Chapter 15.1 - Figures
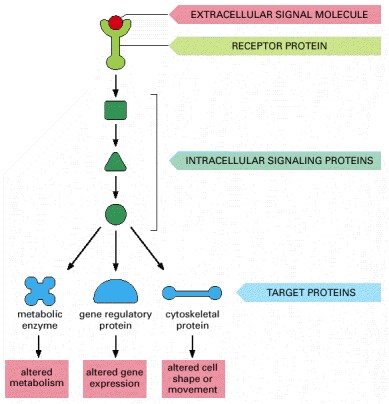
The signal molecule binds to a receptor protein (which is usually embedded in the plasma membrane), thereby activating an intracellular signaling pathway that is mediated by a series of signaling proteins. Finally, one or more of these intracellular signaling proteins interacts with a target protein, altering the target protein so that it helps to change the behavior of the cell.
Chapter 15.2 - Figures
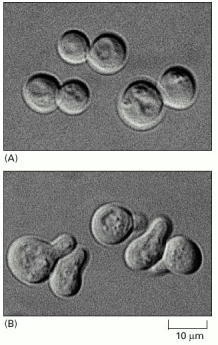
(A) The cells are normally spherical. (B) In response to mating factor secreted by neighboring yeast cells, they put out a protrusion toward the source of the factor in preparation for mating. (Courtesy of Michael Snyder.)
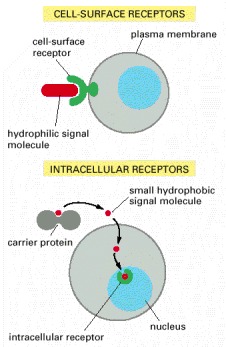
Most signal molecules are hydrophilic and are therefore unable to cross the plasma membrane directly; instead, they bind to cell-surface receptors, which in turn generate one or more signals inside the target cell. Some small signal molecules, by contrast, diffuse across the plasma membrane and bind to receptors inside the target cell—either in the cytosol or in the nucleus (as shown here). Many of these small signal molecules are hydrophobic and nearly insoluble in aqueous solutions; they are therefore transported in the bloodstream and other extracellular fluids after binding to carrier proteins, from which they dissociate before entering the target cell.
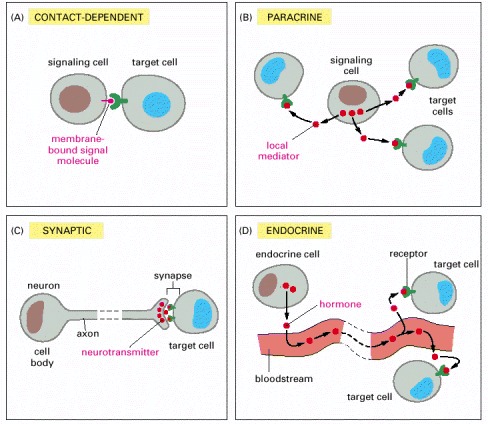
(A) Contact-dependent signaling requires cells to be in direct membrane-membrane contact. (B) Paracrine signaling depends on signals that are released into the extracellular space and act locally on neighboring cells. (C) Synaptic signaling is performed by neurons that transmit signals electrically along their axons and release neurotransmitters at synapses, which are often located far away from the cell body. (D) Endocrine signaling depends on endocrine cells, which secrete hormones into the bloodstream that are then distributed widely throughout the body. Many of the same types of signaling molecules are used in paracrine, synaptic, and endocrine signaling; the crucial differences lie in the speed and selectivity with which the signals are delivered to their targets.
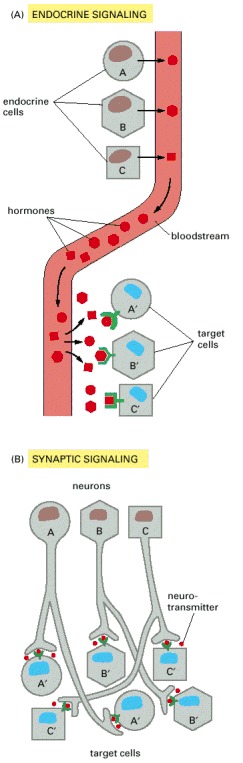
In complex animals, endocrine cells and nerve cells work together to coordinate the diverse activities of the billions of cells. Whereas different endocrine cells must use different hormones to communicate specifically with their target cells, different nerve cells can use the same neurotransmitter and still communicate in a highly specific manner. (A) Endocrine cells secrete hormones into the blood, which signal only the specific target cells that recognize them. These target cells have receptors for binding a specific hormone, which the cells “pull” from the extracellular fluid. (B) In synaptic signaling, by contrast, specificity arises from the synaptic contacts between a nerve cell and the specific target cells it signals. Usually, only a target cell that is in synaptic communication with a nerve cell is exposed to the neurotransmitter released from the nerve terminal (although some neurotransmitters act in a paracrine mode, serving as local mediators that influence multiple target cells in the area).
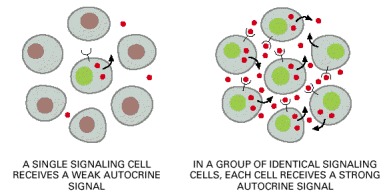
A group of identical cells produces a higher concentration of a secreted signal than does a single cell. When this signal binds back to a receptor on the same cell type, it encourages the cells to respond coordinately as a group.
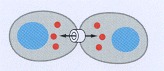
Cells connected by gap junctions share small molecules, including small intracellular signaling molecules, and can therefore respond to extracellular signals in a coordinated way.
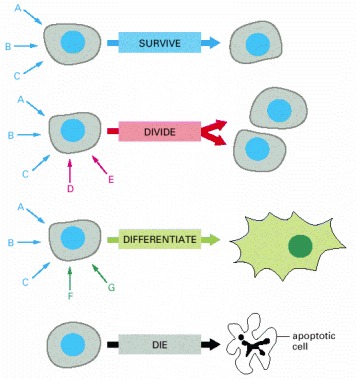
Each cell type displays a set of receptors that enables it to respond to a corresponding set of signal molecules produced by other cells. These signal molecules work in combinations to regulate the behavior of the cell. As shown here, an individual cell requires multiple signals to survive (blue arrows) and additional signals to divide (red arrow) or differentiate (green arrows). If deprived of appropriate survival signals, a cell will undergo a form of cell suicide known as programmed cell death, or apoptosis.
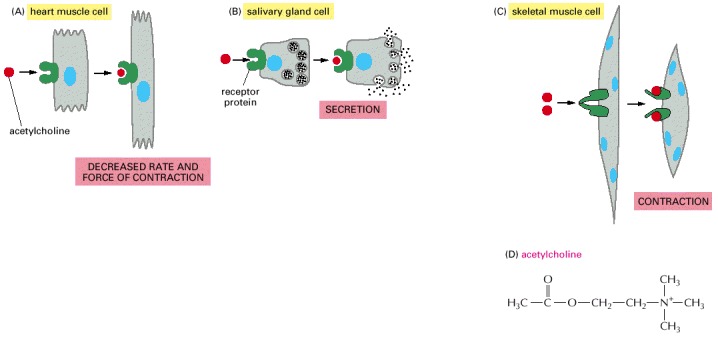
Different cell types are specialized to respond to acetylcholine in different ways. (A and B) For these two cell types, acetylcholine binds to similar receptor proteins, but the intracellular signals produced are interpreted differently in cells specialized for different functions. (C) This muscle cell produces a distinct type of receptor protein for acetylcholine, which generates different intracellular signals from the receptor shown in (A) and (B), and results in a different effect. (D) The chemical structure of acetylcholine.
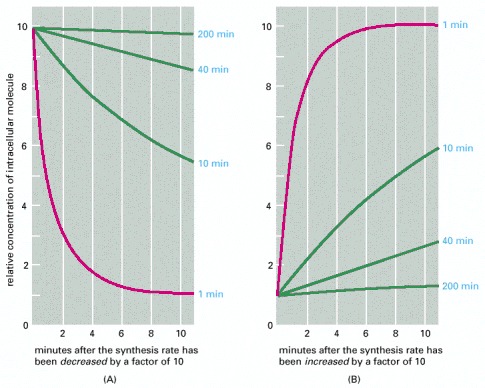
The graphs show the predicted relative rates of change in the intracellular concentrations of molecules with differing turnover times when their synthesis rates are either (A) decreased or (B) increased suddenly by a factor of 10. In both cases, the concentrations of those molecules that are normally being rapidly degraded in the cell (red lines) change quickly, whereas the concentrations of those that are normally being slowly degraded (green lines) change proportionally more slowly. The numbers (in blue) on the right are the half-lives assumed for each of the different molecules.
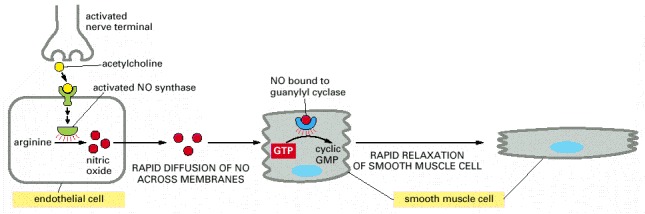
Acetylcholine released by nerve terminals in the blood vessel wall activates NO synthase in endothelial cells lining the blood vessel, causing the endothelial cells to produce NO. The NO diffuses out of the endothelial cells and into the underlying smooth muscle cells, where it binds to and activates guanylyl cyclase to produce cyclic GMP. The cyclic GMP triggers a response that causes the smooth muscle cells to relax, enhancing blood flow through the blood vessel.
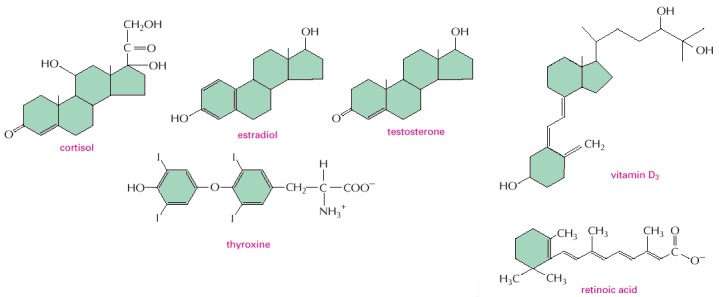
Note that all of them are small and hydrophobic. The active, hydroxylated form of vitamin D3 is shown. Estradiol and testosterone are steroid sex hormones.
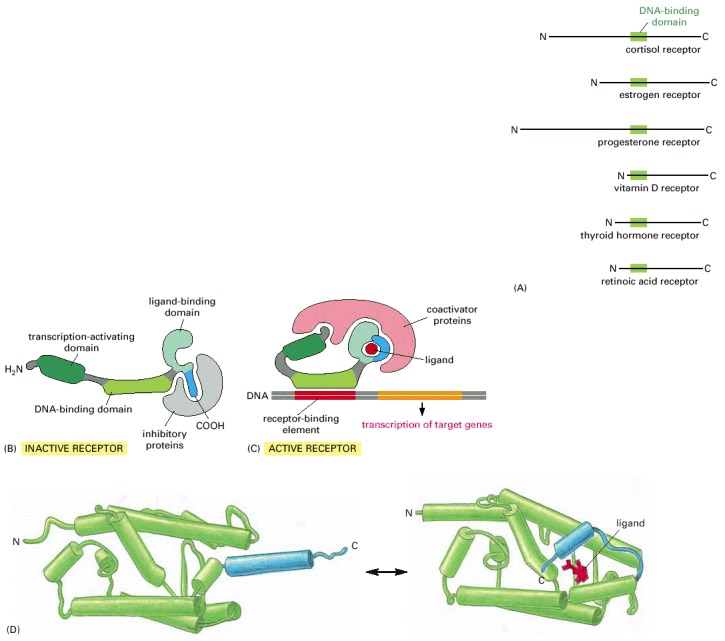
All nuclear hormone receptors bind to DNA as either homodimers or heterodimers, but for simplicity we show them as monomers here. (A) The receptors all have a related structure. The short DNA-binding domain in each receptor is shown in green. (B) A receptor protein in its inactive state is bound to inhibitory proteins. Domain-swap experiments suggest that many of the ligand-binding, transcription-activating, and DNA-binding domains in these receptors can function as interchangeable modules. (C) The binding of ligand to the receptor causes the ligand-binding domain of the receptor to clamp shut around the ligand, the inhibitory proteins to dissociate, and coactivator proteins to bind to the receptor's transcription-activating domain, thereby increasing gene transcription. (D) The three-dimensional structure of a ligand-binding domain with (right) and without (left) ligand bound. Note that the blue α helix acts as a lid that snaps shut when the ligand (shown in red) binds, trapping the ligand in place.
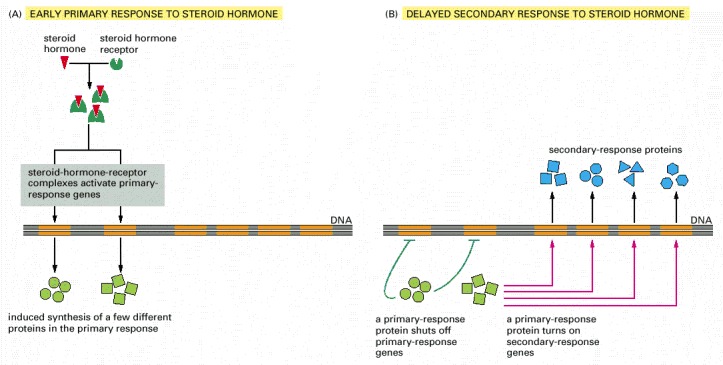
(A) Early primary response and (B) delayed secondary response. The figure shows the responses to a steroid hormone, but the same principles apply for all ligands that activate this family of receptor proteins. Some of the primary-response proteins turn on secondary-response genes, whereas others turn off the primary-response genes. The actual number of primary- and secondary-response genes is greater than shown. As expected, drugs that inhibit protein synthesis suppress the transcription of secondary-response genes but not primary-response genes, allowing these two classes of gene transcription responses to be readily distinguished.
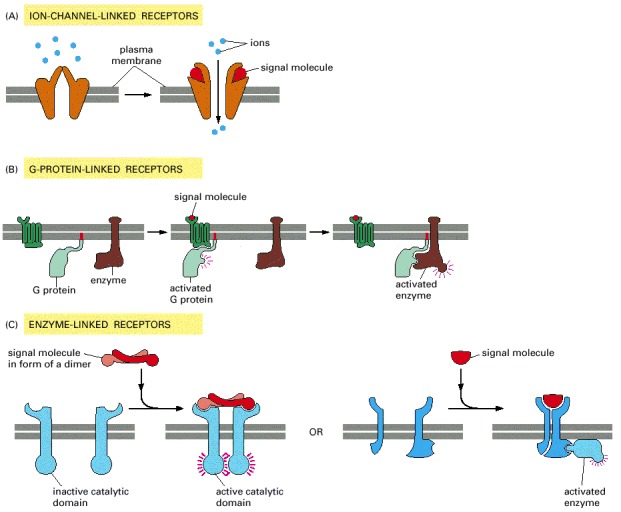
(A) Ion-channel-linked receptors, (B) G-protein-linked receptors, and (C) enzyme-linked receptors. Although many enzyme-linked receptors have intrinsic enzyme activity, as shown on the left, many others rely on associated enzymes, as shown on the right.
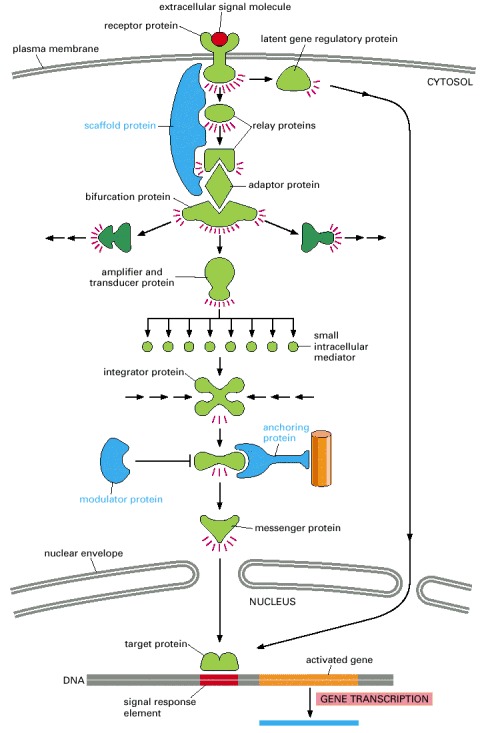
In this example, a series of signaling proteins and small intracellular mediators relay the extracellular signal into the cell, causing a change in gene expression. The signal is amplified, altered (transduced), and distributed en route. Many of the steps can be modulated by other extracellular and intracellular signals, so that the final result of one signal depends on other factors affecting the cell (see Figure 15-8). Ultimately, the signaling pathway activates (or inactivates) target proteins that alter cell behavior. In this example, the target is a gene regulatory protein.
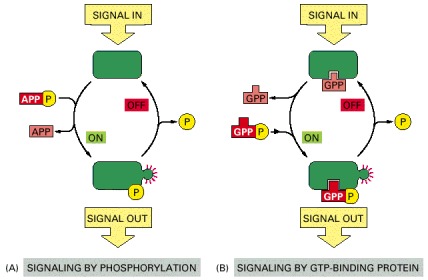
In both cases, a signaling protein is activated by the addition of a phosphate group and inactivated by the removal of the phosphate. (A) The phosphate is added covalently to the signaling protein by a protein kinase. (B) A signaling protein is induced to exchange its bound GDP for GTP. To emphasize the similarity in the two mechanisms, ATP is shown as APPP, ADP as APP, GTP as GPPP, and GDP as GPP.
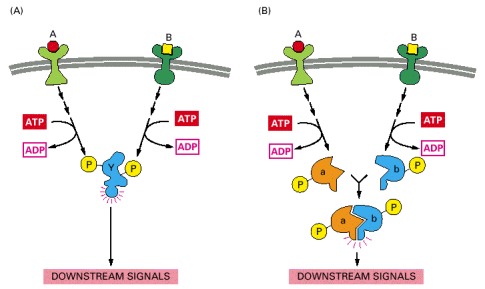
(A) Extracellular signals A and B both activate a different series of protein phosphorylations, each of which leads to the phosphorylation of protein Y but at different sites on the protein. Protein Y is activated only when both of these sites are phosphorylated, and therefore it becomes active only when signals A and B are simultaneously present. For this reason, integrator proteins are sometimes called coincidence detectors. (B) Extracellular signals A and B lead to the phosphorylation of two proteins, a and b, which then bind to each other to create the active protein. In both of the examples illustrated, the proteins themselves are phosphorylated. An equivalent form of control can also occur, however, by the exchange of GTP for GDP on GTP-binding proteins (see Figure 15-17).
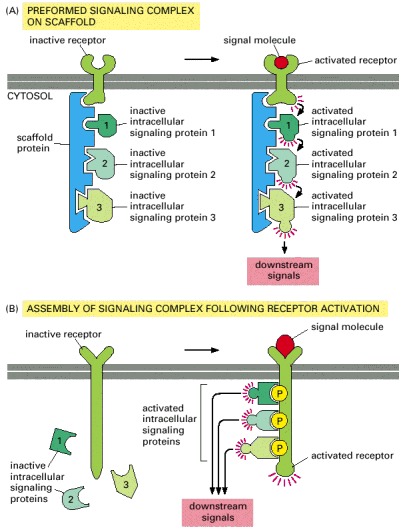
(A) A receptor and some of the intracellular signaling proteins it activates in sequence are preassembled into a signaling complex by a large scaffold protein. (B) A large signaling complex is assembled after a receptor has been activated by the binding of an extracellular signal molecule; here the activated receptor phosphorylates itself at multiple sites, which then act as docking sites for intracellular signaling proteins.
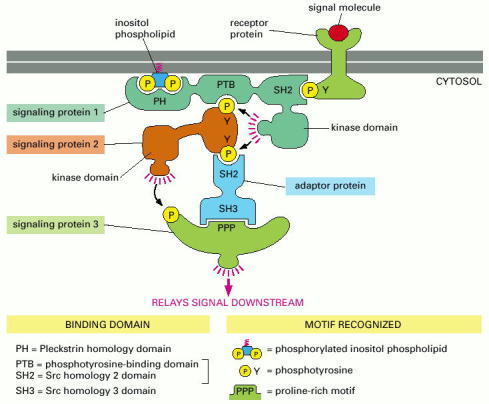
Signaling protein 1 contains three different binding domains, plus a catalytic protein kinase domain. It moves to the plasma membrane when extracellular signals lead to the creation of various phosphorylated docking sites on the cytosolic face of the membrane. Its SH2 domain binds to phosphorylated tyrosines on the receptor protein, and its PH domain binds to phosphorylated inositol phospholipids in the inner leaflet of the lipid bilayer. Protein 1 then phosphorylates signaling protein 2 on tyrosines, which allows protein 2 to bind to the PTB domain on protein 1 and to the SH2 domain on an adaptor protein. The adaptor protein then links protein 2 to protein 3, causing the phosphorylation of protein 3 by protein 2. The adaptor protein shown consists of two binding domains—an SH2 domain, which binds to a phosphotyrosine on protein 2, and an SH3 domain, which binds to a proline-rich motif on protein 3.
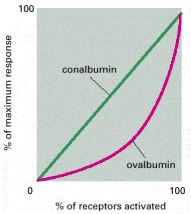
When activated, estradiol receptors turn on the transcription of several genes. Dose-response curves for two of these genes are shown, one coding for the egg protein conalbumin and the other coding for the egg protein ovalbumin. The linear response curve for conalbumin indicates that each activated receptor molecule that binds to the conalbumin gene increases the activity of the gene by the same amount. In contrast, the lag followed by the steep increase in the response curve for ovalbumin suggests that more than one activated receptor (in this case, two receptors) must bind simultaneously to the ovalbumin gene to initiate its transcription. (Adapted from E.R. Mulvihill and R.D. Palmiter, J. Biol. Chem. 252:2060–2068, 1977.)
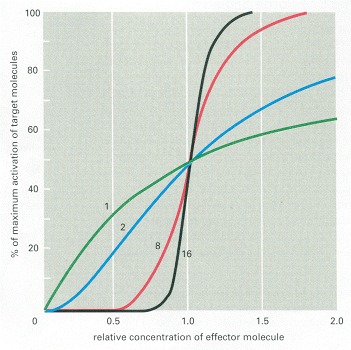
The curves show how the sharpness of the response increases with an increase in the number of effector molecules that must bind simultaneously to activate a target macromolecule. The curves shown are those expected if the activation requires the simultaneous binding of 1, 2, 8, or 16 effector molecules.
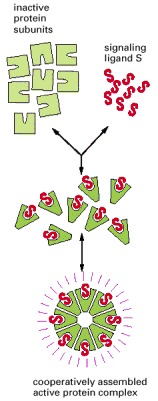
Here, the simultaneous binding of eight molecules of a signaling ligand to a set of eight protein subunits is required to form an active protein complex. The ability of the subunits to assemble into the active complex depends on an allosteric conformational change that the subunits undergo when they bind their ligand. The binding of the ligand in the formation of such a complex is generally a cooperative process, causing a steep response as the ligand concentration is changed, as explained in Chapter 3. At low ligand concentrations, the number of active complexes increases roughly in proportion to the eighth power of the ligand concentration.
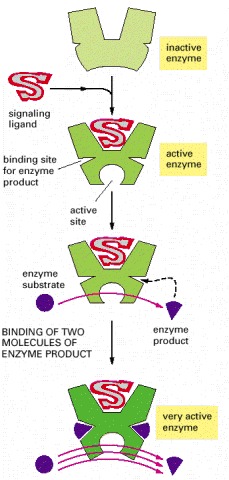
In this example, the initial binding of the signaling ligand activates the enzyme to generate a product that binds back to the enzyme, further increasing the enzyme's activity.
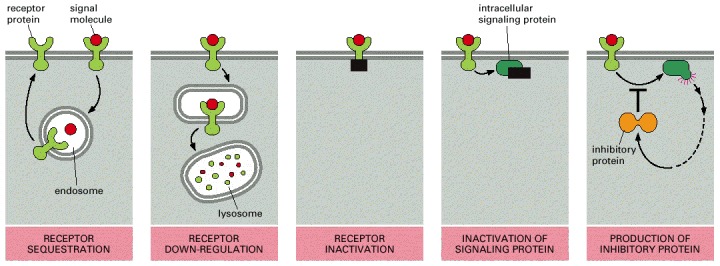
The inactivation mechanisms shown here for both the receptor and the intracellular signaling protein often involve phosphorylation of the protein that is inactivated, although other types of modification are also known to occur. In bacterial chemotaxis, which we discuss later, desensitization depends on methylation of the receptor protein.
Chapter 15.3 - Figures
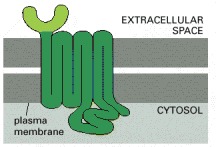
Receptors that bind protein ligands have a large extracellular domain formed by the part of the polypeptide chain shown in light green. This domain, together with some of the transmembrane segments, binds the protein ligand. Receptors for small ligands such as adrenaline have small extracellular domains, and the ligand usually binds deep within the plane of the membrane to a site that is formed by amino acids from several transmembrane segments.
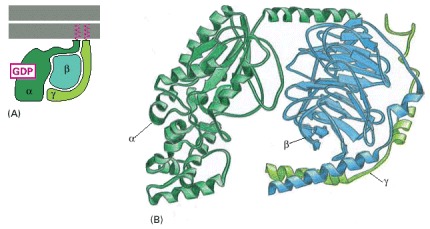
(A) Note that both the α and the γ subunits have covalently attached lipid molecules (red) that help to bind them to the plasma membrane, and the α subunit has GDP bound. (B) The three-dimensional structure of an inactive G protein, based on transducin, the G protein in visual transduction (discussed later). The α subunit contains the GTPase domain and binds to one side of the β subunit, which locks the GTPase domain in an inactive conformation that binds GDP. The γ subunit binds to the opposite side of the β subunit. (B, based on D.G. Lombright et al., Nature 379:311–319, 1996.)
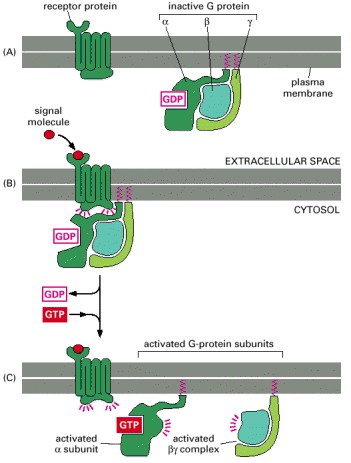
(A) In the unstimulated state, the receptor and the G protein are both inactive. Although they are shown here as separate entities in the plasma membrane, in some cases, at least, they are associated in a preformed complex. (B) Binding of an extracellular signal to the receptor changes the conformation of the receptor, which in turn alters the conformation of the G protein that is bound to the receptor. (C) The alteration of the α subunit of the G protein allows it to exchange its GDP for GTP. This causes the G protein to break up into two active components—an α subunit and a βγ complex, both of which can regulate the activity of target proteins in the plasma membrane. The receptor stays active while the external signal molecule is bound to it, and it can therefore catalyze the activation of many molecules of G protein.
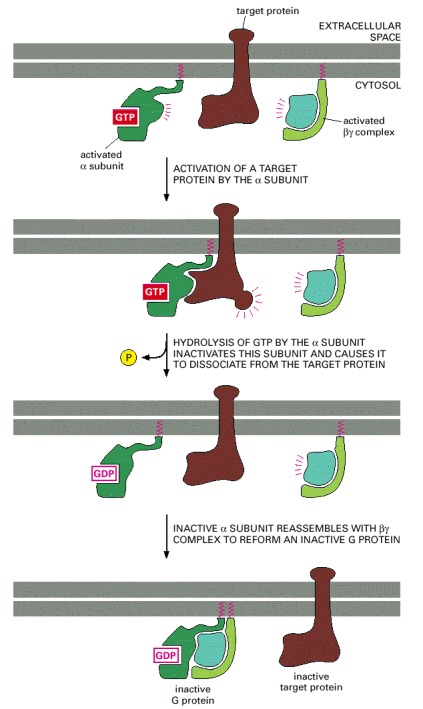
After a G-protein α subunit activates its target protein, it shuts itself off by hydrolyzing its bound GTP to GDP. This inactivates the α subunit, which dissociates from the target protein and reassociates with a βγ complex to re-form an inactive G protein. Binding to the target protein or to a membrane-bound RGS protein (not shown) usually stimulates the GTPase activity of the α subunit; this stimulation greatly speeds up the inactivation process shown here.
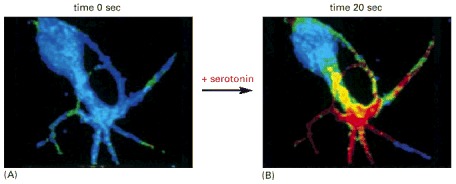
This nerve cell in culture is responding to the neurotransmitter serotonin, which acts through a G-protein-linked receptor to cause a rapid rise in the intracellular concentration of cyclic AMP. To monitor the cyclic AMP level, the cell has been loaded with a fluorescent protein that changes its fluorescence when it binds cyclic AMP. Blue indicates a low level of cyclic AMP, yellow an intermediate level, and red a high level. (A) In the resting cell, the cyclic AMP level is about 5 × 10-8 M. (B) Twenty seconds after the addition of serotonin to the culture medium, the intracellular level of cyclic AMP has increased to more than 10-6 M, an increase of more than twentyfold. (From Brian J. Bacskai et al., Science 260:222–226, 1993. © AAAS.)
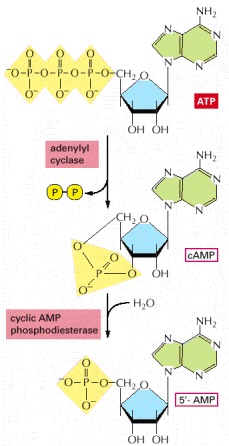
In a reaction catalyzed by the enzyme adenylyl cyclase, cyclic AMP (cAMP) is synthesized from ATP through a cyclization reaction that removes two phosphate groups as pyrophosphate ( —
— ); a pyrophosphatase drives this synthesis by hydrolyzing the released pyrophosphate to phosphate (not shown). Cyclic AMP is unstable in the cell, because it is itself hydrolyzed by a specific phosphodiesterase to form 5′-AMP, as indicated.
); a pyrophosphatase drives this synthesis by hydrolyzing the released pyrophosphate to phosphate (not shown). Cyclic AMP is unstable in the cell, because it is itself hydrolyzed by a specific phosphodiesterase to form 5′-AMP, as indicated.
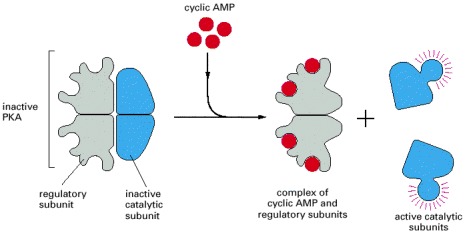
The binding of cyclic AMP to the regulatory subunits induces a conformational change, causing these subunits to dissociate from the catalytic subunits, thereby activating the kinase activity of the catalytic subunits. The release of the catalytic subunits requires the binding of more than two cyclic AMP molecules to the regulatory subunits in the tetramer. This requirement greatly sharpens the response of the kinase to changes in cyclic AMP concentration, as discussed earlier. Mammalian cells have at least two types of PKAs: type I is mainly in the cytosol, whereas type II is bound via its regulatory subunit and special anchoring proteins to the plasma membrane, nuclear membrane, mitochondrial outer membrane, and microtubules. In all cases, however, once the catalytic subunits are freed and active, they can migrate into the nucleus (where they can phosphorylate gene regulatory proteins), while the regulatory subunits remain in the cytoplasm. The three-dimensional structure of the protein kinase domain of the PKA catalytic subunit is shown in Figure 3-64.
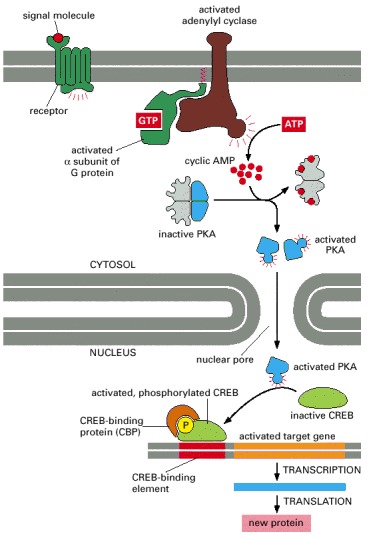
The binding of an extracellular signal molecule to its G-protein-linked receptor leads to the activation of adenylyl cyclase and a rise in cyclic AMP concentration. The increase in cyclic AMP concentration activates PKA in the cytosol, and the released catalytic subunits then move into the nucleus, where they phosphorylate the CREB gene regulatory protein. Once phosphorylated, CREB recruits the coactivator CBP, which stimulates gene transcription. This signaling pathway controls many processes in cells, ranging from hormone synthesis in endocrine cells to the production of proteins required for long-term memory in the brain. We shall see later that some kinases that are activated by a rise in intracellular Ca2+ can also phosphorylate and thereby activate CREB.
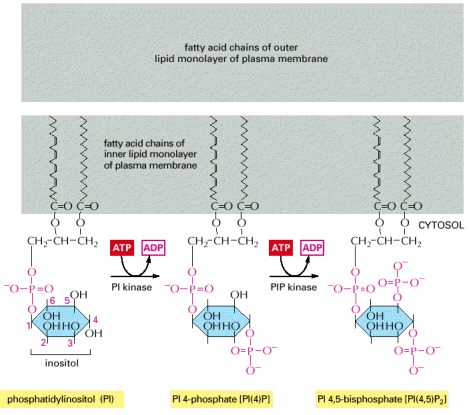
The polyphosphoinositides—PI(4)P and PI(4,5)P2—are produced by the phosphorylation of phosphatidylinositol (PI) and PI(4)P, respectively. Although all three inositol phospholipids may be broken down in a signaling response, it is the breakdown of PI(4,5)P2 that is most critical because it generates two intracellular mediators, as shown in the next two figures. Nevertheless, PI(4,5)P2 is the least abundant, constituting less than 10% of the total inositol lipids and less than 1% of the total phospholipids in a cell. The conventional numbering of the carbon atoms in the inositol ring is shown in red numbers on the PI molecule.
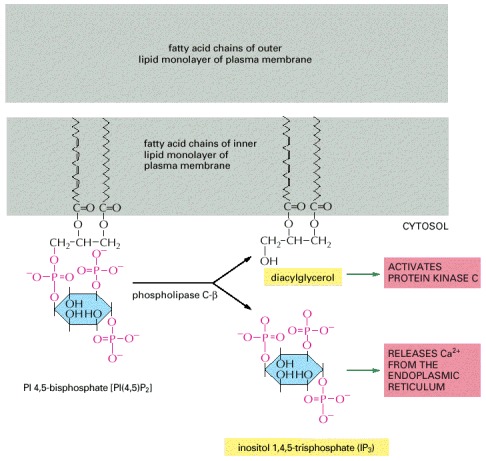
Two intracellular mediators are produced when PI(4,5)P2 is hydrolyzed: inositol 1,4,5-trisphosphate (IP3), which diffuses through the cytosol and releases Ca2+ from the ER, and diacylglycerol, which remains in the membrane and helps to activate the enzyme protein kinase C (see Figure 15-36). There are at least three classes of phospholipase C—β, γ, and σ—and it is the β class that is activated by G-protein-linked receptors. We shall see later that the γ class is activated by a second class of receptors, called receptor tyrosine kinases, that activate the inositol phospholipid signaling pathway without an intermediary G protein.
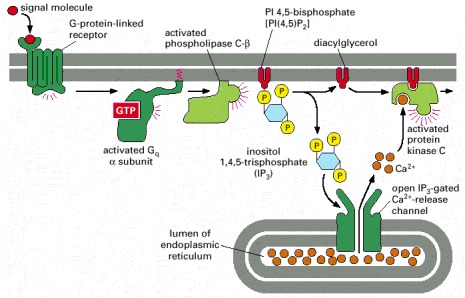
The activated receptor stimulates the plasma-membrane-bound enzyme phospholipase C-β via a G protein. Depending on the isoform of the enzyme, it may be activated by the α subunit of Gq as shown, by the βγ complex of another G protein, or by both. Two intracellular messenger molecules are produced when PI(4,5)P2 is hydrolyzed by the activated phospholipase C-β. Inositol 1,4,5-trisphosphate (IP3) diffuses through the cytosol and releases Ca2+ from the endoplasmic reticulum by binding to and opening IP3-gated Ca2+-release channels in the endoplasmic reticulum membrane. The large electrochemical gradient for Ca2+ across this membrane causes Ca2+ to escape into the cytosol. Diacylglycerol remains in the plasma membrane and, together with phosphatidylserine (not shown) and Ca2+, helps to activate the enzyme protein kinase C, which is recruited from the cytosol to the cytosolic face of the plasma membrane. Of the 11 or more distinct isoforms of PKC in mammals, at least four are activated by diacylglycerol.
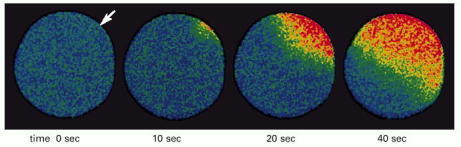
This starfish egg was injected with a Ca2+-sensitive fluorescent dye before it was fertilized. A wave of cytosolic Ca2+ (red), released from the endoplasmic reticulum, is seen to sweep across the egg from the site of sperm entry (arrow). This Ca2+ wave provokes a change in the egg cell surface, preventing the entry of other sperm, and it also initiates embryonic development (discussed in Chapter 20). (Courtesy of Stephen A. Stricker.)
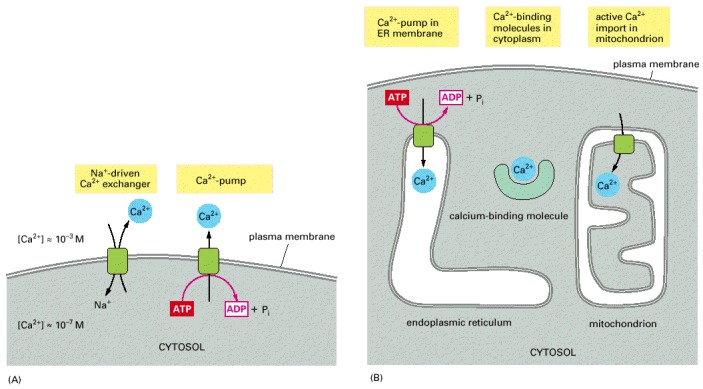
(A) Ca2+ is actively pumped out of the cytosol to the cell exterior. (B) Ca2+ is pumped into the ER and mitochondria, and various molecules in the cell bind free Ca2+ tightly.
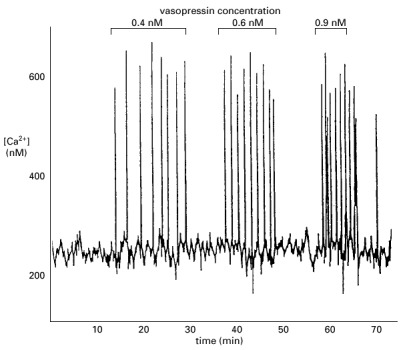
The cell was loaded with the Ca2+-sensitive protein aequorin and then exposed to increasing concentrations of vasopressin. Note that the frequency of the Ca2+ spikes increases with an increasing concentration of vasopressin, but that the amplitude of the spikes is not affected. (Adapted from N.M. Woods, K.S.R. Cuthbertson, and P.H. Cobbold, Nature 319:600–602, 1986.)
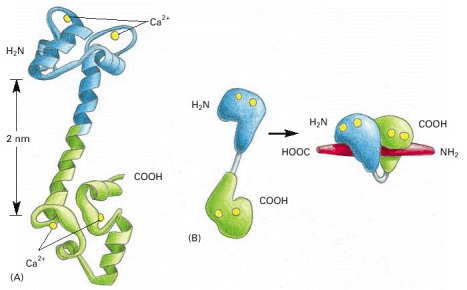
(A) The molecule has a “dumbbell” shape, with two globular ends connected by a long, exposed α helix. Each end has two Ca2+-binding domains, each with a loop of 12 amino acids, in which aspartic acid and glutamic acid side chains form ionic bonds with Ca2+. The two Ca2+-binding sites in the carboxyl-terminal part of the molecule have a tenfold higher affinity for Ca2+ than the two in the amino-terminal part. In solution, the molecule is flexible, displaying a range of forms, from extended (as shown) to more compact. (B) The major structural change in Ca2+/calmodulin that occurs when it binds to a target protein (in this example, a peptide that consists of the Ca2+/calmodulin-binding domain of a Ca2+/calmodulin-dependent protein kinase). Note that the Ca2+/calmodulin has “jack-knifed” to surround the peptide. (A, based on x-ray crystallographic data from Y.S. Babu et al., Nature 315:37–40, 1985; B, based on x-ray crystallographic data from W.E. Meador, A.R. Means, and F.A. Quiocho, Science 257:1251–1255, 1992, and on NMR data from M. Ikura et al., Science 256:632–638, 1992. © AAAS.)
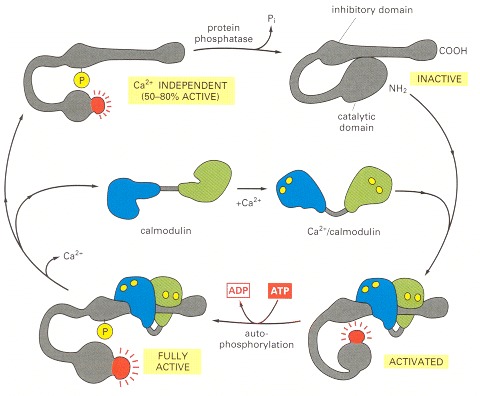
The enzyme is a large protein complex of about 12 subunits, although, for simplicity, only one subunit is shown. The subunits are of four homologous kinds (α, β, γ, and σ), which are expressed in different proportions in different cell types. In the absence of Ca2+/calmodulin, the enzyme is inactive as the result of an interaction between the inhibitory domain and the catalytic domain. The binding of Ca2+/calmodulin alters the conformation of the protein, allowing the catalytic domain to phosphorylate the inhibitory domain of neighboring subunits in the complex, as well as other proteins in the cell (not shown). The autophosphorylation of the enzyme complex (by mutual phosphorylation of its subunits) prolongs the activity of the enzyme in two ways. First, it traps the bound Ca2+/calmodulin so that it does not dissociate from the enzyme complex until cytosolic Ca2+ levels return to basal values for at least 10 seconds (not shown). Second, it converts the enzyme to a Ca2+-independent form so that the kinase remains active even after the Ca2+/calmodulin dissociates from it. This activity continues until the autophosphorylation process is overridden by a protein phosphatase.
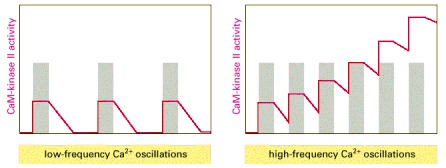
(A) At low frequencies of Ca2+ spikes (gray bars), the enzyme becomes inactive after each spike, as the autophosphorylation induced by Ca2+/calmodulin binding does not maintain the enzyme's activity long enough for the enzyme to remain active until the next Ca2+ spike arrives. (B) At higher spike frequencies, however, the enzyme fails to inactivate completely between Ca2+ spikes, so its activity ratchets up with each spike. If the spike frequency is high enough, this progressive increase in enzyme activity would continue until the enzyme is autophosphorylated on all subunits and is therefore maximally activated. Once enough of its subunits are autophosphorylated, the enzyme can be maintained in a highly active state even with a relatively low frequency of Ca2+ spikes (a form of cell memory). The binding of Ca2+/calmodulin to the enzyme is enhanced by the CaM-kinase II autophosphorylation (a form of positive feedback), causing the response of the enzyme to repeated Ca2+ spikes to exhibit a steep threshold in its frequency response, as discussed earlier.
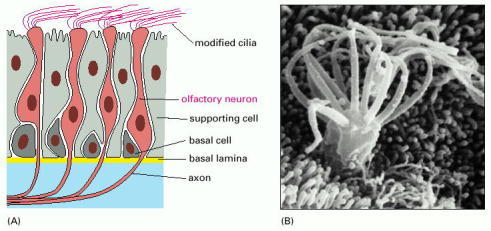
(A) This drawing shows a section of olfactory epithelium in the nose. Olfactory receptor neurons possess modified cilia, which project from the surface of the epithelium and contain the olfactory receptors, as well as the signal transduction machinery. The axon, which extends from the opposite end of the receptor neuron, conveys electrical signals to the brain when the cell is activated by an odorant to produce an action potential. The basal cells act as stem cells, producing new receptor neurons throughout life, to replace the neurons that die.
(B) A scanning electron micrograph of the cilia on the surface of an olfactory neuron. (B, from E.E. Morrison and R.M. Costanzo, J. Comp. Neurol. 297:1–13, 1990. © Wiley-Liss, Inc.)
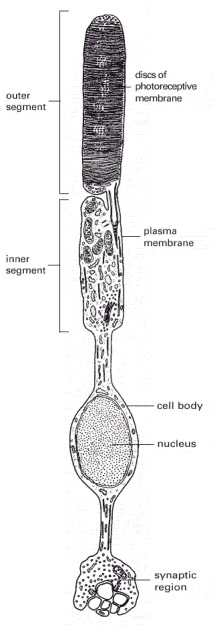
There are about 1000 discs in the outer segment. The disc membranes are not connected to the plasma membrane.
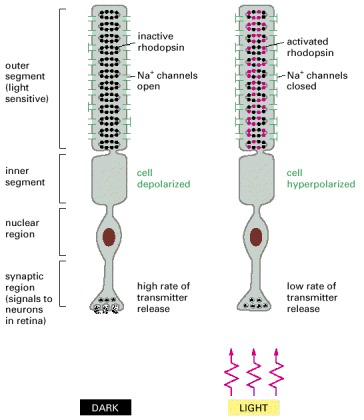
Rhodopsin molecules in the outer-segment discs absorb photons. Photon absorption leads to the closure of Na+ channels in the plasma membrane, which hyperpolarizes the membrane and reduces the rate of neurotransmitter release from the synaptic region. Because the neurotransmitter acts to inhibit many of the postsynaptic retinal neurons, illumination serves to free the neurons from inhibition and thus, in effect, excites them.
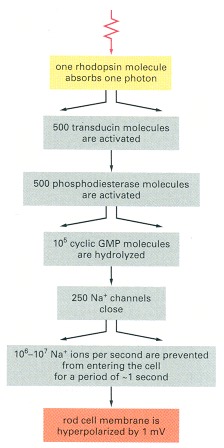
The divergent arrows indicate the steps where amplification occurs.
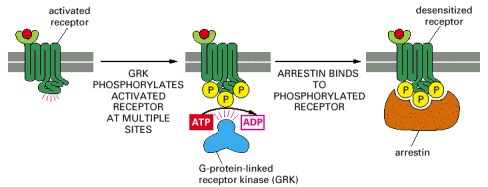
The binding of an arrestin to the phosphorylated receptor prevents the receptor from binding to its G protein and can direct its endocytosis. Mice that are deficient in one form of arrestin fail to desensitize in response to morphine, for example, attesting to the importance of arrestins for desensitization.
Chapter 15.4 - Figures
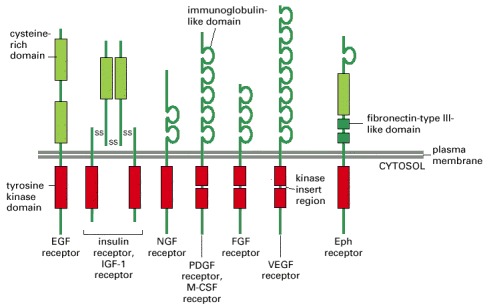
Only one or two members of each subfamily are indicated. Note that the tyrosine kinase domain is interrupted by a “kinase insert region” in some of the subfamilies. The functional roles of most of the cysteine-rich, immunoglobulin-like, and fibronectin-type III-like domains are not known. Some of the ligands and responses for the receptors shown are listed in Table 15-4.
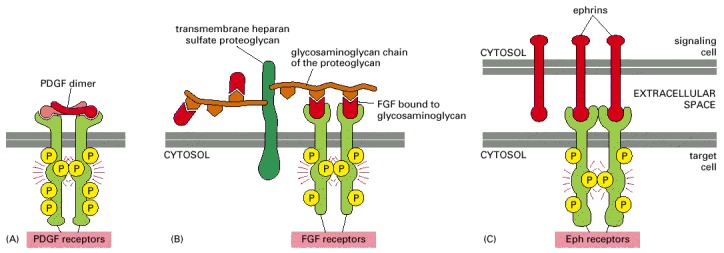
When the receptor chains are cross-linked, the kinase domains of adjacent receptors cross-phosphorylate each other, stimulating the kinase activity of the receptor and creating docking sites for intracellular proteins. (A) Platelet-derived growth factor (PDGF) is a covalently linked dimer with two receptor-binding sites, so it can directly cross-link adjacent receptors to initiate the intracellular signaling process. The PDGF dimers are composed of A and B chains in different combinations, and they can activate different combinations of two types of PDGF receptor chains (α and β), which have somewhat different signaling properties. (B) Some monomeric ligands, such as fibroblast growth factors (FGFs), bind in clusters to proteoglycans, enabling the ligands to cross-link their receptors. The proteoglycans can be in the extracellular matrix or, as shown here, on the cell surface. There are more than 20 types of FGFs and more than 4 types of FGF receptors. (C) Membrane-bound signaling proteins, such as ephrins, can cross-link their receptors even though they are monomeric, because they cluster in the plasma membrane of the signaling cell. Some ephrins (B-type) are transmembrane proteins (as shown), whereas others (A-type) are linked to the membrane by a glycosylphosphatidylinositol (GPI) anchor (see Figure 12-56). For the most part, A-type ephrins activate A-type Eph receptors, and B-type ephrins activate B-type Eph receptors.
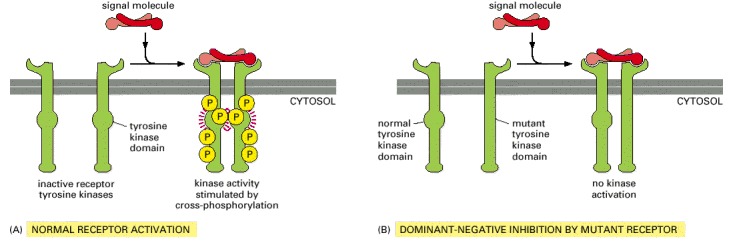
(A) In this example, the normal receptors dimerize in response to ligand binding. The two kinase domains cross-phosphorylate each other, increasing the activity of the kinase domains, which can now further activate the receptor dimer by phosphorylating other sites on the receptors. (B) The mutant receptor with an inactivated kinase domain can dimerize normally, but it cannot cross-phosphorylate a normal receptor in a dimer. For this reason, the mutant receptors, if present in excess, will block signaling by the normal receptors—a process called dominant-negative inhibition. Cell biologists frequently use this strategy to inhibit a specific type of receptor tyrosine kinase in a cell to determine its normal function.
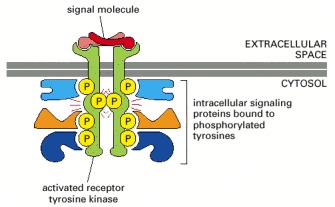
The activated receptor and its bound signaling proteins form a signaling complex that can then broadcast signals along multiple signaling pathways.
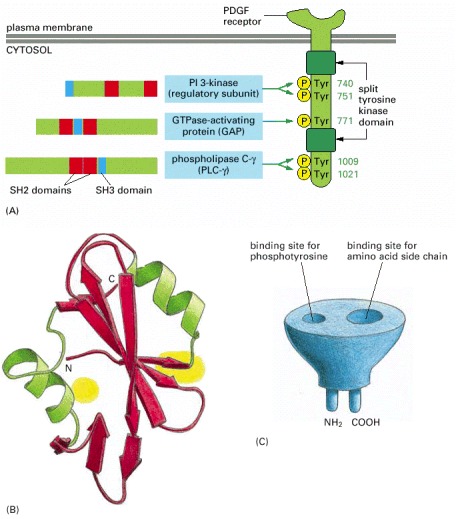
(A) This drawing of a PDGF receptor shows five of the tyrosine autophosphorylation sites, three in the kinase insert region and two on the C-terminal tail, to which the three signaling proteins shown bind as indicated. The numbers on the right indicate the positions of the tyrosines in the polypeptide chain. These binding sites have been identified by using recombinant DNA technology to mutate specific tyrosines in the receptor. Mutation of tyrosines 1009 and 1021, for example, prevents the binding and activation of PLC-γ, so that receptor activation no longer stimulates the inositol phospholipid signaling pathway. The locations of the SH2 (red) and SH3 (blue) domains in the three signaling proteins are indicated. (Additional autophosphorylation sites on this receptor are not shown, including those that serve as binding sites for the cytoplasmic tyrosine kinase Src and the adaptor proteins Grb2 and Shc, discussed later.) (B) The three-dimensional structure of an SH2 domain, as determined by x-ray crystallography. The binding pocket for phosphotyrosine is shown in yellow on the right, and a pocket for binding a specific amino acid side chain (isoleucine, in this case) is shown in yellow on the left (see also Figure 3-40). (C) The SH2 domain is a compact, “plug-in” module, which can be inserted almost anywhere in a protein without disturbing the protein's folding or function (see Figure 3-19). Because each domain has distinct sites for recognizing phosphotyrosine and for recognizing a particular amino acid side chain, different SH2 domains recognize phosphotyrosine in the context of different flanking amino acid sequences. (B, based on data from G. Waksman et al., Cell 72:1–20, 1993. © Elsevier.)
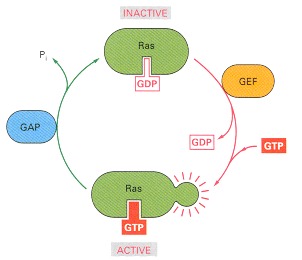
GTPase-activating proteins (GAPs) inactivate Ras by stimulating it to hydrolyze its bound GTP; the inactivated Ras remains tightly bound to GDP. Guanine nucleotide exchange factors (GEFs) activate Ras by stimulating it to give up its GDP; the concentration of GTP in the cytosol is 10 times greater than the concentration of GDP, and Ras rapidly binds GTP once GDP has been ejected. Several Ras-regulating GAPs (Ras GAPs) have been characterized in mammalian cells, including p120 GAP and neurofibromin (so named because it is encoded by the gene that is mutated in the common human genetic disease neurofibromatosis, which is associated with tumors of nerves). The Ras GAPs maintain most of the Ras protein (~95%) in unstimulated cells in an inactive GDP-bound state.
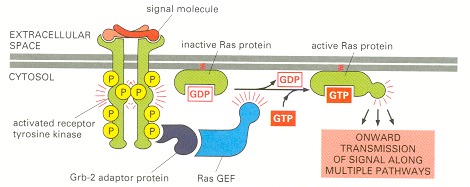
Most of the signaling proteins bound to the activated receptor are omitted for simplicity. The Grb-2 adaptor protein binds to a specific phosphotyrosine on the receptor and to the Ras guanine nucleotide exchange factor (GEF), which stimulates Ras to exchange its bound GDP for GTP. The activated Ras then activates several downstream signaling pathways, one of which is shown in Figure 15-56.
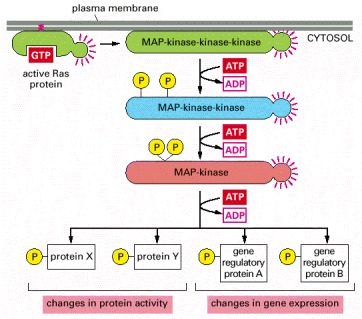
Multiple such pathways involving structurally and functionally related proteins operate in all eucaryotes, each coupling an extracellular stimulus to a variety of cell outputs. The pathway activated by Ras begins with a MAP-kinase-kinase-kinase called Raf, which activates the MAP-kinase-kinase Mek, which then activates the MAP-kinase called Erk. Erk in turn phosphorylates a variety of downstream proteins, including other kinases, as well as gene regulatory proteins in the nucleus. The resulting changes in gene expression and protein activity cause complex changes in cell behavior.
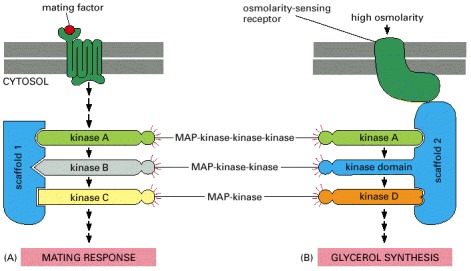
Budding yeast have at least six three-component MAP-kinase modules involved in a variety of biological processes, including the two responses illustrated here—a mating response and the response to high osmolarity. (A) The mating response is triggered when a mating factor secreted by a yeast of opposite mating type binds to a G-protein-linked receptor. This activates a G protein, and the βγ complex of the G protein indirectly activates the MAP-kinase-kinase-kinase (kinase A), which then relays the response onward. Once activated, the MAP-kinase (kinase C) phosphorylates and thereby activates several proteins that mediate the mating response, in which the yeast cell stops dividing and prepares for fusion. The three kinases in this module are bound to scaffold protein 1. (B) In a second response, a yeast cell exposed to a high-osmolarity environment is induced to synthesize glycerol to increase its internal osmolarity. This response is mediated by a transmembrane, osmolarity-sensing, receptor protein and a different MAP-kinase module bound to a second scaffold protein. (Note that the kinase domain of scaffold 2 provides the MAP-kinase-kinase activity of this module.) Although both pathways use the same MAP-kinase-kinase-kinase (kinase A, green), there is no cross talk between them, because the kinases in each module are tightly bound to different scaffold proteins, and the osmosensor is bound to the same scaffold protein as the particular kinase it activates.
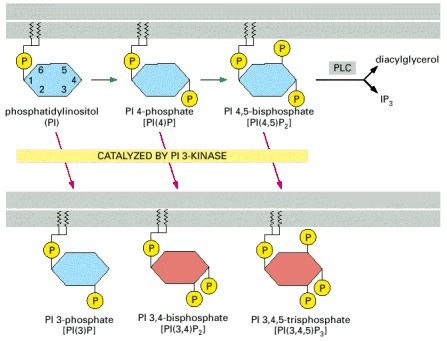
PI 3-kinase phosphorylates the inositol ring on carbon atom 3 to generate the inositol phospholipids shown at the bottom of the figure; the two lipids shown in red can serve as docking sites for signaling proteins with PH domains. The phosphorylations indicated by the green arrows are catalyzed by other inositol phospholipid kinases. As discussed earlier, phospholipase C (PLC-β or PLC-γ) can cleave PI(4,5)P2 to produce the two small signaling molecules diacylglycerol and inositol 1,4,5-trisphosphate (IP3).
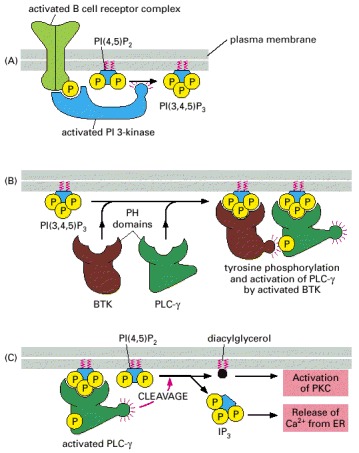
(A) PI 3-kinase binds to a phosphotyrosine on the activated B cell receptor complex and is thereby activated to phosphorylate the inositol phospholipid PI 4,5-bisphosphate [PI(4,5)P2], generating PI 3,4,5-trisphosphate [PI(3,4,5)P3]. (B) The PI 3,4,5-trisphosphate serves as a docking site for two signaling proteins with PH domains that then interact with each other. This causes the cytoplasmic tyrosine kinase BTK to phosphorylate and thereby activate PLC-γ at the membrane. (C) The activated PLC-γ then cleaves PI(4,5)P2 to generate diacylglycerol and IP3, which relay the signal onward.
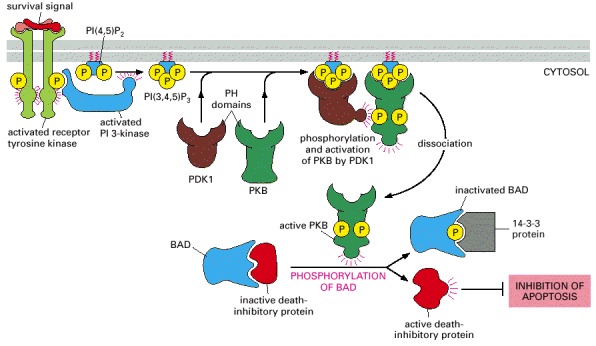
An extracellular survival signal activates a receptor tyrosine kinase, which recruits and activates PI 3-kinase. The PI 3-kinase produces PI(3,4,5)P3 and PI(3,4)P2 (not shown), both of which serve as docking sites for two serine/threonine kinases with PH domains—protein kinase B (PKB) and the phosphoinositol-dependent kinase PDK1. The binding of PKB to the inositol lipids alters its conformation so that the protein can be phosphorylated and activated by PDK1. The activated PKB now dissociates from the plasma membrane and phosphorylates the BAD protein, which, when unphosphorylated, holds one or more death-inhibitory proteins in an inactive state. Once phosphorylated, BAD releases the inhibitory proteins, which now can block programmed cell death (apoptosis) and thereby promote cell survival.
As shown, once phosphorylated, BAD binds to a ubiquitous cytosolic protein called 14-3-3, which keeps BAD out of action. There are about 20 14-3-3 proteins in human cells, all of which bind to specific phosphoserine-containing motifs in proteins. The activation of other signaling pathways can also lead to BAD phosphorylation and the promotion of cell survival (not shown).
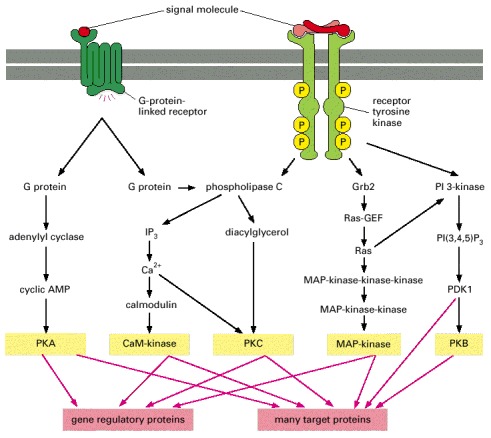
In this schematic example, the five kinases (shaded yellow) at the end of each pathway phosphorylate target proteins (shaded red), some of which are phosphorylated by more than one of the kinases. The specific phospholipase C activated by the two types of receptors is different: G-protein-linked receptors activate PLC-β, whereas receptor tyrosine kinases activate PLC-γ (not shown).
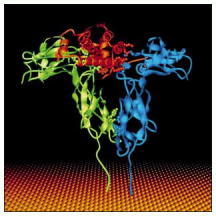
The hormone (red) has cross-linked two identical receptors (one shown in green and the other in blue). Hormone binding activates cytoplasmic tyrosine kinases that are tightly bound to the cytosolic tails of the receptors (not shown). The structures shown were determined by x-ray crystallographic studies of complexes formed between the hormone and extracellular receptor domains produced by recombinant DNA technology. It was entirely unexpected that a monomeric ligand such as growth hormone would cross-link its receptors, as it requires that the two identical receptors recognize different parts of the hormone. As mentioned earlier, EGF does the same thing. (From A.M. deVos, M. Ultsch, and A.A. Kossiakoff, Science 255:306–312, 1992. © AAAS.)
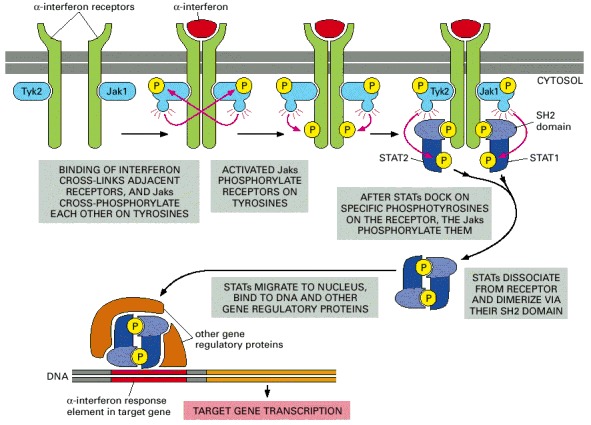
The binding of interferon either causes two separate receptor polypeptide chains to dimerize (as shown) or reorients the receptor chains in a preformed dimer. In either case, the associated Jaks are brought together so that they can cross-phosphorylate each other on tyrosines, starting the signaling process. The two different receptor chains are associated with different Jaks (Tyk2 and Jak1), and they recruit different STATs (STAT1 and STAT2). The STATs dissociate from the receptors and form heterodimers when activated by phosphorylation, and they bind to specific DNA sequences in the cell nucleus, where, together with other gene regulatory proteins, they induce the transcription of adjacent genes.
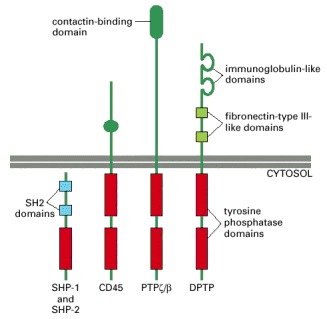
The cytoplasmic tyrosine phosphatases SHP-1 and SHP-2 have similar structures, with two SH2 domains. The three transmembrane receptorlike tyrosine phosphatases have two tandemly arranged intracellular phosphatase domains, with the one closest to the membrane providing most or all of the catalytic activity. DPTP is a Drosophila protein; the others in the figure are mammalian proteins.
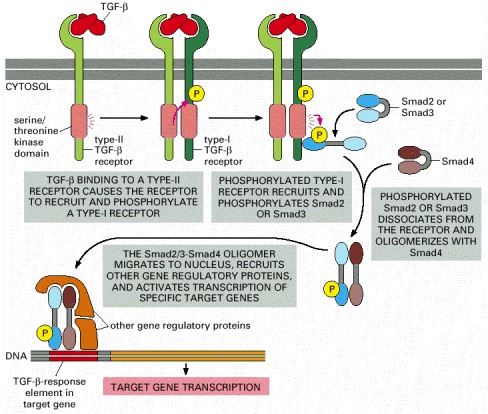
Note that TGF-β is a dimer and that Smads open up to expose a dimerization surface when they are phosphorylated. Several features of the pathway have been omitted for simplicity, including the following: (1) The type-I and type-II receptor proteins are both thought to be dimers. (2) The type-I receptors are normally associated with an inhibitory protein, which dissociates when the type-I receptor is phosphorylated by a type-II receptor. (3) The individual Smads are thought to be trimers. (4) An anchoring protein (called SARA, for Smad anchor for receptor activation) helps to recruit Smad2 or Smad3 to the activated type I receptor by binding to the receptor, to the Smad, and to inositol phopholipid molecules in the plasma membrane. (5) The function of certain Smads is regulated by enzymes that enhance their ubiquitylation and thereby their degradation.
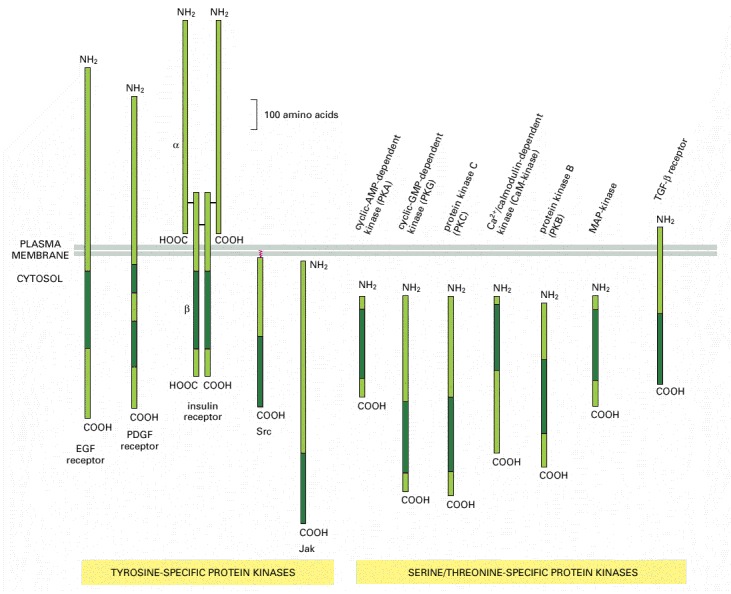
The size and location of their catalytic domains (dark green) are shown. In each case the catalytic domain is about 250 amino acids long. These domains are all similar in amino acid sequence, suggesting that they have all evolved from a common primordial kinase (see also Figure 3-65). Note that all of the tyrosine kinases shown are bound to the plasma membrane (Jaks are bound by their association with cytokine receptors), whereas most of the serine/threonine kinases are in the cytosol.
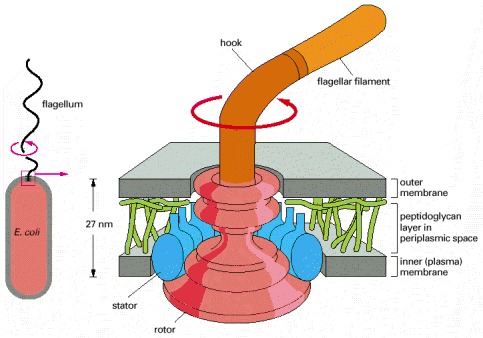
The flagellum is linked to a flexible hook. The hook is attached to a series of protein rings (shown in red), which are embedded in the outer and inner (plasma) membranes. The rings form a rotor, which rotates with the flagellum at more than 100 revolutions per second. The rotation is driven by a flow of protons through an outer ring of proteins (see Figure 14-17), the stator, which also contains the proteins responsible for switching the direction of rotation. (Based on data from T. Kubori et al., J. Mol. Biol. 226:433–446, 1992, and N.R. Francis et al., Proc. Natl. Acad. Sci. USA 89:6304–6308, 1992.)
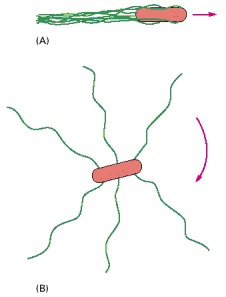
(A) When the flagella rotate counterclockwise, they are drawn together into a single bundle, which acts as a propeller to produce smooth swimming. (B) When the flagella rotate clockwise, they fly apart and produce tumbling.
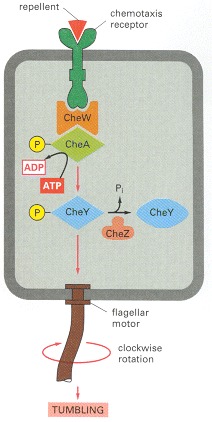
The histidine kinase CheA is stably bound to the receptor via the adaptor protein CheW. The binding of a repellent increases the activity of the receptor, which stimulates CheA to phosphorylate itself on histidine. CheA quickly transfers its covalently bound, high-energy phosphate directly to CheY to generate CheY-phosphate, which then diffuses away, binds to the flagellar motor, and causes the motor to rotate clockwise, which results in tumbling. The binding of an attractant has the opposite effect: it decreases the activity of the receptor and therefore decreases the phosphorylation of CheA and CheY, which results in counterclockwise flagellar rotation and smooth swimming. CheZ accelerates the autodephosphorylation of CheY-phosphate, thereby inactivating it. Each of the phosphorylated intermediates decays in about 10 seconds, enabling the bacterium to respond very quickly to changes in its environment (see Figure 15-10).
Chapter 15.5 - Figures
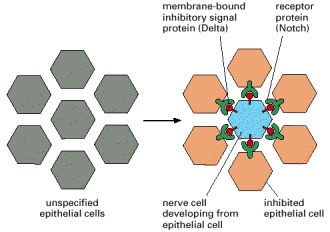
When individual cells in the epithelium begin to develop as neural cells, they signal to their neighbors not to do the same. This inhibitory, contact-dependent signaling is mediated by the ligand Delta that appears on the surface of the future nerve cell and binds to Notch proteins on the neighboring cells. In many tissues, all the cells in a cluster initially express Delta and Notch, and a competition occurs, with one cell emerging as winner, expressing Delta strongly and inhibiting its neighbors from doing likewise. In other cases, additional factors interact with Delta or Notch to make some cells susceptible to the lateral inhibition signal and others deaf to it.
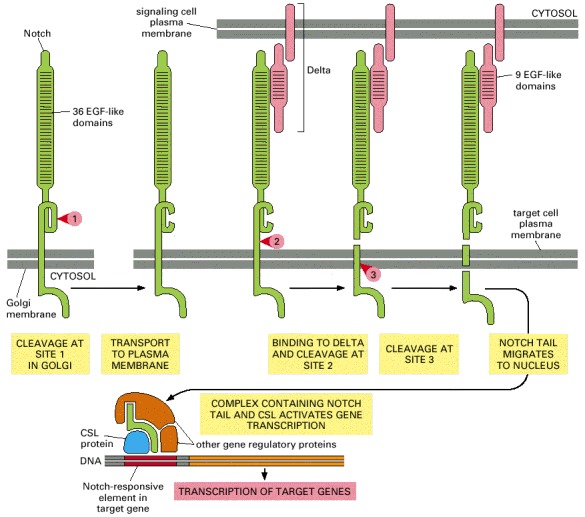
The numbered red arrowheads indicate the sites of proteolytic cleavage. The first proteolytic processing step occurs within the trans Golgi network to generate the mature heterodimeric Notch receptor that is then displayed on the cell surface. The binding of Delta, which is displayed on a neighboring cell, triggers the next two proteolytic steps. Note that Notch and Delta interact through their repeated EGF-like domains. Some evidence suggests that the tension exerted on Notch by the endocytic machinery of the interacting cells triggers the cleavage at site 2.
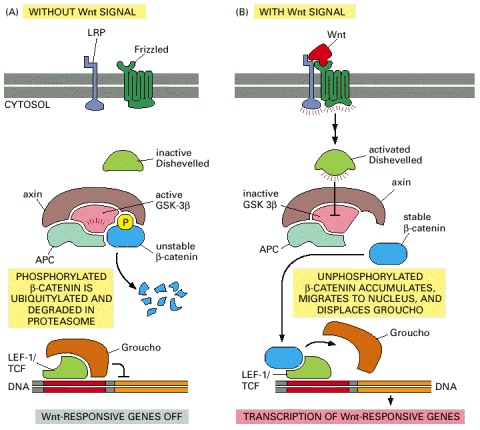
(A) In the absence of a Wnt signal, some β-catenin is bound to the cytosolic tail of cadherin proteins (not shown) and any cytosolic β-catenin becomes bound by the APC-axin-GSK-3β degradation complex. In this complex, β-catenin is phosphorylated by GSK-3β, triggering its ubiquitylation and degradation in proteasomes. Wnt-responsive genes are kept inactive by the Groucho corepressor protein bound to the gene regulatory protein LEF-1/TCF. (B) Wnt binding to Frizzled and LRP activates Dishevelled by an unknown mechanism. By an equally mysterious mechanism, which requires casein kinase 1 (not shown), this leads to the inactivation of GSK-β3 in the degradation complex. As a result, the phosphorylation and degradation of β-catenin is inhibited, and β-catenin accumulates in the cytoplasm and nucleus. In the nucleus, β-catenin binds to LEF-1/TCF, displaces Groucho, and acts as a coactivator to stimulate the transcription of Wnt target genes.
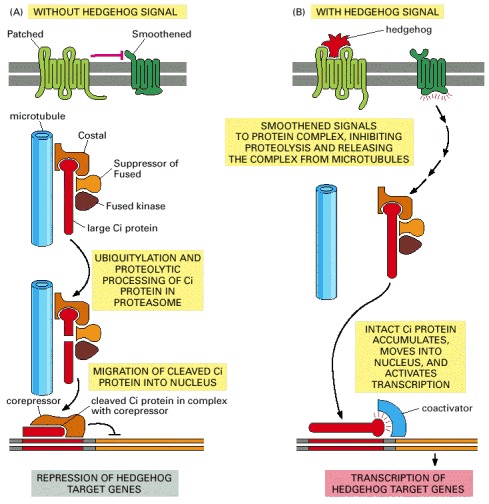
(A) In the absence of Hedgehog, the Patched receptor inhibits Smoothened probably by promoting the degradation or intracellular sequestration of Smoothened. The Ci protein is located in a protein complex and is cleaved to form a transcriptional repressor, which accumulates in the nucleus to help keep Hedgehog target genes inactive. The protein complex includes the serine/threonine kinase Fused, the anchoring protein Costal (which binds the complex to microtubules), and the adaptor protein Suppressor of Fused. (B) Hedgehog binding to Patched relieves the inhibition of Smoothened, which now signals to the protein complex to stop processing Ci, to dissociate from microtubules, and to release the unprocessed Ci so it can accumulate in the nucleus and activate the transcription of Hedgehog-responsive genes. Most of the molecular events in the pathway are unknown.
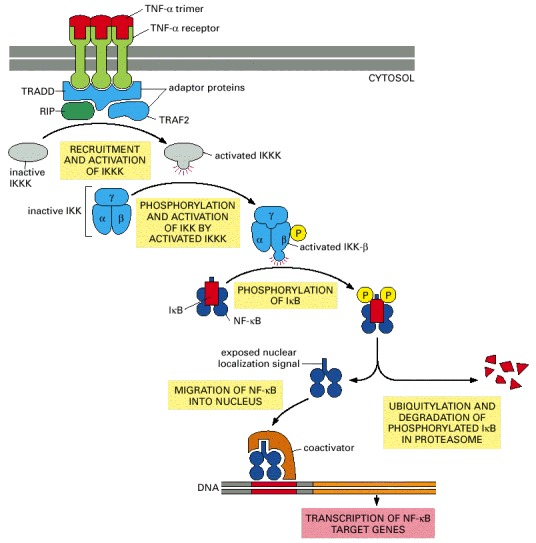
Both TNF-α and its receptors are trimers. The binding of TNF-α causes a rearrangement of the clustered cytosolic tails of the receptors, which now recruit a number of intracellular signaling proteins, including the receptor-interacting protein kinase (RIP) and two adaptor proteins, TNF-associated death-domain protein (TRADD) and TNF-receptor-associated factor 2 (TRAF2). These then recruit and activate an unidentified kinase, IκB kinase kinase kinase (IKKK), which phosphorylates and activates IκB kinase kinase (IKK). IKK is a heterotrimer composed of two kinase subunits (IKK-α and IKK-β) and a regulatory adaptor subunit called IKK-γ. The IKK-β then phosphorylates IκB on two serines, which marks the protein for ubiquitylation and degradation in proteasomes. The nuclear localization signal on the free NF-κB now directs the transport of this protein into the nucleus where, in collaboration with coactivator proteins, it stimulates the transcription of its target genes. In addition to target genes involved in the inflammatory response, NF-κB also activates the IκB gene, providing negative feedback (not shown). The role of RIP is unclear: although it is required for TNF signaling, its kinase domain is not.
Chapter 15.6 - Figures
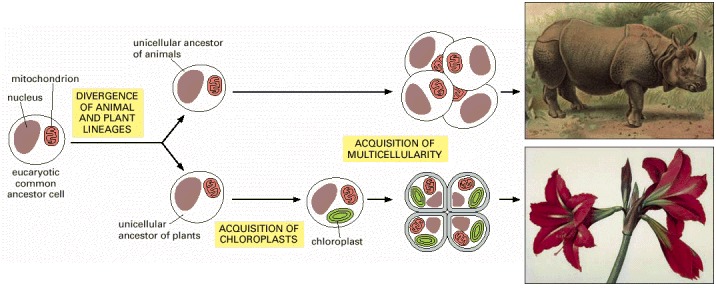
The plant lineage acquired chloroplasts after the two lineages diverged. Both lineages independently gave rise to multicellular organisms—plants and animals. (Paintings courtesy of John Innes Foundation.)
(Courtesy of David Lawson.)
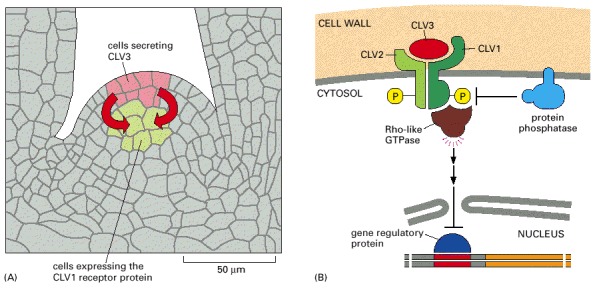
(A) Cells in the outer layer of the meristem secrete CLV3 protein, which binds to CLV1 receptor proteins on target cells in an adjacent, more central region of the meristem, presumably stimulating the differentiation of the target cells. (B) Some parts of the intracellular signaling pathway activated by CLV3 binding. The CLV receptor protein is thought to be a homodimer or heterodimer, which phosphorylates itself on serines and threonines, thereby activating the receptor and leading to the activation of a Rho-like GTPase. The signaling pathway after this point is unclear, but it leads to the inhibition of a gene regulatory protein in the nucleus, thereby blocking the transcription of genes that otherwise might inhibit differentiation. The phosphatase dephosphorylates the receptor and thereby negatively regulates the signaling pathway.
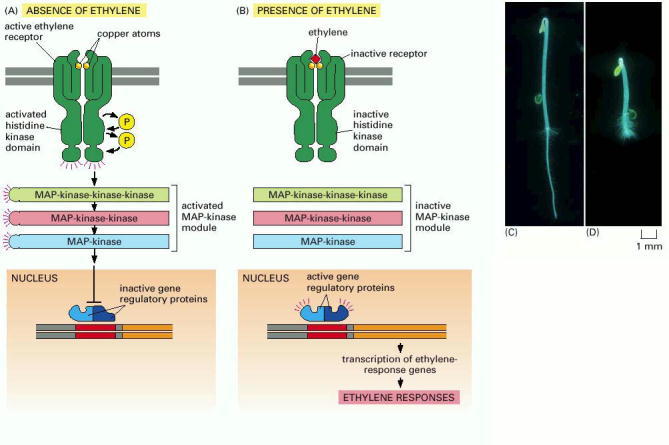
(A) In the absence of ethylene, the receptors and the MAP-kinase module are active, leading to inhibition of the gene regulatory proteins in the nucleus that are responsible for the transcription of ethylene-responsive genes. (B) In the presence of ethylene, the receptors and the MAP-kinase module are inactive, so the ethylene-responsive genes are transcribed. (C and D) The ethylene-mediated “triple response” that occurs when the growing shoot of a germinating seedling encounters an obstacle underground. After such an encounter (D), the shoot thickens, and the protective hook (at top) increases its curvature to protect the tip of the shoot. (C and D, courtesy of Melanie Webb.)
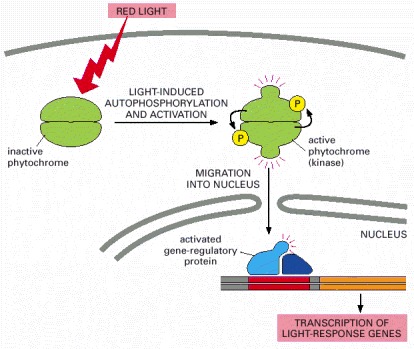
When activated by light, the phytochrome, which is a dimer, phosphorylates itself and then moves into the nucleus, where it activates gene regulatory proteins to stimulate the transcription of specific genes.