Exam - 1 - Key Topics
1.) Be Able to Classify Reactions from Diagram
Deamination
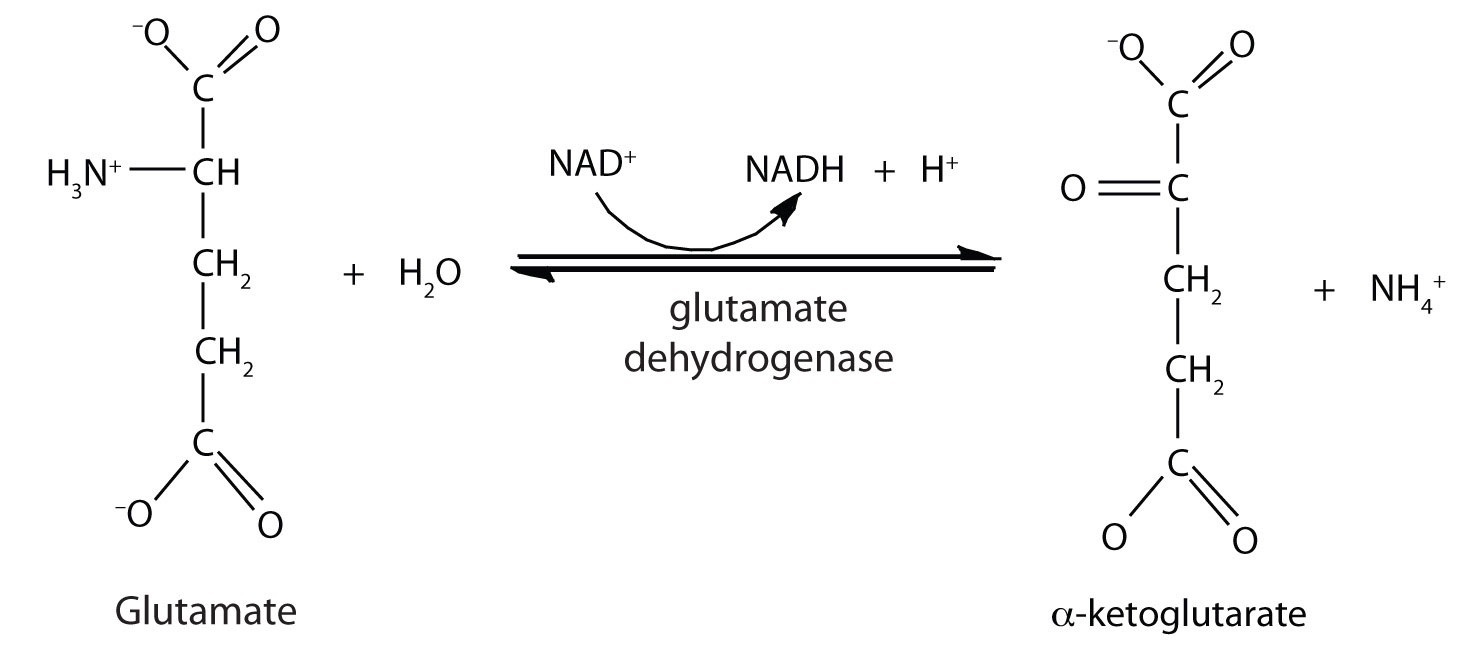
Decarboxylation
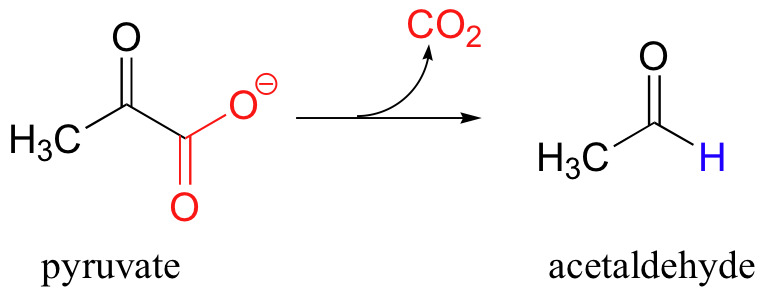
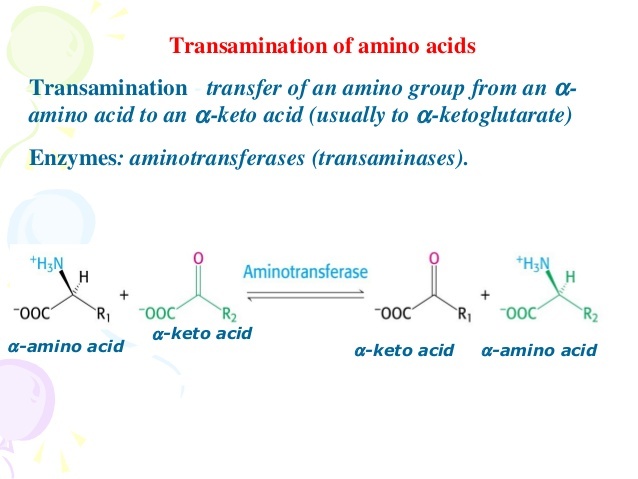
Transamination
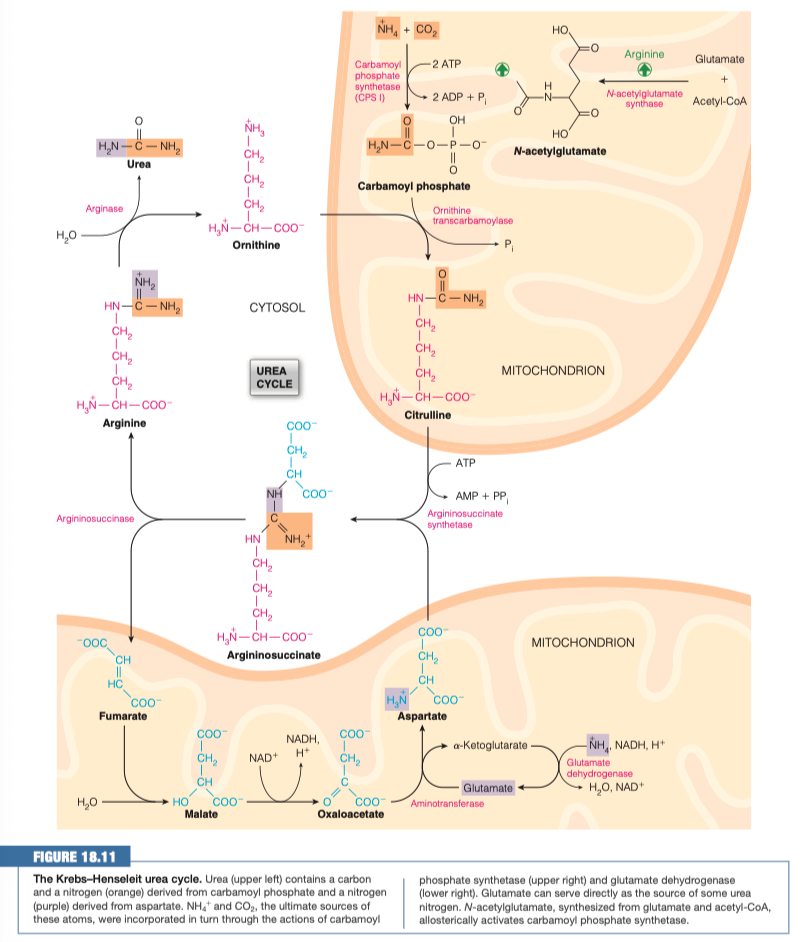
2.) What is the Purpose of the Urea Cycle?
- Ammonia (NH4+) is toxic, and must be removed from the body
- Urea Cycle is the process of converting ammonia into non-toxic urea, which can then be secreted via the kidneys.
3.) How is the urea cycle regulated?
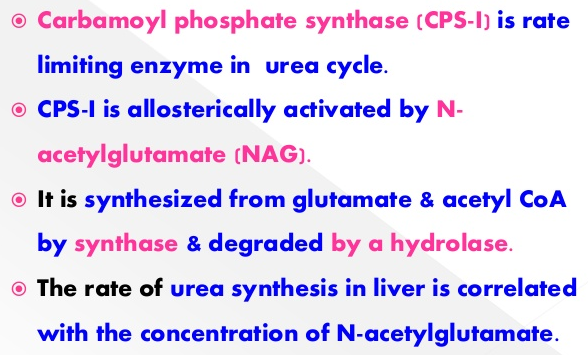
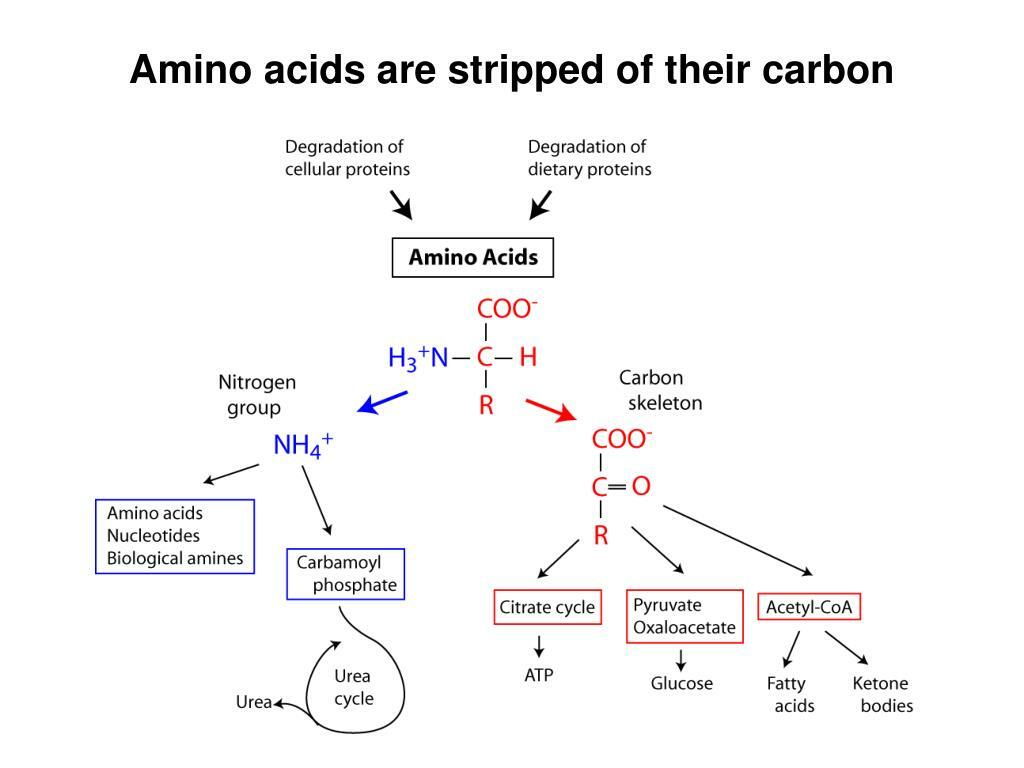
4.) What is the Process of Amino Acid Degredation?
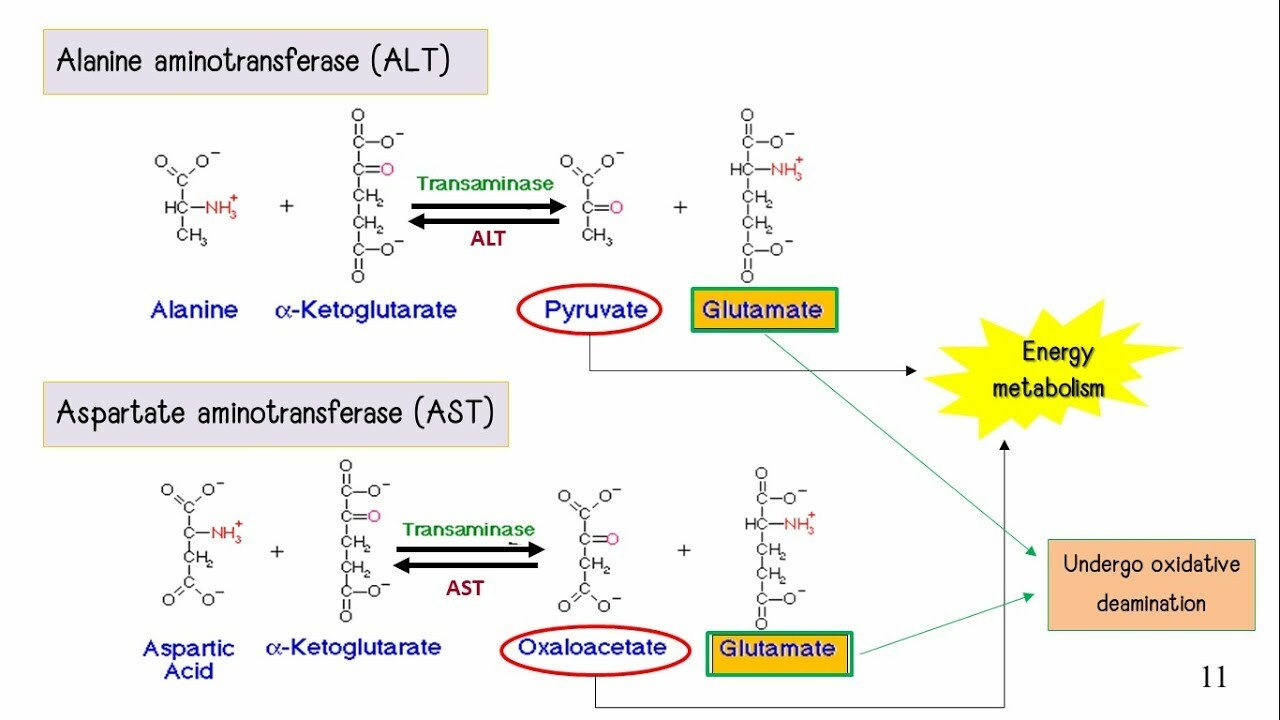
- Step 1 = Transamination = Nitrogen is removed from the carbon skeleton and transferred to α-ketoglutarate, which yields glutamate
- Step 2 = The Carbon Skeleton gets recycled into glucose or ketone bodies via their respective pathways.
- Step 2 = Ammonium is converted into urea via Urea Cycle
5.) Where Can Nitrogen Enter the Urea Cycle?
1.) Free NH4+ in Mitochondria --> Glutamate --> Aspartate
2.) Free NH4+ in Mitochondria --> Carbamoyl Phosphate --> Citrulline
6.) Study Figure 18.7
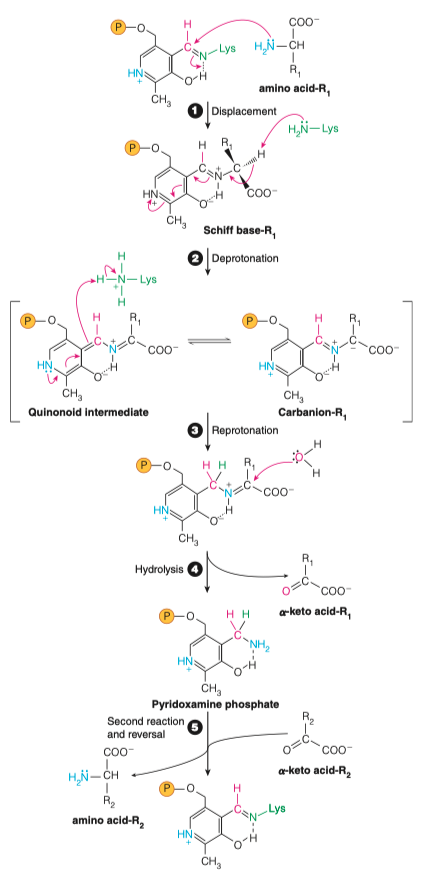
Summary 1
1.) Lysine is displaced to form a Schiff Base
2.) Schiff base is deprotonated
3.) Quinonoid intermediate is re-protonated
4.) Carboxylate is hydrolyzed
5.)
a. The transamination reaction with a second a-keto acid (R2) b. Conversion, by reversal of steps 1-4, to enzyme-bound PLP and amino acid (R2).
Summary - 2
- Incoming AA1 displaces the connection between PLP and enzyme
- Hydrogen is given to enzyme from AA1
- Hydrogen is taken from enzyme and placed onto PLP skeleton at C4
- Water is used to split an alpha keto acid from PLP intermediate making PMP
- a. The transamination reaction with a second a-keto acid (R2) b. Conversion, by reversal of steps 1-4, to enzyme-bound PLP and amino acid (R2).
7.) Study Figure 18.10, 18.11 and 18.12
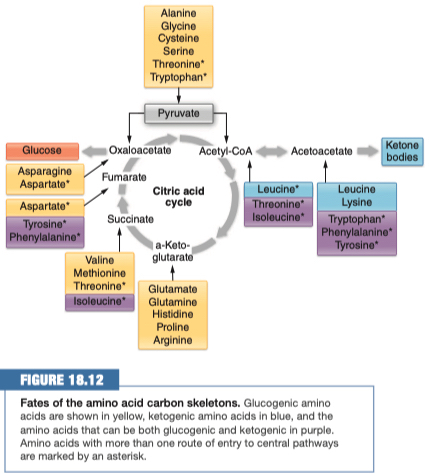
Yellow = Glucogenic
Ketogenic = Blue
Purple = Glucogenic and Ketogenic
7.) What does it mean that some amino acids are glucogenic and some are ketogenic?
Some amino acids will break apart into two sections (skeletons), where one section goes into the glucose pathway, while the other section goes into the ketone pathway.
8.) What are sources of amino acids inside the body?
1.) Digestion / Free Ammonia
2.) DeNovo Synthesis
3.) Protein Turnover/Breakdown
9.) What are the main uses of amino acids?
- To Make Energy
- To Make Neurotransmitters
- To Make Hormones
- To Make Biological regulators
10.) What is the role of PLP?
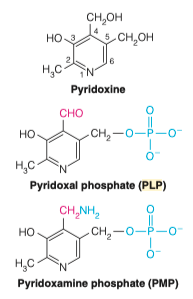
- PLP is used to catalyze different transamination reactions
- Vitamin B6 is the precursor to PLP
- PLP serves to hold the nitrogen from AA1 to give to AA2 as they cannot directly pass it from one to the other
11.) What is the role of THF?
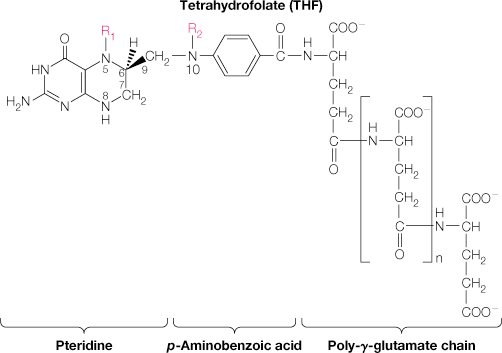
Folate is precursor for THF
THF is the single carbon donor
There are a lot of molecules in your body that need a carbon atom.
Difference between Uracil vs Thiamine is a methyl. And this methyl has to come from THF.
The main difference between the THF versions is which oxidation level the THF is carrying. These various forms can be used in many different metabolic reactions
12.) What happens during the degradation of asparagine into oxaloacetate?
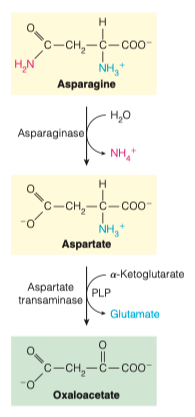
Asparaginase catalyzes hydrolytic cleavage of the asparagine amide to aspartate and ammonium.
Aspartate is then transaminated directly to oxaloacetate.
Amino Acid Acceptor = ⍺-Ketoglutarate
Coenzyme = PLP
13.) What are the four main products created when nitrogen is assimilated into the body?
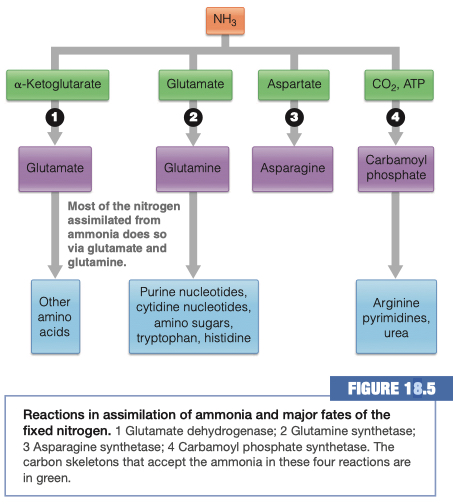
- 1.) Glutamate
- 2.) Glutamine
- 3.) Asparagine
- 4.) Carbamoyl Phosphate
14.) Why is glutamate production directly related to glutamine production?
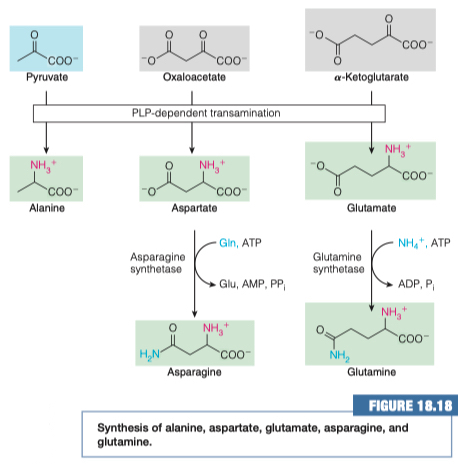
- Glutamate can accept a second ammonia to form glutamine.
- You can't make glutamine without first having the glutamate substrate
- ⍺-Ketogluterate is being doubly animated to build glutamine.
15.) What does it mean for an amino acid to be essential? How do we obtain these amino acids? What originally makes them?
- Essential Amino Acids = ones we can not synthesize, and must get from external sources
- We get essential amino acids from diet.
- Plants and bacteria originally synthesize them
16.) How is arginine synthesized?
- Citrulline and Aspartate Combine to form --> Argininosuccinate
- Argininosuccinate then splits into Fumarate and Arginine
- Argininosuccinate --> Arginine --> Urea and Ornithine ( cytosol )
- Argininosuccinate --> Fumarate --> Malate --> Oxaloacetate --> Aspartate ( mitochondria )
- Arginine is an Essential amino acid because only a catalytic amount is produced in the urea cycle.
17.) What is an 𝛼-Keto acid?
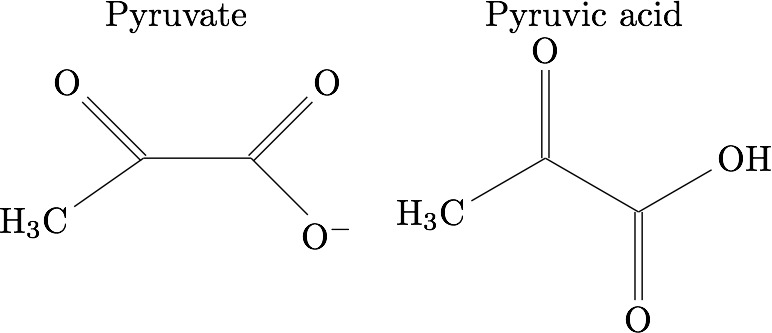
- Has carboxylic acid and ketone
18.) Why do you think glutamine is used to transport ammonia and not glutmate?
- Glutamic acid has an acidic side chain.
- Glutamine, has a neutral NH2 group
19.) Which 𝛼-amino acids are not used for protein synthesis in the urea cycle?
- Ornithine
- Citruline
- Argininosuccinate
20.) The enzymes that catalyze reactions in the urea cycle are induced during high protein diets and during starvation. Explain
- If you are ingesting a lot of protein, you have to break it down
- If you are starving, you have to break it down protein to survive.
21.) Give an example how how the biosynthesis pathway is different than degradation
Similarity 1: ⍺-Ketoglutarate can form Glutamate which can form Glutamine, Proline, Arginine. All the products of ⍺-Ketoglutarate can be broken back down into ⍺-Ketoglutarate
Similarity 2: Oxaloacetate can form Aspartate which can then form Asparagine. Asparagine can be broken back down into oxaloacetate.
Similarity 3: Pyruvate can form Alanine and Isoleucine. Alanine and Isoleucine can be broken back down into Pyruvate
The other pathways have completely different synthesis and decomposition routes.
Example Difference 1: Histidine can form ⍺-Ketoglutarate , but you can't do the reverse process where ⍺-Ketoglutarate forms Histidine.
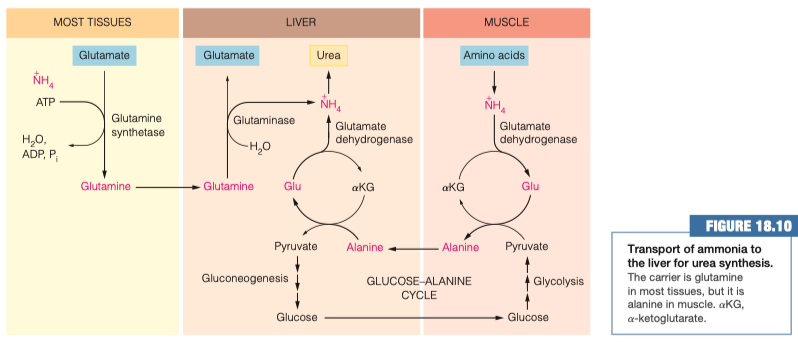
22.) The Glucose-Alanine Cycle - Real World Example
Titin, a protein in human muscle cells, plays a role in muscle elasticity. The cytosolic protein contains 30,000 amino acids. Once the protein stops working how will it be degraded, utilized, and excreted?
The dysfunctional protein in the muscle cytosol is tagged by ubiquitin.
A proteasome comes along and recognizes the ubiquitin tags, and starts breaking it down to amino acids
The breakdown of amino acids can follow different routes.
Some go into energy production, some get remade into other proteins, some are used to synthesize non-amino acids, some enter the urea cycle.
Focusing just on the ones that enter urea cycle.

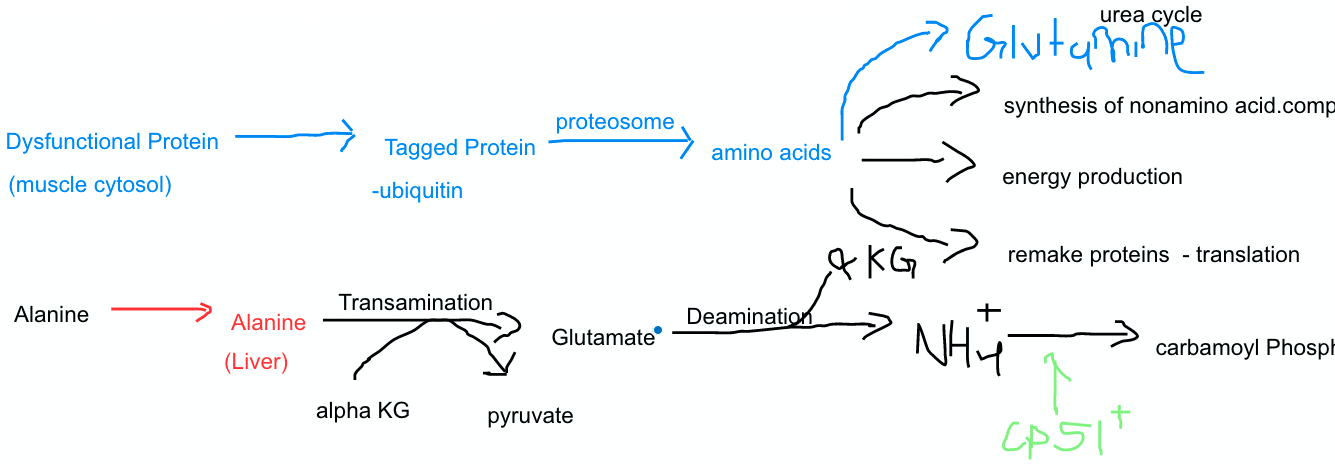
Preparation for the Urea Cycle
We need to somehow get NH4+ into the liver, so we can begin the urea cycle.
Since we are starting in the muscle tissue, we will enter via alanine part of the Glucose-Alanine Cycle
Free Floating NH4+ in Muscle Tissue --> Glutamate --> Alanine --> Liver
Now we have the nitrogen that was in the muscle tissue converted into alanine, inside the liver.
This alanine is then transaminated by ⍺-Ketogluterate and split into glutamate and pyruvate
Pyruvate will be recycled, but the glutamate will undergo deamination and split into NH4+ and ⍺-Ketogluterate.
Now we have our NH4+ inside the liver, and it can finally start the urea cycle.
Steps 1-6 were necessary in order to transfer the reactive NH4+ from of the muscle tissue to the liver cells.
Urea Cycle Time
There are two routes we can take to convert NH4+ into intermediates that are safe to leave the mitochondria
Route 1:
a.
b.
Route 2:
a.
b.
The intermediates we built in Step 1 leave the mitochondria and combine.
Argininosuccinate then breaks apart into Fumarate which is recycled into the mitochondria, and Arginine.
Arginine then uses its enzyme arginase, to break apart into ornithine which is recycled into the mitochondria, and our final product, Urea
Chapter 18 Summary
- Although inorganic nitrogen is abundant, metabolism of most organisms is limited by nitrogen bioavailability. Reduction of N2 in biological nitrogen fixation and reduction of nitrate in plant and bacterial metabolism generate ammonia, which all organisms can utilize.
- Proteins are in a continual state of turnover and replacement. The turnover is partly for replacement of damaged proteins and partly as the result of normal cellular regulatory mechanisms. Most amino acids released by protein turnover are reutilized for protein synthesis.
- Transamination and numerous additional reactions undergone by amino acids use pyridoxal phosphate as a coenzyme. Tetrahydrofolate binds one-carbon units at three different oxidation states, interconverts them, and transfers them in the synthesis of purine nucleotides, thymidine nucleotides, and several amino acids. B-12 coenzymes include methylcobalamin, which participates in methionine biosynthesis, and 5′-deoxyadenosylcobalamin, the coenzyme for methylmalonyl-CoA mutase. Folate metabolism presents various chemotherapeutic targets, and folate and B-12 deficiencies both have important clinical consequences.
- When amino acids are degraded, either for catabolism of an oversupply or when needed for energy generation, the first step is usually removal of the α-amino group, either through transamination or oxidative deamination. The resultant ammonia is excreted directly (in fish), converted to uric acid (in most reptiles, insects, and birds), or converted to urea (in mammals). Urea synthesis is a cyclic pathway involving ornithine and arginine as intermediates
- The capacity for amino acid synthesis varies greatly among organisms. Mammals require about half of the 20 common amino acids in the diet. Amino acids are synthesized from intermediates in the citric acid cycle, glycolysis, and the pentose phosphate pathway.
- Amino acids play numerous roles as intermediates in the biosynthesis of other metabolites. Amino acids are involved in the biosynthesis of purine nucleotides (glutamine, glycine, serine), pyrimidine nucleotides (aspartate, glutamine), polyamines and methyl groups (methionine), glutathione (glutamate, cysteine, glycine), creatine phosphate (arginine), neurotransmitters (tyrosine, tryptophan, glutamate, arginine), lignin, aromatic compounds and pigments (phenylalanine), hormones (tyrosine, histidine), porphyrins (glycine and glutamate in plants), and other amino acids. The roles of amino acids as neurotransmitters and neurotransmitter precursors are particularly important, as are their roles in porphyrin synthesis.