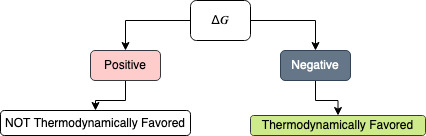
$$\Delta G = Heat\ of\ Reaction (\Delta H) - \left(\ Temperature (T_{kelvin}) * Entropy\ Change\ (\Delta S)\ \right)$$
$$\Delta G = \Delta H - \left(\ T * \Delta S\ \right)$$

$$\Delta G = Heat\ of\ Reaction (\Delta H) - \left(\ Temperature (T_{kelvin}) * Entropy\ Change\ (\Delta S)\ \right)$$